Abstract
The anti-inflammatory, analgesic, antipyretic and antithrombotic activities of aspirin confer its wide therapeutic application. The three former activities require higher doses of aspirin, whereas the latter can be achieved through a lower, thus safer dose of the drug. Low-dose, long-term aspirin is used as an antithrombotic therapy to prevent cardiovascular disease. Such therapy is used by millions of people worldwide, including those suffering from age-related macular degeneration (AMD); thus, questions have arisen as to whether such treatment has any impact on the development and course of AMD. This editorial addresses the important issue of possible beneficial and adverse effects of long-term, low-dose aspirin treatment of AMD patients. Special emphasis is given to the ability of aspirin to acetylate cyclooxygenases (especially COX-2) and thus to initiate a biochemical pathway leading to the generation of anti-inflammatory pro-resolving mediators synthesized from both ω-3 and ω-6 long-chain polyunsaturated fatty acids. Such mediators (e.g., resolvins, lipoxins) may be of therapeutic value in retarding the development of dry form AMD.
Therapeutic use of aspirin has its roots in ancient times when willow tree bark was recognized as a remedy for fever and pain. Although willow-based preparations were in common curative use for ages, it was not until the 1820s that the willow's active ingredient was identified as salicin, which later served a substrate for salicylic acid and next acetylsalicylic acid (ASA). At the end of the nineteenth century, ASA was achieved in powder form and named ‘aspirin’; it was registered as a drug and available in tablet form. Since then, aspirin has started its staggering career Citation[1,2].
At present, aspirin is perhaps the most widely used medicine with millions of people using it acutely or chronically for different purposes: to reduce pain and fever, to treat inflammatory diseases and to prevent cardiovascular diseases (CVD) Citation[3]. Some authors also consider the role of aspirin in cancer prevention Citation[4]. Acute or short-term treatment (e.g., against pain, fever) usually requires larger doses (0.5 – 1 g to maximally 4 g daily) in divided oral doses; in rheumatic fever, up to 5 – 8 g/day in divided doses may be given. Conversely, long-term CVD ‘preventive’ therapy requires lower doses – usually 75 – 150 mg/day (325 mg/day is sometimes prescribed, but 75 – 100 mg doses are preferred) Citation[5]. Aspirin is available over-the-counter (without prescription), and it has the reputation of a safe drug. However, aspirin, in addition to its therapeutic potential, may produce an array of undesired effects, from mild to unpleasant (including dyspepsia) and trying sensation originating from alimentary system to more serious gastrointestinal problems. These gastrointestinal problems include deterioration/induction of ulcer disease, perforation or local bleeding as well as bleeding from injured or pathological (including new formed) blood vessels anywhere in the body due to its antithrombotic activity. Furthermore, aspirin is capable of precipitating asthmatic attacks, so it cannot be used in patients with bronchial asthma. There are many other unwanted symptoms and side effects resulting from either over-use/dose of aspirin or hypersensitivity to the drug Citation[5]. Such unwanted/adverse symptoms may occur irrespective of the mode and dose of aspirin treatment, depending on individual sensitivity to aspirin (and other NSAIDs), age and health condition of patients receiving the drug, as well as the existence of accompanying diseases and pharmacotherapies (possible drug-drug interactions). Therefore, safety concern while using aspirin especially on a long-term regimen cannot be neglected. It should be emphasized that antithrombotic (anti-platelet) activity of aspirin can be considered either as an awaited (therapeutic) effect or side (unwanted) effect, depending on the aim of therapy The ratio between positives (benefit) vs negatives (risk) must always be carefully considered before making a decision about introduction, continuation or withdrawal of aspirin-based therapy.
This editorial focuses on long-term, low-dose use of aspirin and age-related macular degeneration (AMD), a progressive retinal disease that leads to severe loss of central vision, including legal blindness. The issue is important now and interest is likely to increase in coming decades as the occurrence of AMD throughout the world shows growing tendency. Many actual and potential AMD patients use aspirin, mainly as a prevention or treatment of cardiovascular malfunctions and/or diseases (CVD). It is therefore important to consider whether such treatment has any impact on AMD. Three possibilities should be taken into consideration: i) increasing the risk of the disease progression (dry form, dry→-wet shift, wet form aggravation, with bleeding from newly formed pathological blood vessels); ii) slowing down or prevention of the disease development (curative or preventive potential); and iii) negligible or virtually no effect on functional aspects of the macular physiology or pathology.
A meaningful answer to such questions may come from careful analysis, combined with adequate methodology of the study, in patients suffering from or being at risk of AMD (dry, wet, dry-→wet) and taking more or less regularly aspirin. There are several published epidemiologic studies in this field; however, the presented results considered collectively are inconclusive Citation[6-12]. Some studies have demonstrated that regular aspirin may be associated with an increased risk of neovascular AMD Citation[8,11], others have not found any association Citation[10,12], while two studies have reported modest beneficial effect of long-term, low-dose treatment with aspirin in reducing the risk of visually significant AMD Citation[6,7]. As the latter option came from large cohort studies (in total > 60,000 individuals), a suggestion that the low-dose, long-term aspirin use may exert (or be associated with) beneficial effect in AMD patients requires attention and deeper insight into the problem, taking into account both the mechanism underlying aspirin action (A) and the pathogenesis of AMD (B).
