Abstract
Drug discovery scientists, faced with the myriad challenges involved in developing novel therapeutics as medicines, have tended to overlook the question of the most beneficial time to administer the drug. Recent developments in our understanding of circadian biology and the availability of tools to characterise the molecular clock indicate that time and duration of dosing may have profound consequences for the efficacy and safety of new and existing therapeutic agents. Progress in the field also suggests that many key physiological mechanisms are remarkably dependent on the circadian clock. It has also become clear that a number of diseases with important unmet medical need display marked circadian variation in their symptoms and severity. These discoveries now reveal opportunities for new therapeutic strategies to be developed that act by modulation of biological rhythms. These novel therapeutic approaches are likely to be facilitated by the continuing development of chemical probes and synthetic ligands targeted to an increasing number of the key proteins that regulate the molecular clock.
1. Biological rhythms
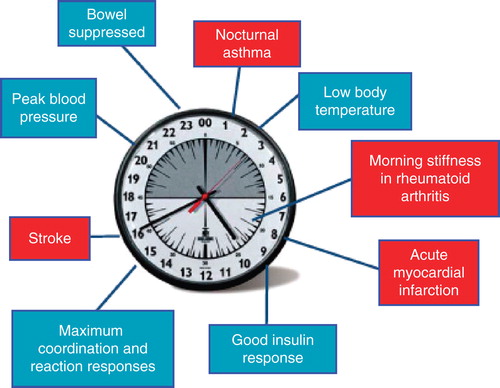
All life has evolved to anticipate the daily changes in environment that are driven by rotation of the planet. In addition to the switch from light to dark that affects sleep/wake cycles, there are changes in nutrient availability that impose additional stress on the circadian cycle. It is now known that many cells in complex organisms contain molecular clocks that control the activity of important signalling pathways. Synchronisation of these clocks to the circadian environment allows for the optimal function of cells and tissues within all animals. It is well established that many physiological processes in humans are influenced by this circadian timing system in which the suprachiasmatic nucleus (SCN) in the brain acts as a master pacemaker that synchronises subsidiary clocks in peripheral cells. These biological clocks are constructed around interlocking gene expression loops that function to translate the expression signals into rhythmical physiological and behavioural outputs. From an initial focus on sleep/wake cycles, feeding behaviour and metabolism, an appreciation that circadian oscillations play a critical role in other important physiological mechanisms has emerged ().
This editorial will consider current and future approaches for pharmacological manipulation of circadian oscillators and will also propose that applying newly appreciated circadian principles to drug delivery will lead to significant therapeutic benefit.
2. The molecular clock
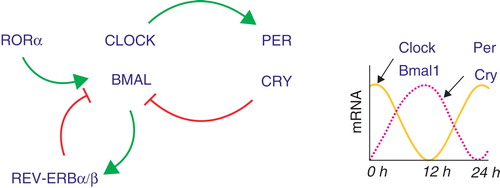
Over the past decade, great advances have been made in understanding the molecular basis of the regulation of cell autonomous circadian clocks in mammals, including humans. A transcriptional–translational feedback loop has been characterised that keeps time in mammalian cells Citation[1]. The positive limb consists of the PAS-domain bHLH transcription factors CLOCK and BMAL1, which heterodimerise to drive expression of Per and Cry (). NPAS2, an orthologue of CLOCK also functions as a BMAL1 heterodimer in the brain. PER and CRY function as transcriptional repressors in the negative limb of the clock by blocking the activity of CLOCK-BMAL1. The oscillating expression of these transcription factors on a daily cycle forms the core molecular clock. Additional transcription factors, notably the nuclear receptors REV-ERBα/REV-ERBβ and RORα, reinforce the core clock by regulating expression of Bmal1. The redundancy in these transcriptional feedback loops is important for maintaining a robust circadian clock.
Clocks in lower organisms are known to sense the availability of nutrients by responding to the availability of foods and signalling molecules. In plants amino acids, sugars and lipids can regulate daily growth cycles. Likewise, in the simplest unicellular organisms redox cycles can control circadian signalling pathways Citation[1].
