Abstract
Nearly 30 years after the discovery in 1964 of the psychoactive ingredient of cannabis (Cannabis sativa), Δ9-tetrahydrocannabinol, its endogenous counterparts were discovered and collectively termed endocannabinoids (eCBs): N-arachidonoylethanolamine (anandamide) in 1992 and 2-arachidonoylglycerol in 1995. Since then, intense research has identified additional eCBs and an ensemble of proteins that bind, synthesize and degrade them, the so-called eCB system. Altogether, these new compounds have been recognized as key mediators of several aspects of human pathophysiology, and in particular of female fertility. Here, the main features of the eCB system are presented, in order to put in a better perspective the relevance of eCB signaling in virtually all steps of human reproduction and to highlight emerging hopes that elements of this system might indeed become novel targets to combat fertility problems.
1. Introduction
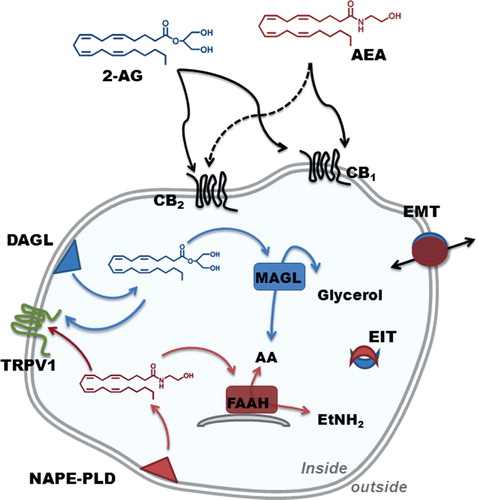
Following the discovery (1964) of the major psychoactive cannabis (Cannabis sativa) constituent, Δ9-tetrahydrocannabinol (THC; ), two 7-transmembrane G-protein-coupled receptors able to bind it were identified (i.e., type 1 [CB1] and type 2 [CB2] cannabinoid receptors), along with their endogenous ligands, called ‘endocannabinoids’ (eCBs). The two best-known members of this quite large family of bioactive lipids are the arachidonic acid derivatives N-arachidonoylethanolamine (anandamide, AEA; ) and 2-arachidonoylglycerol (2-AG; ). Most of the actions of these compounds depend on proteins that bind and metabolize them, and that together with eCBs themselves form the so-called eCB system (ECS). The latter is schematically depicted in , as we know it in reproductive cells. Besides eCBs, CB1 and CB2, the ECS also includes the transient receptor potential vanilloid 1 (TRPV1) ion channel that is activated by AEA and 2-AG at an intracellular binding site and numerous metabolic enzymes Citation[1].
Table 1. Δ9-Tetrahydrocannabinol, endocannabinoids and major eCB system elements in female reproductive cells.
The general view of eCB metabolism is that AEA is synthesized mainly by N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), whereas two diacylglycerol lipases (DAGLα/β) are responsible for the synthesis of 2-AG. The eCB-mediated effects are then terminated by a fast catabolism, mainly through hydrolysis of AEA by fatty acid amide hydrolase (FAAH), and of 2-AG by monoacylglycerol lipase (MAGL) (). Alternate to these hydrolytic routes, AEA and 2-AG can be oxidized by COX-2, distinct lipoxygenases or CYP450 Citation[2], yet the impact of these oxidative pathways on female fertility remains to be established. Incidentally, eCB signaling holds promise also for therapeutic exploitation in male reproductive dysfunctions, a rather complex topic that has been recently reviewed Citation[3].
