Abstract
Sodium/glucose co-transporter 2 inhibitors (SGLT2i) represent a novel class of glucose-lowering agents that lower plasma glucose levels through pharmacological inhibition of glucose reuptake from the kidney, independent of insulin secretion and action. Clinical trials of SGLT2i demonstrated therapeutic benefits on glycemic control and bodyweight in individuals with type 2 diabetes, with few cases of serious adverse events (SAEs). However, a considerable number of SAEs were reported in patients receiving SGLT2i clinically in Japan during the first 3 months of their use. These included urogenital infections, hypoglycemia and dehydration. Unexpectedly, serious skin and subcutaneous disorders, mainly reported as generalized rash or skin eruption, were prominent in patients receiving SGLT2i, but with unknown mechanisms. There is also concern for potential SAEs associated with chronic SGLT2i administration, especially in the non-obese type 2 diabetes characterized by reduced insulin secretion often seen in East Asia. Chronic SAEs may include severe hypoglycemia due to depletion of hepatic glycogen storage, acceleration of diabetes-associated sarcopenia and ketosis/ketoacidosis. The current information on acute SAEs confirms the importance of caution in the appropriate use of SGLT2i. Furthermore, careful long-term observation of patients receiving SGLT2i is essential to avoid SAEs and for better clinical use of this drug class.
Sodium/glucose co-transporter 2 inhibitors (SGLT2i) were recently developed as novel class glucose-lowering agents for management of type 2 diabetes Citation[1,2]. SGLT2i increases renal glucose excretion and lowers plasma glucose levels through pharmacological inhibition of glucose reuptake from kidney, independent of insulin secretion and action. Although various oral glucose-lowering agents, including insulin-sensitizers (metformin, thiazolidinediones), insulin secretagogues (sulfonylureas, glinides, dipeptidyl peptidase-4 inhibitors [DPP-4i]) and an inhibitor of glucose absorption (α-glycosidase inhibitor), are available to treat pathophysiological defects in type 2 diabetes directly, SGLT2i may mitigate several unmet needs related to therapy for type 2 diabetes such as overnutrition due to abnormal eating behaviors and massive insulin resistance in insulin-receiving patients. In fact, clinical trials of SGLT2i demonstrate therapeutic benefits on glycemic control and bodyweight in individuals with type 2 diabetes Citation[1,2]. In addition, somewhat acceptable incidence rates of serious adverse events (SAEs) associated with SGLT2i during the limited observational period were noted Citation[1,2]. However, findings in clinical trials must be refined by clinical practice, and it is important to review SAEs for clues to appropriate use of a new drug such as SGLT2i.
A considerable number of cases of SAEs were reported in patients receiving SGLT2i after the first SGLT2i, ipraglifrozin, emerged in Japanese clinical practice in April 2014, followed in May by three SGLT2i, dapaglifrozin, tofoglifrozin and luseoglifrozin. As of January 2015, 10 deaths were reported with use of SGLT2i (ipraglifrozin, 4; dapagrifrozin 4; tofogrifrozin 1 and luseogrifrozin 1) Citation[3], but a causal relationship is not clear. The estimated incidence of all SAEs was 26.0 per 10,000 patients receiving ipraglifrozin, dapaglifrozin, tofoglifrozin or luseoglifrozin during the first 3 months after the launch of each of these drugs in Japan, and was approximately 6.2-fold higher than that of DPP-4i (sitagliptin, vildagliptin and alogliptin) in the corresponding period (). Post-marketing spontaneous reports of adverse events are influenced by extraneous factors including the Weber effect Citation[4]. However, to diminish any further increase among patients receiving SGLT2i in Japan, a committee of experts in the field (N Inagaki of Kyoto University; K Ueki of University of Tokyo; K Kaku of Kawasaki Medical University; T Kadowaki of University of Tokyo; Y Seino of Kansai Electric Power Hospital; M Haneda of Asahikawa Medical University and S Sato of University of Tokyo) formulated a recommendation on appropriate usage of SGLT2i based on the characteristics of the reported SAE cases associated with SGLT2i treatment ().