3. Regulation of molecular clock function
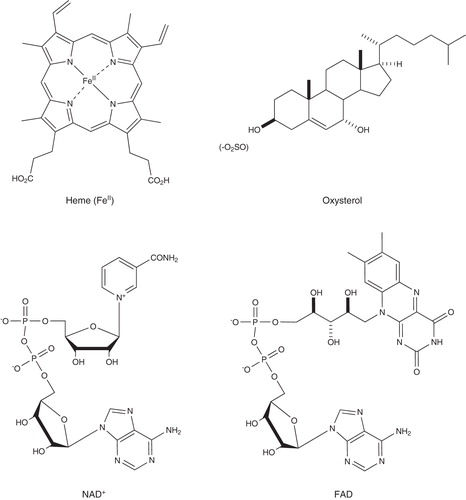
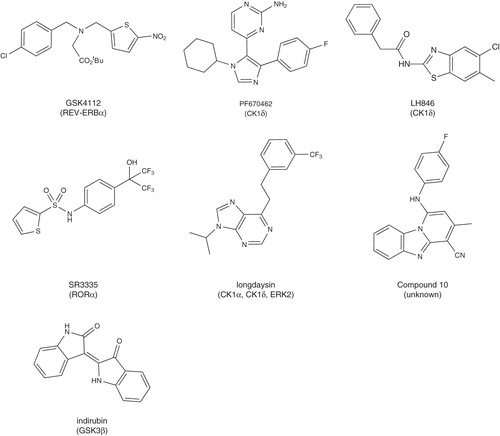
The transcription factors of the mammalian core clock are also dependent on a number of endogenous ligands and cofactors, through which their activity can be modulated (). For example, heme has been identified as the endogenous ligand for REV-ERBα and REV-ERBβ. The biosynthesis of heme is regulated by REV-ERBα through transcriptional repression of expression of its rate-limiting biosynthetic enzyme ALAS1. This feedback loop exists to control heme levels in response to nutrient availability, providing a potential molecular mechanism for the host environment to influence the circadian clock Citation[2]. X-ray crystallography has revealed the details of the REV-ERBβ-heme complex in which the iron (II) is held in a hexacoordinate complex by a histidine and cysteine residues from the nuclear receptor. Elegant biochemical studies have shown that the complex can exist in multiple coordination states and that the monoatomic gases NO and CO decrease receptor activity on binding to the iron porphyrin. Cholesterol and its primary metabolites (oxysterols and sulphated derivatives) have been identified as endogenous ligands for RORα Citation[3]. Little is known about whether the levels of these substances or the enzymes that produce them oscillate in cells. Recently, a series of tertiary sulphonamides were described as synthetic ROR ligands Citation[4], which holds promise for studying the role of the receptor in the circadian clock.
The dinucleotides flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD) have been implicated as signalling molecules in the regulation of the core clock. The activity of mammalian CRY is dependent on the flavoprotein FAD, although it is not photoregulated as in lower organisms Citation[5]. The activity of the CLOCK-BMAL1 heterodimer is regulated by NAD through a feedback loop involving SIRT1, providing an additional mechanism for nutrients to regulate circadian rhythms. Expression of Nampt, the critical enzyme in NAD synthesis is activated by CLOCK-BMAL1. The resulting increase in NAD-dependent Sirt1 deactylase activity results in post-translational modification of BMAL1 with suppression of its activity. The cycles of NAD production and deacetylation of BMAL1 are an elegant example of the interaction of metabolism with the circadian clock Citation[6].
Synthetic ligands for REV-ERBα have also been reported. All of the compounds are analogues of GSK4112 (), a lipophilic tertiary amine that competes with heme for binding to REV-ERBα Citation[7]. The GSK4112-REV-ERBα complex functions as a strong transcriptional repressor to block expression of target genes such as bmal1. In circadian reporter assays using synchronised cells, activation of REV-ERBα by GSK4112 resulted in a 3 h advance or delay of the phase of oscillation depending on the time of its dosing, providing compelling evidence that ligand modulation of the receptor affects timing of the circadian clock.
In addition to the role of endogenous ligands and signalling molecules, it has long been appreciated that phosphorylation of the core clock proteins can regulate their activity Citation[8]. The rare human genetic disorder of familial advanced sleep phase syndrome, in which the affected individuals go to sleep and wake several hours early, was shown to result from point mutations in the per2 gene that removed phosphoylation sites. The casein kinase 1 (CK1) family of kinases are implicated in the phosphoylation of PER2 at these sites. Studies with subtype selective CK1 inhibitors have demonstrated that CK1δ is the dominant isozyme for regulation of circadian period Citation[9]. Inhibition of CK1δ leads to nuclear localisation of PER2 and a decrease in its cellular clearance. Notably, in mice made arrhythmic through deletion of the SCN protein VIPR2, daily administration of a CK1δ inhibitor entrained the mice to a circadian rhythm of locomotor activity. GSK3β is another kinase that has been shown to affect circadian rhythms, however, it phosporylates multiple components of the clock and different inhibitors have shown opposing phenotypes. Lithium, a weak GSK3β inhibitor, caused lengthening of circadian period in cell culture and in vivo, while inhibition by indirubin led to a short period phenotype in cells. REV-ERBα, BMAL1, CLOCK and CRY2 have all been reported as substrates for GSK3β, further complicating the interpretation of its role in circadian regulation Citation[8].
