Abstract
Type 2 diabetes (T2D) – particularly with concurrent obesity (‘diabesity’) – is an intensifying global public-health problem. Medical needs and market opportunities in the T2D space have propelled discovery efforts aimed at inventing new synthetic T2D drugs differentiable by improved safety and efficacy and/or the ability to modulate emerging T2D targets. Particularly for moderately and severely obese individuals, weight-loss (bariatric) surgery offers an effective means of reducing obesity-driven T2D that is superior in many respects to medical T2D management. Yet, not all overweight or obese individuals with T2D qualify for bariatric surgery, and current healthcare resources are inadequate for applying surgical T2D control to more than a very small segment of qualified patients. Bariatric surgery is no guarantee of ‘curative’ T2D abrogation, significant rates of T2D non-remission or re-emergence having been observed in diabesity patients following bariatric procedures. Preoperative glucose control by oral hypoglycemic drugs reduces the chance of T2D recurrence post-surgery, and diabesity patients in whom glycemic indices have been improved by bariatric surgery may still require some level of T2D pharmacotherapy. Laboratory and clinical data indicate that synthetic T2D drugs can improve T2D-related outcomes following bariatric procedures, and current T2D drug-discovery efforts are being informed by the metabolic advantages associated with bariatric surgery. These circumstances intensify the need for and extend the impact of T2D drug discovery by demonstrating multiple levels of interplay between medical and surgical approaches to improve the health of individuals with diabesity and, perhaps, approach the overarching goal of decreasing long-term cardiovascular mortality.
1. Background
In the November, 2013, issue of Expert Opinion on Drug Discovery, Safavi et al. Citation[1] surveyed approved synthetic drugs and late-stage pipeline compounds as pharmacotherapeutics for type 2 diabetes (T2D). Concluding that ‘finding new targets and ... synthetic methods’ is the ‘main goal in T2D drug discovery,’ the authors mention in passing that weight-loss (bariatric) surgery ‘is a highly effective treatment for obesity-related T2D.’ This commentary aims to widen the aperture afforded by Safavi and colleagues by considering the proposition that the inability of bariatric surgery to eradicate the T2D pandemic expands the scope of and presents new opportunities for drug discovery in the T2D space.
2. Bariatric surgery can effectively address, but has not solved, the T2D problem
An intensifying worldwide epidemic, obesity contributes to the development of numerous disabling and lifespan-limiting maladies. One of these, T2D, is well recognized as obesity's principal metabolic comorbidity and one of the fastest-growing noncommunicable diseases, characterized by insulin resistance and insufficient pancreatic β-cell insulin production to maintain acceptable glucose control/euglycemia Citation[2]. Poorly controlled T2D itself increases cardiovascular risk and invites micro- (neuropathy, retinopathy) and macrovascular (obstructive arteriopathy leading to stroke, myocardial infarction) complications, the latter jeopardizing survival Citation[3]. The coordinate escalation in the global incidences of obesity and T2D serves as a signpost for the contemporary pandemic of T2D in the context of obesity (‘diabesity’) Citation[4].
Durable weight loss has long remained both a primary medical recommendation and an elusive goal for most diabesity patients Citation[5]. Traditional T2D medical management involving behavioral modification and individual drugs/drug combinations can elicit salutary effects (), but with relatively poor long-term health benefit Citation[6]. Intensive lifestyle intervention (medically supervised weight loss, increased physical activity), although effective in reducing the body weight of overweight or obese T2D patients, offered no clear evidence of cardiovascular benefit at a median follow-up of 9.6 years in the large, randomized, controlled ‘Look AHEAD’ study Citation[7]. Other prospective studies indicate that only some 50% of patients with moderate-to-severe T2D ever attain successful, sustained glycemic control through drug treatment Citation[8].
Figure 1. A pharmaco-surgical interactome for T2D management. The Venn diagram represents the principal patient populations/beneficial outcomes associated with medical T2D management with insulin and synthetic pharmacotherapeutics (left); surgical T2D management with metabolic/bariatric procedural intervention (right); and a synergistic combination of the two T2D management approaches (center). Treatment impact may vary with specific pharmacotherapeutic regimen and surgical procedure.
