Abstract
Tumor-targeting delivery of drugs has received much attention in the field of drug development. Traditional antitumor agents are often nonspecific for tumor cells; therefore, tumor-targeting drug-delivery design is a promising approach to improve the therapeutic efficacy of chemotherapy. Polyunsaturated fatty acids (PUFAs), such as linoleic acid, α-linolenic acid, arachidonic acid and docosahexaenoic acid, are naturally occurring essential substances which play important roles in cell growth. Due to their lipophilic nature, PUFAs are readily incorporated into the lipid bilayer of cells, especially tumor cells. Therefore, PUFAs can be used as an applicable carrier to increase the therapeutic efficacy of anticancer drugs. In this review, several PUFA–drug conjugates with potent antitumor activities are summarized, and the bright prospects of PUFAs in drug development are also proposed. A general design strategy of PUFA–drug conjugates is provided, as well as a discussion of the recent progress in PUFA–drug conjugates and preclinical therapies. The tumor growth-related PUFAs are expected to play an important role in the development of more efficacious antitumor agents.
1. Introduction
Chemotherapy has made significant progress in the treatment of various cancers for several decades. However, cancer treatment still faces challenges due to its non-selectivity and severe side effects. Traditional antitumor agents rely on the premise that rapidly proliferating cancer cells are more likely to be killed by cytotoxic agents. While in reality, normal cells, that is, red blood cells, hair follicles, gut epithelia, bone marrow and lymphatic cells, also proliferate rapidly and the traditional cytotoxic agents cannot distinguish them from cancerous cells, which causes undesirable systemic toxicity, such as hair loss and damage to the liver, kidney and bone marrow. The actual problem is that the difference in activity between tumor cell/tissue and non-tumor tissue is relatively small, and thus it will result in a similarly small therapeutic window in which normal tissue should not be damaged while all the tumor cells are killed. Clearly, if the drug could be targeted to the tumor more effectively, with less drug reaching critical normal tissues, resulting in fewer life-threatening side effects, cancer therapy could be substantially improved. Tumor-targeting drug design is, therefore, a promising approach to improve the therapeutic efficacy of chemotherapy.
The main physiological characteristic of cancer cells is their enhanced growth and metabolic rate. In order to grow rapidly, tumor requires large numbers of nutrients and vitamins Citation[1]. Therefore, tumor cells highly express many specific membrane receptors which facilitate the uptake of extracellular substance, and thus provide an avenue to improve the selectivity of chemotherapy drugs using the tumor growth-related molecules as a tumor-targeting carrier. In the past decades, the tumor growth-related molecules, such as glucose, folic acid Citation[2,3], aptamers Citation[4], oligopeptides Citation[5] and hyaluronic acid Citation[6,] have been applied as tumor-specific moieties to construct tumor-targeting conjugates (or prodrugs) and their good evidence of effectiveness has been widely reviewed Citation[7,8].
This review is concerned with the unique biological activity of polyunsaturated fatty acids (PUFAs) and their potent applications in improving the therapeutic efficacy of antitumor agents.
2. The biological activity of PUFAs
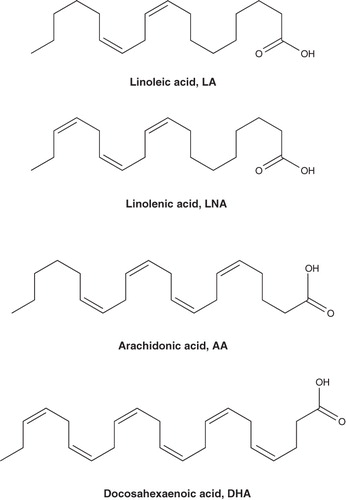
Naturally occurring PUFAs play important roles in cell growth and are used as biochemical precursors and energy source Citation[9,10]. The representative PUFAs (), such as linoleic acid, α-linolenic acid (LNA), arachidonic acid and docosahexaenoic acid (DHA), were defined as 18, 20 and 22 carbons, and 2 – 6 unconjugated cis-double bonds aliphatic compounds. The PUFAs were mainly obtained from milk, vegetable oils and cold-water fish, and they were proven to be safe essential substances. For example, DHA is currently approved as a nutritional additive by the FDA in the US.
As tumor cells grow rapidly, they greedily need nutrients for proliferation. Therefore, PUFAs will preferentially be taken up by tumor cells Citation[11]. In fact, PUFAs are readily incorporated into the lipid bilayer of cells owing to their lipophilic nature, and this will disrupt the membrane structure and fluidity of active cells, and thus the chemosensitivity was likely to be influenced, especially for tumor cells Citation[12,13]. In addition, PUFAs themselves possess anticancer activity against CFPAC, PANC-1 and Mia-Pa-Ca-2 pancreatic and HL-60 leukemia cell lines, and their antitumor activities have been evaluated in preclinical and clinical studies Citation[14,15]. The inhibition of tumor growth by DHA was mediated through a decrease in intracellular cAMP via a Gi-protein-coupled signal transduction pathway Citation[11].