Recently, several laboratories have conducted cell-based phenotypic screens of compound libraries to identify molecules that affect cycling of luciferase reporter genes driven by circadian promoters. Screening of the commercial LOPAC library identified several kinase inhibitors. The period lengthening compounds were shown to inhibit CK1, while the period shortening compounds were GSK3β inhibitors. A subsequent screen of a 120K compound library led to the identification of a compound (designated longdaysin) that had pronounced period lengthening activity through regulation of PER1 stability Citation[10]. Using chemical proteomics, it was demonstrated that longdaysin inhibited multiple kinases including CK1α, CK1δ and ERK2. Administration of longdaysin to zebrafish led to a lengthening of their circadian period. Recently, a third screen of a 500K compound library identified the benzothiazole LH846 with a pronounced period lengthening activity Citation[11]. The chronotypic activity was due to selective inhibition of CK1δ. An independent screen of a 200K compound library in a clock reporter assay Citation[12] also identified CK1 inhibitors with period lengthening activity. More significant was the discovery of several compounds with unknown molecular targets that increased amplitude of rhythms (often with period shortening activity). These compounds demonstrated robust induction of reporter activity in multiple cell types. Drugs with a similar chronotypic activity might be useful for treatment of disorders associated with weakened clock activity.
In summary, the molecular components of the circadian clock are sensitive to a wide range of endogenous signalling molecules ranging from diatomic gas to nutrients, porphyrins and dinucleotides. These substances provide chemical mechanisms to tune the cycling of the clock to the environment. Importantly, the regulation of clock activity with synthetic small molecules that target kinases and nuclear receptors has already demonstrated that there is considerable therapeutic potential through the modulation of circadian oscillations. For example, recent genetic studies on REV-ERBα have shown an important link between the clock and the innate immune system, positioning this nuclear receptor as a key gating mechanism for inflammation Citation[13]. This study utilised the synthetic REV-ERBα ligand GSK4112 to demonstrate that receptor modulation could drive suppression of inflammatory responses. A more recent report described a modified analogue that affected diurnal rhythms and peripheral metabolism in diabetic mice through activation of REV-ERBα and REV-ERBβ Citation[14].
4. Chronotherapy
In the clinical setting, it is recognised that both acute events such as myocardial infarction and the symptoms of chronic diseases such as rheumatoid arthritis cluster at certain times of the day, suggesting that it may be beneficial to deliver drug therapy to coincide with these disease fluctuations. Indeed, the concept of chronopharmacology has already demonstrated therapeutic benefit in the treatment of rheumatoid arthritis and in cancer chemotherapy. It is clear that this approach may also benefit patients suffering from metabolic disorders such as diabetes. Circadian delivery of chemotherapeutic agents has been shown to significantly modify tolerability in experimental models and in cancer patients Citation[15]. Improved efficacy has also been achieved when drugs are administered to coincide with the time of maximum tolerability. This is due to inherently poor circadian entrainment of tumours and persistent circadian entrainment of non-disease tissues. Furthermore, whenever anticancer drugs are administered at their most toxic time, host clocks are disrupted. It has also been established that disruption of the sleep/activity rhythm accelerates cancer growth. The complex circadian time-dependent connection between the host, cancer and therapeutic agents is further impacted by additional factors including gender, patient-to-patient variations and clock gene polymorphisms. Additionally, gender, circadian physiology, clock genes and the cell cycle critically affect the outcome of cancer chronotherapeutics Citation[15]. In other disease settings, chronotherapy has thus far focused on delivery of drugs at the time of peak symptoms, for example, the use of controlled release prednisolone to treat rheumatoid arthritis in the early morning, resulting in improved symptom control as compared with standard therapy Citation[16]. In patients with asthma, symptoms generally worsen during the early hours of the morning, and pulmonary function often deteriorates at the same time. Furthermore, it has been demonstrated that symptom control with inhaled glucocorticoids can be improved by delivery of the drug in the afternoon. These clinical observations have been further explored at the molecular level, with recent data indicating that glucocorticoids have a powerful resetting effect on the peripheral circadian clock in the lung, an activity that may contribute to their efficacy Citation[17]. However, the advances in molecular characterisation of the SCN and peripheral cellular clocks suggest even greater therapeutic opportunities. It has become clear that these molecular oscillators themselves play a direct role in the regulation of inflammation and metabolism, and that disturbance of this interaction can have a significant impact on output pathways and disease pathology.