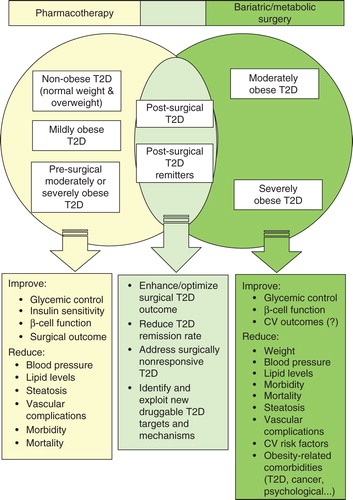
In contrast to medical management alone, multiple retrospective analyses and prospective clinical studies demonstrate that bariatric surgery is the most effective weight reduction treatment for both morbidly (i.e., severely) obese adults (body mass index [BMI] > 40 kg/m2) and moderately obese adults (BMI 35 – 40 kg/m2) with significant weight-related health problems Citation[9-11]. Roux-en-Y gastric bypass (RYGB) accounts for 46.6% of the global number of bariatric procedures worldwide, followed in frequency by sleeve gastrectomy (SG) (27.8%), adjustable gastric banding (AGB) (17.8%) and biliopancreatic diversion (BPD) (2.2%) Citation[12]. As detailed in recent authoritative overviews Citation[9,13], RYGB is the prototypical restrictive-malabsorptive procedure designed to divert ingested nutrients from passage through the upper digestive tract. A restrictive procedure, SG markedly reduces gastric volume by laparoscopic removal of some 70 – 80% of the stomach. An easily reversible, minimally invasive restrictive procedure, AGB reduces the size of the stomach pouch by means of an inflatable band placed around the upper portion of the stomach such that satiety is induced by smaller amounts of food, whereas BPD is a restrictive-malabsorptive procedure whose risk profile has limited its application to the most severely obese. All four bariatric procedures not only elicit substantial and sustained weight loss in moderately or morbidly obese adults Citation[9,10] but also reduce or ameliorate various obesity-associated comorbidities, including T2D, with positive impact on overall health, quality of life and survival Citation[10,11,14-16]. RYGB is generally associated with better weight-reduction outcomes and diabetes control, and the superior T2D-related efficacy is thought to reflect, at least in part, bypass procedure-induced reprogramming of intestinal glucose metabolism () Citation[9,10,17-19]. Notwithstanding post-surgical nutritional liabilities and effects on drug absorption, the outcome success and low procedural risk generally associated with bariatric surgery have engendered interest in its somewhat controversial application to non-obese (BMI < 30 kg/m2) and mildly obese (BMI 30 – 34.9 kg/m2) adults with T2D and to morbidly obese children and adolescents who have failed to achieve treatment targets with conservative weight-loss measures Citation[11,20-22], the escalating rates of youth obesity and obesity-driven T2D being particularly alarming Citation[23]. Indeed, accumulating evidence indicates that bariatric surgery in mildly obese subjects is associated with at least comparable, if not better, outcomes (including T2D remission rates) than in morbidly obese subjects for up to 6 years post surgery Citation[11,24,25].
The acute negative caloric balance consequent to food restriction per se has been considered a likely contributor to improved glucose control within 7 – 14 days post surgery Citation[26]. Although a meta-analysis suggests a proportional relationship between T2D resolution rates and the degree of weight loss after bariatric surgery Citation[27], the mechanisms responsible for the acute therapeutic effects of bariatric surgery on glucose homeostasis, β-cell function and insulin sensitivity are not well understood and appear to be mostly weight-loss independent. Candidate weight-independent mechanisms not mimicked by current pharmacotherapy that may contribute to T2D resolution following bariatric surgery include altered nutrient flow and sensing within regions of the gastrointestinal tract and changes in the production of bile acids and gut hormones Citation[26,28,29].
Mechanistic vagaries aside, bariatric surgery is successful in reducing the incidence of T2D in a significant proportion of diabesity patients. Depending upon such factors as procedure type, definition of remission, endpoint indices of glucose control, patient entry criteria, postoperative care regimen and study limitations (e.g., attrition rates), reductions in T2D-related indices of 6.0 – 95% at 1 – 2 years post surgery and 36% at 10 years post surgery have been reported Citation[15,30,31]. Although circumscribed by a maximum of 2 years of follow-up and a rather limited number of subjects, six clinical trials have demonstrated through direct comparison the superior outcomes of bariatric surgery over conventional medical T2D management with respect to both weight loss and indices of glycemic control () Citation[32-37], a conclusion supported by a recent comparative overview Citation[38] and cross-study meta-analysis Citation[39]. These findings have led to recommendations by at least some sectors of the medical community and prominent diabetological associations that bariatric surgery be considered valid primary therapy for (obesity-driven) T2D and, as such, be incorporated into T2D treatment algorithms as ‘metabolic surgery’ Citation[22,40,41]. Accordingly, the term ‘metabolic/bariatric surgery’ is used henceforth in this presentation.