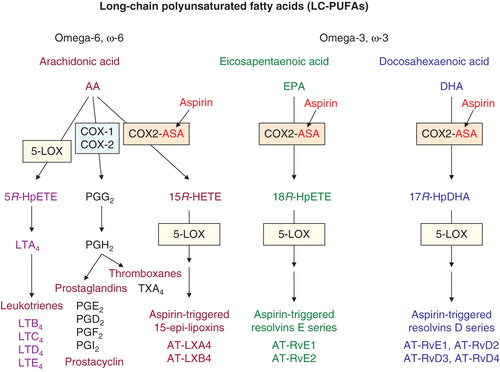
A. Aspirin (ASA), by analogy to other NSAIDs, exerts its action by inhibiting catalytic activity of COX-1 > COX-2, thus blocking arachidonic acid (AA)-driven pathway leading to prostanoids: prostaglandins (mediators of inflammation and pain) and thromboxanes (possessing platelet-aggregating and vasoconstrictor activity). The COX blocking activity of aspirin, which is irreversible in nature, involves acetylation of the hydroxyl group of serine residue: Ser530 in COX-1 and Ser516 in COX-2. Only these mechanisms underlie the anti-inflammatory and anti-thrombotic potential of aspirin Citation[1,2], the latter being responsible for the cardiologic beneficial activity of the drug. Functionally COX isoforms differ; COX-1 is a constitutive enzyme responsible for the production of prostaglandins involved in physiologic processes (e.g., protection of the stomach mucosa, platelet aggregation), whereas COX-2 is an inducible enzyme and produces prostaglandins, mostly PGE2, during inflammatory reactions. In addition to the mentioned functional differences, there are also structural differences in active sites of COX isoenzymes. The active site of COX-2 is slightly larger than that of COX-1, and AA, a COX substrate, can go on to become a substrate for acetylated COX-2 (that is incapable of catalyzing AA into prostaglandins and thromboxanes), initiating the pathway: AA → 15R-HETE (15R-hydroxy-eicosatetraenoic acid), which, with the aid of lipoxygenase (LOX), leads to the generation of lipoxins (). So called epi-lipoxins, 15-epi-LXA4 and 15-epi-LXB4, known as ATL (aspirin-triggered lipoxins), are formed and all lipoxins are endowed with pronounced anti-inflammatory pro-resolving activity. In other words, by acetylating COX-2, aspirin redirects COX-2's catalytic activity from generating the intermediate for prostaglandins and thromboxanes towards producing 15R-HETE, and then to anti-inflammatory mediators. Interestingly, besides AA (ω-6 polyunsaturated fatty acid [PUFA]), other PUFAs representing ω-3 series, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can be converted via aspirin-acetylated COX-2 to their hydroperoxy derivatives, that is, 18R-HpEPE and 17R-HpDHA, respectively. Via LOX-dependent mechanism these are converted to bioactive – anti-inflammatory pro-resolving epimeres of resolvins of E and D series, respectively () Citation[13]. It should be emphasized that DHA concentrations within the retina are the highest in human body, so the formation of aspirin-triggered DHA-dependent anti-inflammatory resolvins (AT-RvD1 – AT-RvD4), and also of DHA-dependent neuroprotectin D1 via LOX-directed pathway, may be relatively pronounced, particularly in an inflamed tissue.
Figure 1. Aspirin and major metabolic pathways involved in the generation of biologically active mediators from long-chain polyunsaturated fatty acids: ω-6 AA, ω-3 EPA and ω-3 DHA. Aspirin (ASA) by acetylating (COX1, COX2) blocks COX-dependent generation of proinflammatory prostaglandins and platelet-aggregating thromboxanes; simultaneously, acetylated COX-2 (COX2-ASA) activates new biochemical pathways leading to the formation of AT anti-inflammatory pro-resolving mediators: AA → lipoxins, EPA → E-resolvins, DHA → D-resolvins. For other explanations see text.
B. AMD is a disease that develops slowly and insidiously, with clinically meaningful symptoms seen in the elderly (60+ years) population. Clinically, AMD is divided into two forms: atrophic (dry form; > 80% of all AMD cases) and exudative (wet or neovascular form; 10 – 15%). The dry form is typically characterized by a progressive course, leading to the degeneration of retinal pigment epithelial cells (RPE) and then photoreceptors, with the late-stage of the disease also known as geographic atrophy. The exudative form is linked to choroidal neovascularization (CNV) directed to the subretinal macular region, with subsequent bleeding and/or fluid leakage, which may result in a sudden loss of central vision. The pathogenesis of AMD is complex and until now remains unclear, likely due to multifactorial and age-dependent character Citation[14,15]. In addition to genetic predispositions and environmental determinants, at least four specific processes contribute to the development of the pathology: lipofuscinogenesis in RPE cells (linked to oxidative stress and lipid peroxidation), drusogenesis, chronic inflammation (called para-inflammation with a prominent role of hyperactive complement alternative pathway) and CNV (in the case of wet form); the latter process results from a disbalanced ratio between proangiogenic (e.g., VEGF, but not only) and anti-angiogenic factors, with functional predominance of the former. AMD-associated CNV is in majority dependent on VEGF, thus it can be treated by intravitreously applied anti-VEGF agent such as bevacizumab (Avastin), ranibizumab (Lucentis) or aflibercept (Eylea), and in some patients by verteporfin-based photodynamic therapy usually in combination with anti-VEGF agent. In contrast to the approved therapies for the wet form AMD, there are up-to-now no approved effective medicines for dry AMD, although a number of drugs/therapies, including those suppressing inflammation, decreasing oxidative stress, modulating visual cycle or acting as neuroprotectants, are actually in clinical development Citation[16].