Figure 1. Incidence of adverse events associated with sodium/glucose co-transporter 2 inhibitors. (A) Incidences of all and selected serious adverse events (SAEs) in patients receiving sodium/glucose co-transporter 2 inhibitors (SGLT2i) or dipeptidyl peptidase-4 inhibitors (DPP-4i) are shown. The numbers of SAEs in patients receiving SGLT2i (ipraglifrozin, dapaglifrozin, tofoglifrozin or luseoglifrozin) or DPP-4i (sitagliptin, vildagliptin or alogliptin) during the first 3 months after launch of each of the drugs was divided by the number of patients who were initiated to each of the drugs during the corresponding period. (B) Incidences of all and selected SAEs in patients receiving four different SGLT2i are shown. The numbers of SAEs in patients receiving ipraglifrozin, dapaglifrozin, tofoglifrozin or luseoglifrozin during the first 3 months after launch of each of the drugs were divided by the number of patients who were initiated to each of the drugs during the corresponding period. The number of SAEs were obtained from Japanese early post-marketing vigilance data derived from the manufacturers of each of the drugs. SAEs in patients receiving SGLT2i or DPP-4i were categorized by the manufacturers of each of the drugs into preferred terms described in MedDRA/J (MedDRA Japanese Maintenance Organization, Pharmaceutical and Medical Device Regulatory Science Society of Japan, Tokyo, Japan). The numbers of patients who were initiated to each of the SGLT2i (ipraglifrozin 63,870; dapaglifrozin 16,664; tofoglifrozin 14,131; luseoglifrozin 8,159) or DPP-4i (sitagliptin 149,433; vildagliptin 20,075; alogliptin 12,512) were obtained from the Japan Medical Data Center Claims Database, Japan Medical Data Center Co., Ltd, Tokyo, Japan. The SAE incidence was expressed as the number of cases per 104 per 3 months. As differences in launch timing and number of patients receiving each of the drugs might influence the incidence of SAEs, these results should be considered carefully.
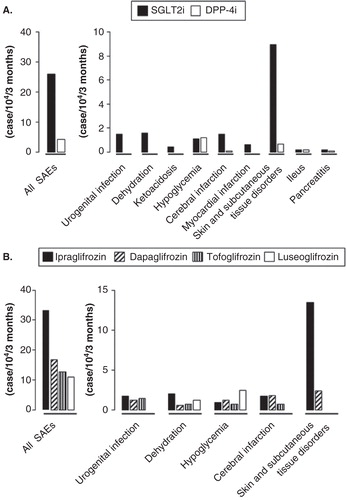
Table 1. Recommendation for appropriate use of sodium/glucose co-transporter 2 inhibitors.
Meta-analysis of clinical trials had demonstrated that the odds ratio (OR) for hypoglycemia was similar between patients with SGLT2i and those receiving placebo (OR: 1.28; 95% confidence interval [CI]: 0.99 – 1.65; I2 = 0%) Citation[5]. Incidence of severe hypoglycemia was comparable between SGLT2i and DPP-4i, which was possibly the major SAE in patients receiving DPP-4i and sulfonylureas () Citation[6]; patients experiencing severe hypoglycemia usually received SGLT2i in conjunction with insulin and/or sulfonylureas. Unlike DPP-4i, severe hypoglycemia also was reported in patients receiving SGLT2i with insulin. Although DPP-4i are thought to have synergistic effects with sulfonylureas on insulin secretion from pancreatic β-cells Citation[6], it is possible that improvement of glycemia by SGLT2i initiation eliminates glucotoxicity in the target tissues of insulin and thereby ameliorates insulin sensitivity to result in hypoglycemia with injected insulin. In addition, SGLT2i enhances glucose excretion even in glucose-demanding conditions Citation[7], and hepatic gluconeogenesis might therefore not adequately respond to hypoglycemia caused by injected insulin. Therefore, it is extremely important to consider dose reduction of sulfonylureas and/or insulin before initiation of SGLT2i as well as to educate patients in the management of hypoglycemia in advance ().
Dehydration and related vascular complications including cerebral and myocardial infarction are major safety concerns in the use of SGLT2i. SGLT2i-induced glycosuria leads to osmotic diuresis (), increasing risk of dehydration. Serious dehydration, cerebral infarctions and myocardial infarctions were reported in patients receiving SGLT2i (). Some cases with cerebral infarction also showed a prominent increase in hematocrit, suggesting association with dehydration. It is unknown whether the incidence of serious dehydration and related vascular complications associated with clinical use of SGLT2i is comparable to those of diuretic drugs. However, careful considerations are required before beginning SGLT2i in the elderly and/or those receiving diuretic drugs, especially during the early stage of SGLT2i initiation. In addition, patients must be instructed to stop SGLT2i during sick days, when they are more easily dehydrated ().