2. Main actions of ECS in female reproduction
There is evidence to suggest that virtually all stages of female reproduction, from oocyte development to parturition, are regulated by eCB signaling through binding to CB1, CB2 and/or TRPV1 receptors, as well as by the activity and expression of the metabolic enzymes that determine the endogenous tone of eCBs (). This topic has been recently covered by comprehensive reviews Citation[3-7], and the main findings can be summarized as follows. It has been shown that CB1 and CB2 are expressed in the preimplantation embryos, and that their activation by uterine AEA can interfere with embryo development. Notably, oviductal transport of the fertilized egg and its development to the morula stage are controlled by a gradient of AEA concentration (increasing from ampulla to isthmus), that is kept by increasing NAPE-PLD and decreasing FAAH expression in epithelial cells of the oviduct Citation[4]. At the same time, FAAH expression increases in embryonic cells that need to be protected against cytotoxicity of AEA Citation[6]. Any alteration of this fine-tuning leads to ectopic pregnancy, a serious condition that occurs at a rate of ∼14 cases per 1000 pregnancies in North America Citation[8] and represents a leading cause of maternal mortality in the first trimester of gestation. In the same line, CB1 is expressed at low levels within both the fallopian tube and endometrium of women with ectopic pregnancy, suggesting that aberrant eCB signaling within the fallopian tubes may lead to this pathology Citation[4]. Additionally, levels of AEA and CB1 are high in non-receptive uteri and interimplantation sites, but they decline to lower levels in receptive uteri and implantation sites, suggesting that optimally balanced eCB signaling is critical to synchronize preimplantation embryo development and prepare the endometrium for implantation (reviewed in Ref. Citation[4]). Again, low NAPE-PLD and high FAAH activity and expression result in low AEA levels at implantation sites, whereas the opposite occurs at interimplantation sites, overall supporting the view that a ‘metabolic control’ of eCB tone has a critical impact on uterine receptiveness Citation[3-7]. In keeping with this concept, trophoblast survival is favored by increased FAAH, and even in maternal lymphocytes activity and expression of this enzyme are upregulated by pro-fertility signals like leptin, progesterone and T helper 2 (Th2) cytokines; instead, they are downregulated by anti-fertility signals like Th1 cytokines Citation[5,6]. Consistently, low FAAH activity and expression Citation[9] and high AEA levels in peripheral blood Citation[10] are associated with pregnancy failure in women with spontaneous or threatened miscarriage, as well as in those undergoing in vitro fertilization-embryo transfer Citation[11]. Finally, placentation is accompanied by reduced CB1 and increased FAAH, whereas parturition requires increased CB1 only Citation[3-7]. More recently, a potential role for N-palmitoylethanolamine (PEA; ), an eCB-like compound endowed with anti-inflammatory properties, and CB2 in female reproductive events has been proposed Citation[12]. Indeed, genetic ablation of CB2 confers resistance to inflammation-induced preterm birth in mice, and the same receptor subtype is preferentially expressed (over CB1) in granulosa cells Citation[13]. Additional investigations will allow to better understand the physiological relevance and the therapeutic potential of these observations.
Table 2. Involvement of eCB system elements in female fertility. See text for details.
Taken together, a tightly regulated eCB signaling threshold across multiple early pregnancy events is critical for female reproductive success Citation[14], and metabolic control of eCB content (especially of AEA through NAPE-PLD and FAAH) seems to be the checkpoint of their CB1-dependent effects on reproductive events.
3. Expert opinion
Based on the available evidence, it can be concluded that eCB signaling definitely holds potential as therapeutic target in multiple female reproductive events. In particular, animal and human data clearly point to CB1, NAPE-PLD and FAAH as potential key regulators of reproduction, suggesting that drugs that are capable of modulating their activity could ameliorate or even influence fertility rates.
In the case of CB1, it appears rather difficult to make of it a therapeutic target, because it is almost ubiquitous and both silencing (by antagonists) and amplification (by agonists) of CB1 signaling can adversely impact female reproductive functions. Thus, the therapeutic utility of agonists and antagonists of this receptor will strongly depend on when and where they are administered. This point raises the concern that, within the ECS, the real challenge is to design magic bullets capable of hitting their specific target at the right time and in the right place, avoiding severe side effects that have so far prevented exploitation of ECS-oriented drugs in many human disease conditions, both centrally and peripherally Citation[15].