It is well known that the conventional chemotherapeutic drugs, such as paclitaxel, 10-hyfroxycamptothecin (HCPT) and doxorubicin (Dox), generally exhibit a serious drawback because of little tumor-specificity in clinic application, and thus cause undesirable systemic side effects. The unique characteristic and urgent requirement for tumor cells will make PUFAs find potent applications in drug development, and naturally there are two aspects to be taken into account: i) the prodrug strategy, which aims to improve the pharmaceutical properties (solubility, permeability, distribution, etc.) through conjugating PUFAs to chemotherapeutic agents and ii) the tumor-targeting conjugates strategy, which will specifically deliver the chemotherapeutic agents to tumor cells based on the preferential uptake of PUFAs by tumor cells.
3. PUFAs conjugate
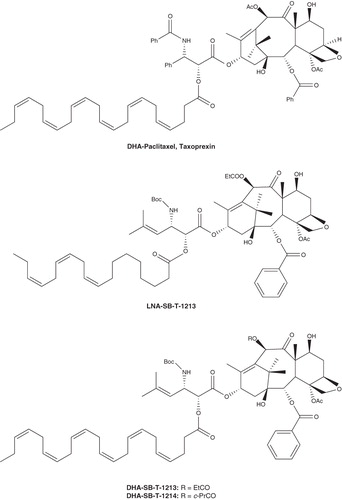
DHA–paclitaxel, a 2′-O-acyl conjugate of DHA and paclitaxel (Taxoprexin, ) reported by Bradley et al., was prepared with the aim of targeting paclitaxel to tumors to decrease toxicity to normal tissues and increase the therapeutic index Citation[16]. Detailed pharmacokinetic studies of DHA–paclitaxel, compared with paclitaxel, in normal rats suggest that it is stable in blood plasma and high concentrations in tumor cells are maintained for a long period of time, whereas paclitaxel is rapidly cleared from plasma and distributed into large volumes of peripheral tissue space.
The plasma concentration of paclitaxel remains above 2 μM in tumors for only 16 h after 20 mg/kg of intravenous (i.v.) paclitaxel, whereas paclitaxel derived from DHA–paclitaxel (120 mg/kg of i.v.) remains above 2 μM for almost 240 h. And the AUC of paclitaxel derived from DHA–paclitaxel (120 mg/kg of i.v.) in either plasma or muscle is almost twice that of paclitaxel at 20 mg/kg, with Cmax ∼ 5.5- and 22-fold, respectively. Although the acylation at 2′-hydroxyl position has been shown to eliminate the microtubule assembly activity of paclitaxel, DHA–paclitaxel exhibits greater antitumor activity than paclitaxel in the M109 tumor model.
DHA–paclitaxel completely eliminated the detectable tumors at 120 mg/kg i.v. for 5 days, whereas paclitaxel at 20 mg/kg did not. In addition, DHA–paclitaxel has decreased side effects such as hind-limb paralysis in mice. Based on the significantly higher therapeutic index, DHA–paclitaxel has been selected as a development drug candidate by the FDA and currently has advanced to human Phase III clinical trials.
Ojima and co-workers reported the conjugates of DHA and LNA using taxoids as highly efficacious antitumor agents (denominated DHA–SB-T-1213, LNA–SB-T-1213 and DHA–SB-T-1214 in ) Citation[17]. The efficacy assayed in vivo against ovarian and colon tumor xenografts suggested that the new generation of PUFAs conjugates exhibit significantly better tumor growth inhibitory activity. In a drug-sensitive human ovarian tumor A121 xenograft (Pgp), DHA–SB-T-1213 (30 mg/kg q3d i.v.) delayed the tumor growth for > 186 days and caused complete regression of tumor in all surviving mice. In a drug-resistant human colon tumor DLD-1 xenograft (Pgp+) in SCID mice, DHA–SB-T-1214 achieved complete regression in five of five mice at 80 mg/kg dose administered on days 5, 8 and 11 (tumor growth delay > 187 days), while LNA–SB-T-1213 exhibited the complete regression in two of five mice (tumor growth delay > 109 days) Citation[17]. The impressive antitumor activity of PUFA–taxoid conjugates against drug-resistant human tumor xenografts will provide bright prospects for the applications of PUFAs in improving therapeutic efficiency of clinical drugs.
