Abstract
Colon-specific delivery systems have attracted considerable attention from the scientific community. One of the distinctions of this site-specific delivery system is its effectiveness in carrying a variety of medicinal agents (required for both localized diseases and systemic therapy). It has been proposed that the biological rhythm of the body may affect the normal physiological as well as biological functions. Diseases such as nocturnal asthma, angina pectoris, inflammation, rheumatoid arthritis, hypertension or cardiac arrhythmia, has been found to follow biological rhythm of the body. For the treatment of these diseases, development of a chronotherapeutic drug delivery system (CrDDS), which delivers a defined dose, at a selected time and chosen rate, and to a targeted site is required. Several CrDDSs have been developed by using various strategies (pH-, time-, microflora-triggered and pressure-controlled systems) with the aim of achieving colon-specific drug delivery. This Editorial article aims to highlight some of the recent advancements that have emerged in the field of colon-targeted drug delivery systems pertaining to the chronotherapy of certain disease conditions.
1. Introduction
“Targeted drug delivery systems are useful for selective and efficient delivery of pharmacologically active compounds to the predetermined targets in therapeutic concentration along with minimizing side effects of the drug” Citation[1]. As far as oral delivery of drugs is concerned, colon-targeted drug delivery systems (CoDDS) have been one of the most preferred field for researchers since past two decades. Colonic delivery deals with the targeted delivery of active agents into the lower part of the gastrointestinal tract (GIT), that is, the large intestine. It can be accomplished either by using the oral route or by rectal administration of drugs. The main hindrances which must be overcome for attaining efficacious delivery of drugs to the colonic region via the oral route are the absorption and degradation of drug in the upper part of the GIT. Further, CoDDS is not only useful for the treatment of diseases (colon cancer, infection, inflammatory bowel disease and irritable bowel syndrome) which requires localized delivery of drugs in the colonic region but it is also useful for the delivery of those molecules which are likely to degrade in the upper part of GIT (e.g., proteins, peptides and vaccines) Citation[2].
Since past two decades, a new type of drug delivery system viz. chronopharmaceuticals has attracted tremendous interest among pharmaceutical research community. It comprises the nitty-gritties and investigation of various features of chronopharmacology, chronopharmacokinetics, chronopathology, chronopharmacodynamics, chronotherapeutics, chronotoxicology, chronophysiology and chronogenetics. Generally, it brings together the concepts of chronobiology and pharmaceutics. Chronobiology is related with the learning of biological rhythms and mechanisms in living organisms Citation[3]. Basically there are three types of biological rhythms in human body viz. circadian, ultradian and infradian. Circardian is originated from Latin terminology ‘Circa’ means ‘about’ and ‘dies’ means ‘day’. Ultradian means alternation of shorter interval (> 1 cycle per day), whereas infradian means alternation extended for > 24 h (< 1 cycle per day) Citation[4]. Ultradian and infradian rhythms are not as useful for chronotherapeutic drug delivery, as they have either > 1 cycle per day or < 1 cycle per day, respectively. On the contrary, many bodily functions have been regulated by circadian rhythm viz. sleep pattern, metabolism, hormone production, physiological behavior, and so on. Chronobiology is based on the principle that the biological processes and functions in all existing creatures demonstrate predictable erraticism over time Citation[5]. On the other hand, pharmaceutics deals with the technological aspects of dosage form designing and manufacturing in order to ensure the safety, efficacy and quality of medicaments. Hence, chronopharmaceutics can be defined as a division of pharmaceutics, which is dedicated to the design, development and evaluation of the drug delivery system, that releases the drug at a pre-programmed pattern (rhythm) in synchronization with the biological clock (certain times of day or night) for the given disease therapy in order to increase the effectiveness and reduce the side effects Citation[3]. As the onset of diseases such as arthritis, asthma, cardiac arrhythmias, hypertension or inflammation is at night time or early in the morning, chronotherapy can be utilized for the treatment of these diseased conditions. Moreover, as chronotherapy is based on the fact that the circardian rhythm should be matched with the drug release profile, it is desirable to have a delayed release delivery system that can provide nocturnal release of a drug. Further, it has been well documented that CoDDS can be useful for achieving such delayed onset of drug release Citation[1]. This Editorial article is not envisioned to offer a comprehensive review on CoDDS, but to highlight some of the recent advancements that emerged in the field of CoDDS pertaining to the chronotherapy of certain disease conditions.