5. Expert opinion
It has been clear for many years that animal and plant physiology is profoundly influenced by circadian rhythms. More recently, we have begun to unravel the molecular machinery itself that makes up the circadian clock and to understand the mechanisms through which many physiological processes are governed by this exquisite molecular machinery. These discoveries have already revealed potential therapeutic applications, and the rapid advances in the field indicate that chronobiology has the potential to uncover many more opportunities for drug discovery and development. To uncover these opportunities and exploit the full potential of the clock, it is necessary to think outside of the conventions of current drug discovery strategies and practices. Two main areas are anticipated where chronobiology will impact on drug discovery. The first is the more conventional approach that is to explore the potential of developing new drugs that act by directly modulating components of the circadian clock itself. The molecular clock has evolved chemical mechanisms to anticipate daily changes in the environment, through sensing circadian variability in nutrients and other organic substances. The pathways regulated by these small molecules include kinases and nuclear receptors that have a rich tradition in pharmacology and drug discovery. In addition, identification of those molecular targets that increase the robustness of the circadian clock may herald a new field of molecular chronotherapy. Many of the tools that are needed are currently available and the greatest challenges are perhaps identifying the best application. Second, further progress is seen with circadian-controlled release or delivery of existing therapeutic agents, providing improved efficacy or reduced toxicity of drugs by delivering them at the most appropriate time and duration for a particular therapeutic indication. There are several reasons why there might be a ‘best’ time to deliver a drug. It is probable that the molecular targets of drugs are themselves subject to strong circadian control, implying that the impact of modulating these targets will vary depending on circadian time. Consequently, there may be no benefit to the patient to be exposed to sustained concentrations of drug over the whole day when the drug target is only expressed or functional for a short period. Indeed, it is likely that conventional 24/7 coverage may increase the total drug burden and contribute to toxicity. Thus, an appreciation of the chronobiology of drug action may allow the development of medicines with improved therapeutic index. This is a radically new way of thinking about drug pharmacokinetics and this new paradigm might see the development of short-acting timed release drugs delivered at the right time to improve efficacy and reduce toxicity, a very different approach as to how the medicines are currently developed. Development of these chrono-optimised medicines may require new diagnostic and continuous monitoring strategies, so that drug administration can be synchronised to the circadian time of the patient. Although such ‘circadian monitors’ do not currently exist, given current advances in the field there is no reason to believe they could not be developed. It is the authors' opinion that such a personalised approach to the dosing of medicines will benefit patients afflicted with many of life's chronic diseases and create new exciting opportunities for drug discovery.
Declaration of interest
The authors of this paper declare funding support from GlaxoSmithKline (GSK). All the authors of this paper are also employees of GSK.
Bibliography
- Dunlap JC. Molecular bases for circadian clocks. Cell 1999;96:271-90
- Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: A heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal 2010;8:e001
- Kallen J, Schlaeppi J-M, Bitsch F, Crystal structure of the human RORalpha ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem 2004;279:14033-8
- Solt LA, Kumar N, Nuhant P, Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 2011;472:491-4
- Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem 2000;69:31-67
- Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 2011;13:125-37
- Grant D, Yin L, Collins JL, GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem Biol 2010;5:925-32
- Reischl S, Kramer A. Kinases and phosphatases in the mammalian circadian clock. FEBS Lett 2011;585:1393-9
- Meng Q-J, Maywood ES, Bechtold DA, Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA 2010;107:15240-5
- Hirota T, Lee JW, Lewis WG, High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol 2010;8:e1000559
- Lee JW, Hirota T, Peters EC, A small molecule modulates circadian rhythms through phosphorylation of the Period protein. Angew Chem Int Ed 2011;50:10608-11
- Chen Z, Yoo S-H, Park Y-S, Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci USA 2012;109(1):101-6
- Gibbs JE, Blaikley J, Beesley S, The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA 2012;109:582-7
- Solt LA, Wang Y, Banerjee S, Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012;485:62-68
- Levi F, Okyar A, Dulong S, Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 2010;50:377-421
- Buttgereit F, Doering G, Schaeffler A, Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 2008;371:2205-14
- Gibbs JE, Beesley S, Plumb J, Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 2009;150:268-76