What about aspirin use by AMD patients? Concerning wet form AMD, there are no literature data that aspirin affects the process of neovascularization; yet, due to its antithrombotic potential aspirin may increase the risk of hemorrhage from pathological CNV or simply prolong the time of bleeding. Therefore, in patients with wet form AMD the use of aspirin should be considered individually, taking into account expected beneficial effects (such as CVD prevention) vs possible negatives related to ASA actions.
It cannot be excluded that low-dose, long-term use of aspirin in selected patients with newly diagnosed early form AMD, or those being at risk of developing the disease, would be advantageous, just because of aspirin ability to acetylate COX-2 and to shift the pathway from proinflammatory (prostaglandins) to anti-inflammatory pro-resolving molecules (AT-lipoxins, AT-resolvins). As already mentioned, one of the driving forces in AMD pathogenesis is chronic non-resolving inflammation (para-inflammation). It is possible that by increasing the production and local (intraretinal) supply of anti-inflammatory pro-resolving mediators, especially those deriving from abundantly occurring DHA in photoreceptor-RPE complex, there will be a chance to retard (or to prevent) the development of the retina/macula devastating and vision-threatening disease such as AMD. In this sense, the cited observations by Christen et al. Citation[6,7], also described by the same authors in a recently published comparative analysis Citation[17], could find reasonable explanation of a small, but anyway beneficial effect of aspirin use on vision in AMD patients, especially in early stages of the disease. Therefore, a possibility of beneficial (rather than harmful; see also Citation[18]) effect of long-term, low-dose aspirin treatment on AMD development and course deserves detailed clinical study.
Conclusion
Among available clinical data concerning aspirin effects, there are some that indicate that low-dose, long-term aspirin, in addition to its accepted CVD-preventive potential, may be associated with reduction of the risk of visually significant AMD. A plausible explanation of such ‘ocular’ observations may be related to the fact that aspirin-evoked acetylation of COX-2 contributes to the generation of anti-inflammatory pro-resolving mediators from long-chain PUFAs (AA, EPA, DHA), a phenomenon that may be of therapeutic value for inflammation-driven dry form AMD, particularly at early stages of the disease development.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
Bibliography
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003;110:255-8
- Fuster V, Sweeny JM; Aspirin. A historical and contemporary therapeutic overview. Circulation 2011;123:786-78
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease. A systematic review. JAMA 2007;297:2018-24
- Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol 2012;9:259-67
- Aspirin. Drug Information Online – Drugs.com. Available from: http://www.drugs.com/monograph/aspirin.html
- Christen WG, Glynn RJ, Ajani UA, et al. Age-related maculopathy in a randomized trial of low dose aspirin among US physicians. Arch Ophthalmol 2001;119:1143-9
- Christen WG, Glynn RJ, Chew EY, Buring JE. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology 2009;116:2386-92
- de Jong PT, Chakravarthy U, Rahu M, et al. Associations between aspirin use and aging macula disorder: the European Eye Study. Ophthalmology 2012;119:112-18
- Klein BE, Howard KP, Gangnon RE, et al. Long-term use of aspirin and age-related macular degeneration. JAMA 2012;308:2460-78
- Cheung N, Tay WT, Cheung GC, et al. Is aspirin intake associated with early age-related macular degeneration? The Singapore Indian Eye Study. Br J Ophthalmol 2013;97:785-8
- Liew G, Mitchell P, Wong TY, et al. The association of aspirin use with age-related macular degeneration. JAMA Intern Med 2013;173:258-64
- Zhu W, Wu Y, Xu D, et al. Aspirin use and risk of age-related macular degeneration: a meta-analysis. PLoS One 2013;8(3):e58821
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and pro-resolving lipid mediators. Annu Rev Pathol 2008;3:279-312
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 2006;58:353-63
- Ambati J, Fowler BJ. Mechanism of age-related macular degeneration. Neuron 2013;75:26-39
- Evans JB, Syed BA. New hope for dry AMD? Nat Rev Drug Discov 2013;12:501-2
- Christen WG, Chew EY. Does long-term aspirin use increase the risk of neovascular age-related macular degeneration? Expert Opin Drug Saf 2014;13(4):421-9
- Kahawita SK, Casson RJ. Aspirin use and early age-related macular degeneration: a meta-analysis. Can J Ophthalmol 2014;49:35-9