2. Conventional versus chronotherapy
Since many decades, conventional dosage forms have been extensively used for the treatment of various diseased conditions. They provide immediate or quick release of drug, which results into frequent administration, in order to maintain the therapeutic concentration of drug. These further result into poor patient compliance, reduced drug efficiency and increased episodes of side effects. Additionally, these types of dosage forms do not find their application in the treatment of diseases which present their symptoms during the night or early morning. Hence, to overcome the drawbacks associated with conventional dosage forms, modified drug release systems have been recommended. These systems were able to deliver the drug at a constant rate over a prolonged period of time which results into improved patient compliance and drug efficiency, and reduction in frequency of drug administration and subsequent side effects. However, apart from these merits, numerous modified release systems offers several difficulties viz. development of resistance and/or drug tolerance (because of continuous delivery of drug), and unavailability of additional amount of drug at the time when the symptoms are aggravated Citation[3].
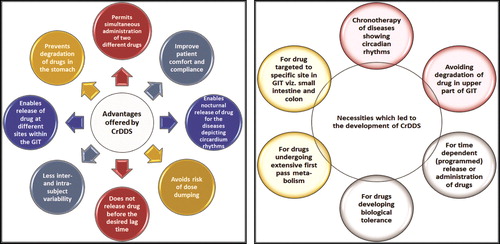
Recently, it has been proposed that the biological rhythm of the body may affect the normal physiological functions viz. Gastric-acid secretion, motility of GIT, blood flow in GIT, hepatic circulation, cardiac output, renal circulation, enzymatic activity in liver, drug–protein binding and urinary pH; and biological functions such as blood pressure, heart rate, platelet aggregation, body temperature, blood-plasma concentration, stroke volume and intraocular pressure Citation[6]. Further, the functions of most of the organs varies as the day passes, specifically when rhythmic and temporal patterns are expressed in a given disease state. Diseases such as nocturnal asthma, angina pectoris, inflammation, rheumatoid arthritis, hypertension or cardiac arrhythmias have been found to follow biological rhythm of the body Citation[3]. Thus, for the treatment of these diseases, development of chronotherapeutic drug delivery system (CrDDS) which delivers a defined dose, at a selected time and a chosen rate and to a targeted site is the need of the hour. The advantages offered by CrDDS is shown in () Citation[3,4].
3. Strategies for attaining CoDDS
CrDDS is envisioned to deliver a quick, or ephemeral, and quantified amount of drug molecule at a pre-programmed time in a predetermined region of GIT Citation[3,4]. Development of CrDDS assists in achieving maximum efficacy of drug molecule against the targeted disease condition, thereby augmenting therapeutic efficiency and improving patient compliance. Further, the lag time for drug release from CrDDS primarily depends on the design aspects of the formulation Citation[3]. The necessities which led to the development of CrDDS is shown in () Citation[3,4].
Over the past many years, several tactics have been used for achieving CrDDS of drugs by applying the concept of CoDDS. The principle strategies were development of pH-dependent systems, time-dependent systems, microbially and/or enzymatically driven drug delivery systems (comprising of biodegradable polymers [polysaccharides] and prodrugs-based CoDDS) and pressure-controlled-based systems Citation[1,2].