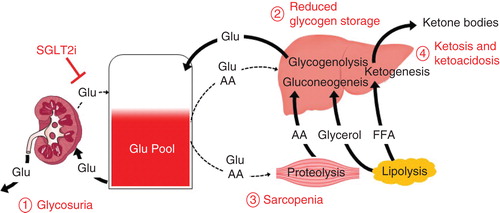
Figure 2. A hypothetical model of possible adverse events and underlying mechanisms associated with chronic use of sodium/glucose co-transporter 2 inhibitors. Glycosuria by sodium/glucose co-transporter 2 (SGLT2i) increases risk of urogenital infections as well as dehydration due to osmotic diuresis (1. Glycosuria). In response to urinary loss of glucose by SGLT2i, insulin levels are reduced and glucagon levels are elevated. Reduced insulin limits uptake of glucose and amino acids into liver and muscle. Elevated glucagon enhances gluconeogenesis and glycogenolysis in liver, proteolysis in muscle and lipolysis in adipose tissues. When patients with non-obese type 2 diabetes inappropriately restrict dietary carbohydrate intake along with their SGLT2i use, hepatic glycogen storage might become depleted due to reduced glucose uptake and enhanced glucogenolysis, making the patient prone to severe hypoglycemia (2. Reduced glycogen storage). Diabetes-associated sarcopenia could be accelerated as the reduced insulin restricts uptake of glucose and amino acids into muscle, and the elevated glucagon enhances proteolysis (3. Sarcopenia). Lipolysis could be enhanced by elevated glucagon, and the release free fatty acids converted into ketone bodies, which might result in ketosis or ketoacidosis (4. Ketosis and ketoacidosis).
Urogenital infection was as frequent in females as in males who received SGLT2i but not DPP-4i (). Because SGLT2i cause glycosuria by their nature (), meta-analysis of clinical trials had already shown that OR for urinary tract and genital infections is higher among patients receiving SGLT2i compared with those receiving placebo (urinary tract infections, OR: 1.34; 95% CI: 1.03 – 1.74; I2 = 0%; genital infection, OR: 3.50; 95% CI: 2.46 – 4.99; I2 = 0%) Citation[5]. It was then recommended to screen for urogenital infection in patients receiving SGLT2i by using questionnaires regarding symptoms related to urogenital infections ().
Unexpectedly, the incidence of skin and subcutaneous tissue disorders, mostly reported as serious generalized rash (34.8%) or drug eruption (27.2%), was prominent in patients receiving SGLT2i (). Other serious skin and subcutaneous disorders include those reported as urticarial (9.8%), systemic erythema (4.3%), erythema (4.3%), eczema (4.3%) and eruption (4.3%). These symptoms were usually seen within 2 weeks after SGLT2i initiation, and sometimes as early as on the first day of SGLT2i initiation. Among the four different SGLT2i, the incidence of skin and subcutaneous tissue disorders was higher in patients receiving ipraglifrozin, while the incidence of hypoglycemia and dehydration were similar (). Only a few cases of serious skin and subcutaneous tissue disorders were reported in clinical trials of SGLT2i, including those of ipraglifrozin, conducted in Japan and worldwide. Due to the limitations of post-marketing spontaneous reports of adverse events, long-term, detailed analysis of any causal relationship of SGLT2i, especially ipraglifrozin, with skin and subcutaneous tissue disorders is required. However, the fact that ipraglifrozin is currently available only in Japan might explain why the skin and subcutaneous disorders are not prominent in countries other than Japan. In addition, preclinical studies in experimental animals suggest that ipraglifrozin is more readily found in skin. It is possible that ipraglifrozin or its metabolites binds to not-yet-identified carrier proteins and serves as a hapten to elicit allergic reactions, or that the metabolites might cause toxicity in the skin. Further investigations to understand the mechanisms of its action are paramount to permit prediction of susceptible patients before SGLT2i initiation.
In addition to the reported acute SAEs discussed above, there are also major concerns for potential adverse effects that may be associated with chronic SGLT2i administration. The pathophysiology of type 2 diabetes involves reduced secretion and action of insulin, together with hypersecretion of glucagon Citation[8-10]. Unlike most glucose-lowering agents developed to ameliorate one or more of these defects to improve glycemic control, SGLT2i does not target any of these pathophysiological problems directly Citation[1,2]. It has been shown recently that SGLT2i elevates plasma glucagon levels, possibly in compensation for the SGLT2i-induced urinary loss of glucose by enhancement of hepatic gluconeogenesis and glycogenolysis Citation[7,11]. Such elevation of glucagon and reduction of insulin, both of which are associated with SGLT2i use, could cause various adverse events, especially in the non-obese type 2 diabetes characterized by reduced insulin secretion often seen in East Asia Citation[12]. When these patients inappropriately restrict dietary carbohydrate intake along with SGLT2i use, hepatic glycogen storage might be depleted due to reduced glucose uptake and enhanced glucogenolysis () Citation[13,14], rendering them prone to severe hypoglycemia. Such conditions might also accelerate diabetes-related sarcopenia Citation[15], since the reduced insulin restricts uptake of glucose and amino acids into muscle and the elevated glucagon enhances proteolysis that releases amino acids for hepatic gluconeogenesis () Citation[9,13]. As the elevated glucagon enhances lipolysis and contributes to reduction of fat mass, free fatty acids released from the adipose tissue are converted into ketone bodies Citation[9,14], which might then result in ketosis or even ketoacidosis (). Healthcare professionals need to bear these possibilities in mind and carefully observe patients receiving SGLT2i and their dietary intake of carbohydrate.