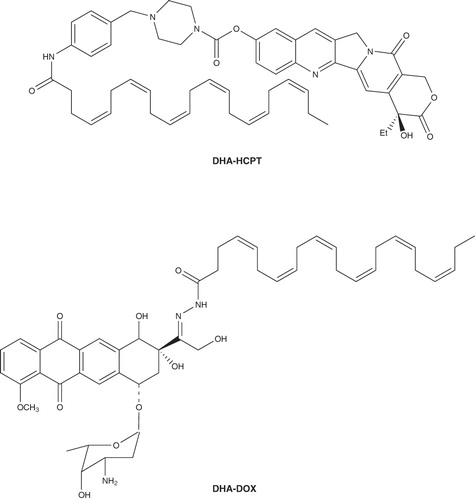
Wang et al. explored the conjugation of DHA with diverse chemotherapeutants such as HCPT and Dox (). The in vivo antitumor evaluation demonstrated that DHA assuredly enhances the therapeutic efficacy of the antitumor agents conjugated. For example, in L1210 leukemia mice model, the DHA–HCPT conjugation had an in life span (ILS) of 154 and 138% at a dose of 180 and 60 mg/kg, respectively, while HCPT, the parent drug, had a 77% increase in life extending. And, it is important to note that DHA–HCPT had a greatly decreased toxicity compared to HCPT (weight loss of 10% for HCPT, 2% for DHA–HCPT) Citation[18]. More interestingly, the carbamoyl bond linked DHA and HCPT, and was deemed to provide a prolonged exposure of the drug to cancer cells, thus resulting in a greatly improved antitumor efficacy of HCPT. According to the same strategy, the conjugation of DHA with Dox demonstrated a result similar to that of DHA–HCPT. In mice bearing L1210 leukemia model, DHA–Dox produced an ILS of 107%, which is twice of that produced by the free Dox (ILS: 53% at 5 mg/kg), and the toxicity of DHA–Dox (weight loss 2%) is much less than that of Dox (weight loss 7%) at the same ILS (53%) produced Citation[19]. Moreover, as reported Citation[20,21], the hydrazone bond used to link DHA and Dox is expected to be readily decomposed in pH-lower tumor cells, leading to tumor-specific through releasing the parent drug Dox. Based on the fact that conjugates of PUFAs such as DHA and LNA with antitumor agents clinically used have better therapeutic efficacy than their corresponding free drugs, it is reasonable to believe that conjugating DHA to existing anticancer drugs may possess superior therapeutic efficacy.
4. Conclusion
PUFA conjugates of clinical anticancer drugs have evoked increasing attention due to their significant potential in preclinical studies, such as enhancing curative effect, reducing side effects and the conceivably tumor-targeting. Most especially, the DHA–paclitaxel described above has received Phase III clinical trials approval, and this will result in further investigations for the applications of those conjugates in cancer chemotherapy.
Due to the lipophilic nature, PUFAs could pass through the lipid bilayer of tumor cells which need vast nutrients for rapid growth. Moreover, PUFAs conjugates have a different pharmacokinetics and biodistribution behavior than low-molecular mass cytotoxic drugs owing to the effects of molecular mass and the biologically stable linkage between PUFA and drug. This can give time for them to be retained at the target site for enhanced periods of time. Consequently, DHA and LNA and other PUFAs may be used as an applicable carrier to increase the therapeutic efficacy of anticancer drugs.
Although PUFAs conjugates have achieved numerous successful trials both in vitro and in vivo evaluations and, as reported, several PUFAs conjugates have advanced to preclinical test or Phase III clinical trial, there are still challenges to be taken into consideration in further investigation.
The PUFAs conjugates generally showed improved therapeutic effects in animal models, while the detailed mechanism of action has not yet been clarified. For example, PUFAs could facilitate uptake of cytotoxic agents conjugated, but the transporting capability of PUFAs, in other words, the structure–activity relationship has not been exploited. Moreover, whether or not the transport across cell membranes of PUFAs conjugates utilizes the same procedure as PUFAs remains unclear.
The linkage conjoining PUFAs with pharmacophores should be carefully introduced. Traditionally, ester or amide bond was used as the linker in conjugates. Because these chemical bonds were slowly disintegrated to release the parent drugs by specific enzymes in the blood circulation, the conjugates will perform a prolonged curative effect. This type of conjugate should be defined as prodrugs. On the other hand, the tumor specific linkers such as imine, hydrazone and disulfide which were cleavable in tumor environment, combined with the action of PUFAs, will lead to superior tumor-targeting effects, and this will be the strategy of tumor-targeting conjugates.
The exceptional efficacy of PUFA conjugates against drug resistance (i.e., multi-drug resistance) provides bright prospects for further investigations for the applications of these conjugates in cancer treatment.
In conclusion, PUFAs conjugates have provided significant advances in improving the drug's anticancer efficacy. Herein, the authors confidently believe that further investigation on new conjugates based on PUFAs will bring about more progresses in drug development.