3.1 pH-dependent systems
The concept of pH-dependent strategy is based on the principle that the pH of the GIT rises gradually from the stomach to the small intestine to the distal ileum. Enteric polymers used for the preparation of these systems tend to prevent dissolution of drug in the upper part of GIT (as they are insoluble in acidic pH). Depending on their chemical composition, these polymers dissolve in the pH range of 5.0 – 7.5, thus preventing release of drug in stomach and providing a delayed release profile. Single-unit (tablets) and multi-unit (pellets) systems are most commonly preferred for enteric coating, as they are easy to manufacture at a comparatively low cost than that of complex systems. Most commonly used enteric polymers are the methacrylic acid copolymers (Eudragit® polymers). Other polymers that can be used for imparting enteric effects are hydroxypropyl methylcellulose phthalate, cellulose acetate phthalate, cellulose acetate trimelliate and polyvinyl acetate phthalate Citation[1,2]. As far as commercial aspect is concerned pH-dependent systems are most extensively used Citation[7]. Regardless of its extensive commercial applicability for achieving CoDDS, there always exists a controversy about their effectiveness for colon targeting Citation[7]. This may be due to high erraticism in pH of GIT (both inter-individual and intra-individual) and dearth of an appropriate coating polymer, which dissolves at preferred pH of the colonic region. It has been well documented that the systems enclosed within pH-dependent polymers lack in providing site-specific release of drug to the colonic region, and they may either tend to provide an early release of medicament in small intestine or there will be no release of medicament in the colonic region Citation[7,8].
3.2 Time-dependent systems
These systems are based on the concept of delaying the release of drug until they reach to the target site, that is, colon. The principle of this scheme is based on resisting the release of drug in acidic milieu of stomach, and providing a lag time of a pre-programmed duration of time, after which the drug release occurs in the colonic region. Here, lag times refers to the time required for transiting from the oral cavity to the colonic region Citation[1,2]. In case of time-dependent drug delivery systems, the site for the initial release of drug largely depends on transit time of GIT. Although the small intestinal transit time is relatively constant (3 – 4 h), a large deviation in gastric evacuation time may tend to either an early release of medicament in small intestine or deferred release of drug way down in descending colon Citation[9-11].
3.3 Microbially and/or enzymatically driven drug delivery systems
Microbially and/or enzymatically driven systems are reported to have greater supremacy over other approaches as far targeting of drugs to the colon is concerned. These systems retain the integrity of the dosage form until it reaches to the colonic region. The drug release in the colonic region is triggered as a result of degradation effect instigated due to the interaction between the microflora and/or enzymes present in the colonic region and the biodegradable polymers (polysaccharides) and prodrugs Citation[1]. Naturally occurring polysaccharides are the most commonly used carriers which are specifically hydrolyzed by the microflora of colonic region. Unfortunately, due to the hydrophilic nature of majority of natural polysaccharides, controlling the release of drug from these material possess a key challenge.
As, as discussed, no single system is found to be precise and accurate enough to deliver active moiety into the colonic region, recently, a novel concept of using the di-dependent drug delivery system has been proposed. In these systems, two factors, that is, pH and time, and pH and microflora of the colon control the release of drug Citation[1].
3.4 Pressure-controlled-based systems
Drug targeting to the colonic region using GI pressure has been proposed as one of the tool for achieving CoDDS. Contractions of muscular intestinal walls (for churning and propulsion of luminal contents) lead to establishment of this luminal pressure, which diverges throughout the GIT with respect to its strength and extent. The highest pressure is being generated by the colonic luminal region as a result of haustral contractions and viscous milieu Citation[12]. There were many methods proposed for the development of these systems viz. by dipping method, by applying ethylcellulose coat on inner surface of gelatin capsule or by forming a coat on capsule-structured portions of suppository base at reduced temperature Citation[13]. The application of GI pressure offers a state-of-the-art strategy for targeting drugs to various sites in GIT. However, there are inadequate data pertaining to the intestinal pressures in different parts of GIT, and these may either have an inter- and intra-subject variability as in the case of pH and transit time. Hence, it is a matter of time which will ascertain whether the pressure-controlled based systems will signify itself as a feasible means for CoDDS Citation[12].