In the case of NAPE-PLD, it seems apparent that inhibitors of this enzyme could be beneficial in treating/preventing ectopic pregnancies and implantation defects, where high AEA concentrations are detrimental for embryo survival and impair gestation. An important first step toward the development of such inhibitors has been recently made Citation[16], paving the way to the possibility of ascertaining their efficacy in preclinical studies and clinical trials.
Finally, FAAH seems the most ideal target for drug development in combating infertility, because it is clear that it acts in vivo as a molecular integrator of signals that drive female fertility (). Altogether, it seems that the best strategy is to aim at enhancing FAAH activity that is defective in so many disturbances of reproduction. Remarkably, low FAAH activity in peripheral blood is an early marker of spontaneous miscarriage, a condition that has no other clinical symptoms Citation[9]. Unfortunately, over the past 10 years we have learned how to make very selective and efficient FAAH inhibitors (see Ref. Citation[15], for a recent review), but we are far from designing effective FAAH activators. In this context, it should be recalled that faah gene expression can be increased by upregulation of its promoter, leading to higher enzyme activity; indeed, this is the means used by several pro-fertility signals (i.e., Th2 cytokines, progesterone and leptin) to enhance FAAH activity in vivo Citation[5,6]. Thus, any drug capable of mimicking the effect of these signals holds a sound therapeutic potential. In the era of ‘epigenetic drugs’, it can be anticipated that the way to develop faah gene amplifiers may not be too long.
Figure 2. FAAH is a molecular integrator of female fertility signals. Compounds that act at the immune interface as pro-fertility signals (e.g., progesterone, leptin or T helper 2 [Th2] cytokines) also enhance FAAH activity. Conversely, molecules that can impair reproduction by acting on immune cells (e.g., Th1 cytokines) or on the uterine epithelium (e.g., progesterone and estrogen) inhibit FAAH.
![Figure 2. FAAH is a molecular integrator of female fertility signals. Compounds that act at the immune interface as pro-fertility signals (e.g., progesterone, leptin or T helper 2 [Th2] cytokines) also enhance FAAH activity. Conversely, molecules that can impair reproduction by acting on immune cells (e.g., Th1 cytokines) or on the uterine epithelium (e.g., progesterone and estrogen) inhibit FAAH.](/cms/asset/2f99a489-d182-45ac-8784-c169b9604e5c/iett_a_1062878_f0002_c.jpg)
On a final note, it should be stressed that little (if any) is known on the role that other ECS elements like 2-AG and its metabolic enzymes MAGL and DAGL Citation[3-7], as well as new players in eCB signaling like lysophosphatidic acid Citation[17], could play in female reproductive events in vivo; more investigations are deemed necessary to further understand their physiological impact. In addition, an emerging concept (already demonstrated for AEA) is that the biological activity of eCBs also depends on their intracellular trafficking by distinct carriers, collectively called eCB intracellular transporters (EITs) Citation[18]. Therefore, in order to understand the many-faceted actions elicited by eCBs (and hence by eCB-related drugs), it appears now crucial to understand how EITs can drive the same eCB to trigger different signaling pathways in different cellular contexts. In keeping with this concept, recent evidence based on pharmacological and genetic manipulation has demonstrated that different EITs may drive AEA signaling at different receptors and/or AEA to be metabolized by different enzymes Citation[19]. Thus, compounds selectively directed at one or more of these novel EITs could lead to the development of intensely searched ECS-based therapeutics with limited side effects and abuse liability. Incidentally, it can be recalled that at present the only ECS-based drug found to be effective in reproductive disturbances like vulvodynia and proctodynia is the anti-inflammatory eCB-like compound PEA Citation[20].
Endocannabinoids (eCBs) (e.g., anandamide) are bioactive lipids (most often arachidonic acid derivatives) that affect several aspects of human pathophysiology.
The biological activity of eCBs occurs through an ensemble of receptor targets (e.g., type 1 cannabinoid receptor [CB1]) and metabolic enzymes (e.g., anandamide synthase N-acyl-phosphatidylethanolamine-specific phospholipase D [NAPE-PLD] and anandamide hydrolase fatty acid amide hydrolase [FAAH]) that together with eCBs themselves constitute the so-called eCB system.