In conclusion, SGLT2i is thought to be useful as a novel anti-diabetic drug because of its ability to lower glucose levels with less risk of hypoglycemia and its action in reducing bodyweight Citation[1,2]. SGLT2i potentially solves several unmet needs related to type 2 diabetes. However, the current information on acute SAEs in patients receiving SGLT2i, including urogenital infections, hypoglycemia and dehydration and subsequent vascular complications, confirms the importance of the appropriate use of SGLT2i by considering risk–benefits for each individual patient with type 2 diabetes. Important remaining questions include analyses of incidence of SAEs, including skin and subcutaneous disorders, in other countries and ethnicities and clarification of the mechanisms underlying the acute SAEs reported in Japan. Careful, long-term observation of patients receiving SGLT2i is essential to avoid SAEs and for better use of this drug class.
Expert opinion
SGLT2i was developed as a novel anti-diabetic drug to ameliorate glucose levels with less hypoglycemic risk and also reduce body weight. While clinical trials demonstrated that SGLT2i is relatively safe, many patients receiving SGLT2i in Japanese clinical practice experienced SAEs. These included urogenital infection, hypoglycemia and dehydration; however, most of these SAEs might have been prevented through triage care of patients by healthcare professionals before initiating SGLT2i therapy and more thorough patient education in advance. The many SAEs involved in skin and subcutaneous disorders seen in patients receiving SGLT2i, especially ipraglifrozin, in Japanese clinical practice must have their underlying mechanism elucidated. There is also a major concern for chronic adverse effects associated with SGLT2i administration, especially in individuals with non-obese type 2 diabetes who might restrict their carbohydrate intake inappropriately. Such chronic SAEs may include severe hypoglycemia due to depletion of hepatic glycogen storage, diabetes-associated sarcopenia and ketosis/ketoacidosis. Healthcare professionals need to bear these concerns in mind and carefully observe patients receiving SGLT2i in order to avoid SAEs and to better use this drug class.
Acknowledgments
The authors thank T Kurose, H Kuwata and T Mitani of Kansai Electric Power Hospital and K Sugawara and I Mori of Kobe University for discussion, and M Yamane of Kansai Electric Power Hospital for secretarial assistance.
Declaration of interest
D Yabe has received a Grant-in-Aid for Young Scientists (B) from Japan Society for Science Promotion and Grants for young researchers from Japan Association for Diabetes Education and Care. Y Seino has received grants from Japan Vascular Disease Research Foundation. D Yabe has received speaker fees from Eli Lilly, MSD, Sanofi, Novo Nordisk, Boehringer Ingelheim, Takeda and Taisho Pharmaceutical. Y Seino has received consulting and/or speaker fees from Eli Lilly, Sanofi, Novo Nordisk, GlaxoSmithKline, Taisho Pharmaceutical, Astellas Pharma, BD, Boehringer Ingelheim, Johnson & Johnson and Takeda. M Kaneko and R Nishikino are employees of the Japan Medical Data Center. M Iwasaki declares no conflict of interest. D Yabe and Y Seino take responsibility for the contents of the article. D Yabe and Y Seino collected and analyzed data, and wrote the manuscript. M Kaneko and R Nishikino contributed to data collection and discussion. M Iwasaki contributed to discussion on a hypothetical model on chronic use of sodium/glucose co-transporter 2 inhibitors.
Notes
Bibliography
- Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig 2014;5(3):265-75
- Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract 2014;104(3):297-322
- Tauchi K. 10 deaths reported following use of newly marketed diabetes drugs. Available from: http://ajw.asahi.com/article/sci_tech/medical/AJ201501090058 [Last accessed 17 January 2015]
- Hoffman KB, Dimbil M, Erdman CB, et al. The Weber effect and the United States food and drug administration’s adverse event reporting system (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug safety 2014;37(4):283-94
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159(4):262-74
- Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabet Investig 2014;5(5):475-7
- Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124(2):499-508
- Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104(6):787-94
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122(1):4-12
- Yabe D, Kuroe A, Watanabe K, et al. Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015;29(3):413-21
- Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124(2):509-14
- Yabe D, Seino Y, Fukushima K, et al.β-cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Current Diabetes Report; 10.1007/s11892-015-0602-9
- Dimitriadis G, Mitrou P, Lambadiari V, et al. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 2011;93(Suppl 1):S52-9
- Ramnanan CJ, Edgerton DS, Kraft G, et al. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 2011;13(Suppl 1):118-25
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2(10):819-29