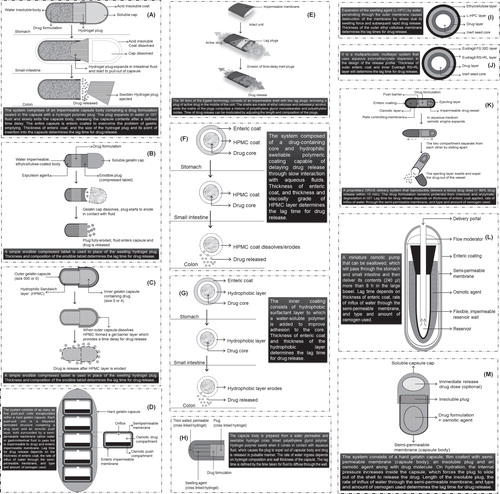
Oral delayed release systems envisioned for achieving chronotherapy for the treatment of diseases viz. nocturnal asthma, angina pectoris, inflammation, rheumatoid arthritis, hypertension or cardiac arrhythmias, when provided with an outer enteric coating (in order to overcome erratic gastric evacuation time), offer a remarkable potential for delivering drug to the colonic region. As the onset of these diseases is early in the morning or at night time, it is obligatory to have a delayed release system which can offer a nocturnal release of drug, which in turn offers a substantial respite to the patient during resting Citation[2,7]. Taking into consideration the advantages and limitations of various approaches, using a di-dependent approach, one can ensure that there is delayed release of the drug. Further, the pharmacokinetic studies will provide proof of concept that the drug is absorbed and therapeutic concentrations are achieved, which will give an idea that the drug binds to the receptor and produces the effect. reveals several examples related to various strategies for achieving chronotherapy of different disease conditions using the CoDDS approach. Additionally, a detailed schematic diagram of some of the platform technologies developed and their functioning is portrayed in () Citation[14].
Table 1. Recently developed CrDDS for achieving CoDDS.
Figure 2. Schematic diagram of (A) Pulsincap®, (B) Erodible plug time-delayed capsule, (C) Hydrophilic sandwich capsule, (D) OROS®-CT, (E) Egalet®, (F) Chronotopic® system, (G) Time Clock® system, (H) Pulsatile hydrogel capsule, (I) Time controlled explosion system, (J) Eudracol®, (K) Chronset™, (L) Osmet™, (M) PORT® system.
4. Expert opinion
Since past 20 years, the pharmaceutical literatures have been deluged with several investigational methodologies for achieving CrDDS through oral route. Regardless of such a colossal volume of investigation being carried out by pharmaceutical researchers for developing CrDDS, very few of them prospered in reaching the doors of clinical phase (the details of which have been discussed elsewhere) Citation[3,5,14]. This may be attributed to some of the facts such as lack of industrial applicability, scale-up difficulties, limited availability of resources (materials), and overall cost of dosage forms development. This points out that much attention needs to be focused on these aspects. Further, development of more refined technologies for the large-scale implementation of these systems is the need of an hour.
An enormous variety of oral CrDDS reported in the literature depicts the interest of formulation scientist in this specific arena of pharmaceutics. As circadian rhythms have been described extensively for numerous diseases, a continual rise in the demand for the development of CrDDS has been forecasted. This leads to the increase in the attempts being made by the scientific community for the development of drug delivery system according to patient requirements, both with respect to compliance and therapeutic efficacy. CrDDS is such a delivery system that makes the drug available at right place, at precise time and in accurate quantity. Moreover, as the understanding regarding the number of diseases following biological rhythm of the body increases, the research in the field of CrDDS will be strengthened to a greater extent, leading to a better treatment and patient compliance.
The regulatory aspects should be taken in consideration right from the early stage of CrDDS development. Implementation of Quality by Design element in product development and increment in the availability of advanced technology for formulation development and processability will help in overcoming the hurdles associated with the development of CrDDS.