Cells and tissues of female reproductive organs are endowed with a fully functional eCB system.
Virtually all steps of female reproductive events are affected by one or more elements of the eCB system, to such an extent that low activity and expression of FAAH, and conversely high levels of its substrate anandamide, in human blood are diagnostic markers of spontaneous abortion.
Overall, available data suggest that enhancement of FAAH (a molecular integrator of fertility signals in vivo), inhibition of NAPE-PLD and agonism/antagonism of the eCB-binding CB1 receptor could be beneficial to promote successful pregnancy in humans.
Acknowledgments
The author wishes to thank Professor Alessandro Finazzi Agrò (Campus Bio-Medico University of Rome, Italy) for his continuing interest and support, and Dr Monica Bari (Tor Vergata University of Rome, Italy) for the artwork.
Declaration of interest
The author gratefully acknowledge financial support from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2010 – 2011 grant). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
This box summarizes key points contained in the article.
Bibliography
- Fezza F, Bari M, Florio R, et al. Endocannabinoids, related compounds and their metabolic routes. Molecules 2014;19(11):17078-106
- Rahman IA, Tsuboi K, Uyama T, Ueda N. New players in the fatty acyl ethanolamide metabolism. Pharmacol Res 2014;86(1):1-10
- Rapino C, Battista N, Bari M, Maccarrone M. Endocannabinoids as biomarkers of human reproduction. Hum Rep Update 2014;20(4):501-16
- Wang H, Dey SK, Maccarrone M. Jekyll and hyde: two faces of cannabinoid signaling in male and female fertility. Endo Rev 2006;27(5):427-48
- Maccarrone M. Endocannabinoids: friends and foes of reproduction. Prog Lipid Res 2009;48(6):344-54
- Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Rep Update 2011;17(3):347-61
- Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chem Neurosci 2012;3(5):349-55
- Stulberg DB, Cain LR, Dahlquist I, Lauderdale DS. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004-2008. Fertil Steril 2014;102(6):1671-6
- Maccarrone M, Valensise H, Bari M, et al. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 2000;355(9212):1326-9
- Habayeb OM, Taylor AH, Finney M, et al. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 2008;299(10):1135-6
- Maccarrone M, Bisogno T, Valensise H, et al. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod 2002;8(2):188-95
- Sun X, Cappelletti M, Li Y, et al. Cnr2 deficiency confers resistance to inflammation-induced preterm birth in mice. Endocrinology 2014;155(10):4006-14
- El-Talatini MR, Taylor AH, Elson JC, et al. Localisation and function of the endocannabinoid system in the human ovary. PLoS One 2009;4(2):e4579
- Xie H, Sun X, Piao Y, et al. Silencing or amplification of endocannabinoid signaling in blastocysts via CB1 compromises trophoblast cell migration. J Biol Chem 2012;287(38):32288-97
- Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin Drug Discov 2013;8(5):509-22
- Scott SA, Spencer CT, O’Reilly MC, et al. Discovery of desketoraloxifene analogues as inhibitors of mammalian, Pseudomonas aeruginosa, and NAPE phospholipase D enzymes. ACS Chem Biol 2015;10(2):421-32
- Beltrame JS, Sordelli MS, Cella M, et al. Lysophosphatidic acid increases the production of pivotal mediators of decidualization and vascularization in the rat uterus. Placenta 2013;34(9):751-6
- Maccarrone M, Dainese E, Oddi S. Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci 2010;35(11):601-8
- Yu S, Levi L, Casadesus G, et al. Fatty acid binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferatoractivated receptor β/δ (PPARβ/δ) in the brain. J Biol Chem 2014;289(18):12748-58
- Keppel Hesselink JM, Kopsky DJ, Sajben NL. Vulvodynia and proctodynia treated with topical baclofen 5% and palmitoylethanolamide. Arch Gynecol Obstet 2014;290(2):389-93