5. Expert opinion
PUFA conjugates have received increasing attention due to their significant potential in preclinical studies. DHA–paclitaxel conjugate reported by Bradley et al. Citation[16] exhibited increasing therapeutic efficacy with sustained high concentrations for a long period of time in tumor cells. Thus, DHA–paclitaxel has currently advanced to a Phase III clinical trial. The conjugates of DHA and LNA with taxoids reported by Ojima and co-workers Citation[17] showed potent antitumor activity against drug-resistant human tumor xenografts. Also, the in vivo antitumor evaluation of conjugate of DHA with HCPT and Dox (reported by Wang et al. Citation[18]) demonstrated greatly improved antitumor efficacy. More interestingly, the carbamoyl bond and hydrazone bond linkage used in the conjugates will generate the tumor-sensitive drug release in the physiological carboxylate esterase or lower pH of tumor cells.
Based on the successful trials both in in vitro and in vivo evaluations, PUFA conjugates will evoke various investigations for the applications in cancer chemotherapy. Further researches will mainly focus on the effect of PUFAs in enhancing therapeutic index and overcoming the MDR as well as the mechanism of action. Moreover, due to its ready entry into lipid bilayer of tumor cells, PUFAs may be used as a tumor-targeting carrier to increase the therapeutic efficacy of anticancer drugs. Thus, the tumor-targeting design strategy based on PUFAs–drug conjugates will also be explored. The authors confidently believe that, in the future, researches on PUFA conjugates will doubtlessly yield more exciting results in drug development.
Declaration of interest
The paper was sponsored by the Key Scientific and Technological Projects of Henan province (Nos. 092102310025, 102102210075, and 102102310195) and the Co-construction Projects of Henan University (No. SBGJ090518).
Notes
Bibliography
- Jaracz S, Chen J, Kuznetsova LV, Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem 2005;13:5043-54
- Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev 2004;56:1127-41
- Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev 2002;54:675-93
- Chu TC, Marks JW III, Lavery LA, Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res 2006;66:5989-92
- Nagy A, Schally AV, Halmos G, Synthesis and biological evaluation of cytotoxic analogs of somatostatin containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin. Proc Natl Acad Sci USA 1998;95:1794-9
- Luo Y, Bernshaw NJ, Lu Z-R, Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm Res 2002;19:396-402
- Monsigny M, Roche A-C, Midoux P, Glycoconjugates as carriers for specific delivery of therapeutic drugs and genes. Adv Drug Deliv Rev 1994;14:1-24
- Ojima I. Guided molecular missiles for tumor-targeting chemotherapy-case studies using the second-generation taxoids as warheads. Acc Chem Res 2008;41:108-19
- Sauer LA, Dauchy RT. The effect of omega-6 and omega-3 fatty acids on 3H-thymidine incorporation in hepatoma 7288CTC perfused in situ. Br J Cancer 1992;66:297-303
- Sauer LA, Nagel WO, Dauchy RT, Stimulation of tumor growth in adult rats in vivo during an acute fast. Cancer Res 1986;46:3469-75
- Sauer LA, Dauchy RT, Blask DE. Mechanism for the antitumor and anticachectic effects of n-3 fatty acids. Cancer Res 2000;60:5289-95
- Grammatikos SI, Subbaiah PV, VictorTA, n-3 and n-6 Fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br J Cancer 1994;70:219-27
- Diomede L, Colotta F, Piovani B, Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-racglycero-3-phosphocholine. A possible basis for its selective action. Int J Cancer Res 1993;53:124-30
- Wigmore SJ, Ross JA, Falconer JS, The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27-30
- Hawkins RA, Sangster K, Arends MJ. Apoptotic death of pancreatic cancer cells induced by polyunsaturated fatty acids varies with double bond number and involves an oxidative mechanism. J Pathol 1998;185:61-70
- Bradley MO, Webb NL, Anthony FH, Tumor targeting by covalent conjugation of a natural fatty acid to taxol. Clin Cancer Res 2001;7:3229-38
- Kuznetsova LV, Chen J, Sun L, Syntheses and evaluation of novel fatty acid-2ndgeneration taxoid conjugates as promising anticancer agents. Bioorg Med Chem Lett 2006;16:974-7
- Wang Y, Li L, Jiang W, Synthesis and evaluation of a DHA and 10-hydroxycamptothecin conjugate. Bioorg Med Chem 2005;13:5592-9
- Wang Y, Li L, Jiang W, Synthesis and preliminary antitumor activity evaluation of a DHA and doxorubicin conjugate. Bioorg Med Chem Lett 2006;16:2974-7
- Kaneko T, Willner D, Monkovic I, New hydrazone derivatives of adriamycin and their immunoconjugates-a correlation between acid stability and cytotoxicity. Bioconjug Chem 1991;2:133
- Kratz F, Warnecke A, Scheuermann K, Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J Med Chem 2002;45:5523