Declaration of interest
The author state no conflict of interest and has received no payment in preparation of this manuscript.
Notes
Bibliography
- Patel MM. Drug delivery: oral colon-specific. In: Swarbrick J, Editor. Encyclopedia of pharmaceutical science and technology. Taylor & Francis Group, LLC, UK; 2014. p. 1091-121.
- Patel M, Shah T, Amin A. Therapeutic opportunities in colon specific drug delivery systems. Crit Rev Ther Drug Carrier Syst 2007;24:147-202
- Lin SY, Kawashima Y. Current status and approaches to developing press-coated chronodelivery drug systems. J Control Release 2012;157:331-53
- Gandhi BR, Mundada AS, Gandhi PP. Chronopharmaceutics: as a clinically relevant drug delivery system. Drug Deliv 2011;18:1-18
- Mandal AS, Biswas N, Karim KM, et al. Drug delivery system based on chronobiology - a review. J Control Release 2010;147:314-25
- Labrecque G, Belanger PM. Biological rhythms in the absorption, distribution, metabolism and excretion of drugs. Pharmacol Ther 1991;52:95-107
- Patel MM, Amin AA. Design and optimization of colon targeted system of theophylline for chronotherapy of nocturnal asthma. J Pharm Sci 2011;100:1760-72
- McConnell EL, Short MD, Basit AW. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J Control Release 2008;130:154-60
- Patel MM, Shah TJ, Amin AF, et al. Design, development and optimization of a novel time and pH-dependent colon targeted drug delivery system. Pharm Dev Technol 2009;14:62-9
- Patel MM, Patel SL, Bhadani MN, et al. A synchronous colon specific drug delivery system for orally administered mesalamine. Acta Pharm Sci 2009;51:251-60
- Patel MM, Amin AA. Formulation and development of release modulated colon targeted system of meloxicam for potential application in the prophylaxis of colorectal cancer. Drug Deliv 2011;18:281-93
- Basit AW. Advances in colonic drug delivery. Drugs 2005;65:1991-2007
- Jeong YI, Ohno T, Hu ZP, et al. Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J Control Release 2001;71:175-82
- Patel MM. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin Drug Deliv 2011;8:1247-58
- Yalavarthi PR, Vulava J, Vadlamudi HC, et al. Modified pulsincap of ibuprofen--a novel approach for chronotherapy. Curr Drug Deliv 2013;10:299-308
- Kadam VD, Gattani SG. Development of colon targeted multiparticulate pulsatile drug delivery system for treating nocturnal asthma. Drug Deliv 2010;17:343-51
- Yassin AE, Aodah AH, Al-Suwayeh S, et al. Theophylline colon specific tablets for chronotherapeutic treatment of nocturnal asthma. Pharm Dev Technol 2012;17:712-18
- Lee Y, Kim IH, Kim J, et al. Evaluation of dextran-flufenamic acid ester as a polymeric colon-specific prodrug of flufenamic acid, an anti-inflammatory drug, for chronotherapy. J Drug Target 2011;19:336-43
- Ramasamy T, Ruttala HB, Shanmugam S, et al. Eudragit-coated aceclofenac-loaded pectin microspheres in chronopharmacological treatment of rheumatoid arthritis. Drug Deliv 2013;20:65-77
- Soni ML, Namdeo KP, Jain SK, et al. pH-enzyme di-dependent chronotherapeutic drug delivery system of theophylline for nocturnal asthma. Chem Pharm Bull (Tokyo) 2011;59:191-5
- Jose S, Prema MT, Chacko AJ, et al. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf B Biointerfaces 2011;83:277-83
- Barakat NS, Al-Suwayeh SA, Taha EI, et al. A new pressure-controlled colon delivery capsule for chronotherapeutic treatment of nocturnal asthma. J Drug Target 2011;19:365-72