Table 1. Randomized controlled clinical trials comparing T2D response to conventional medical versus surgical treatment in obese individuals.
3. Multiple (surgery-related) niches for T2D synthetic drugs
Does the unique and impressive ability of metabolic/bariatric surgery to remit (obesity-driven) T2D marginalize medical treatment for diabesity such that the intensive, ongoing search for new T2D drugs/drug targets Citation[1,6,42] risks – if not merits – de-emphasis? In the following paragraphs of this section, the author offers several considerations supporting an answer in the negative.
In Africa (Nigeria), Europe (UK), North America (USA) and Asia (Taiwan), the prevalence of T2D has been reported to be 7.1 – 20.4% in moderately obese and 13.8 – 44% in morbidly obese adults Citation[43,44], patient populations considered eligible for metabolic/bariatric surgery by current clinical, regulatory and specialist-association guidelines Citation[9-11,22]. Thus, most individuals who undergo bariatric surgery do not have T2D Citation[45]. These statistics substantiate conclusion that the overarching therapeutic goal of metabolic/bariatric surgery remains weight reduction and not glycemic control such that T2D population-based treatment continues to be centered around synthetic drugs (with or without insulin). Nonetheless, in moderately or morbidly obese, nondiabetic subjects, metabolic/bariatric surgery does exert a strong T2D prevention effect, reducing the risk of developing T2D at 2 (by 75 – 96%), 10 (by 75 – 84%) and 15 (by 78%) years post surgery Citation[46].
Notwithstanding the fact that obesity is an independent pro-inflammatory risk factor for T2D Citation[2], obesity and T2D do not co-occur only in the moderately and morbidly obese. Some 3 – 4% of normal-weight (BMI < 25 kg/m2), 3 – 4% overweight (BMI 25 – 30 kg/m2), and 10 – 12% mildly obese individuals in the USA have T2D and would be considered ineligible for metabolic/bariatric surgery by current guidelines, but could qualify as candidates for synthetic anti-T2D drugs Citation[10,22,43].
Metabolic/bariatric surgery is by no means a ‘magic-bullet cure’ for either obesity or T2D in the sense that the signs and symptoms of these diseases inevitably remit post procedure without risk of re-emergence. Results from both meta-analyses and randomized trials show 1- and 2-year T2D remission rates in morbidly obese subjects from 57% after the less invasive AGB procedure to 80% after the more invasive RYGB, that is, metabolic/bariatric surgery proved unable to resolve T2D in some 20% to over 40% of diabesity patients within 1 – 2 years post procedure Citation[30,32-34,47]. At 3 years after RYGB, T2D re-emerged or worsened in 24% of diabesity patients Citation[48] and in 35% at 5 years post surgery Citation[49]. Other studies report that recurrence of T2D after initial surgical remission was evident in 19% within a 5 – 9-year follow-up range across various procedures Citation[15] and 31.4% within 7 – 9 years after RYGB Citation[50]. Among morbidly obese patients with remission of diabetes at 2 years after metabolic/bariatric surgery involving RYGB, AGB and other procedures, T2D was reported to have reoccurred in 50% by 10 years' follow-up Citation[30,46]. Re-emergence of T2D after metabolic/bariatric surgery has been potentially associated with several preoperative factors, including poor glycemic control, severity of β-cell dysfunction, T2D duration and insulin use as well as a host of postoperative factors, such as maladaptive eating leading to weight regain, time after surgery and definition of clinical T2D remission Citation[51,52]. Even significant, surgically-induced weight loss need not be accompanied by any signs of improved glycemic control whatsoever Citation[53]. These clinical data substantiate the existence of a substantial number of diabesity patients who had undergone metabolic/bariatric surgery, yet would still be candidates for medical treatment with synthetic drugs to keep T2D in-check and help maintain glucose homeostasis due to lack of post-surgical T2D resolution.
The resolution of T2D after metabolic/bariatric surgery is more likely to occur in diabesity patients whose T2D had been controlled by oral hypoglycemic medications, and poor preoperative glycemic control and insulin use are associated with increased chance of T2D recurrence after surgically improved glucose control Citation[15,51,52]. These results suggest an important role for synthetic drugs in a positive post-surgical prognosis by limiting the degree of preoperative glucose dysregulation.
Although variable among studies, criteria for complete (surgical) remission of T2D generally include both evidence of normal glycemic control and discontinuation of all diabetes medications Citation[9,16,52,54]. Yet, diabesity patients who show acute surgical improvement in glycemic indices may need to remain on some (usually, reduced) level of T2D pharmacotherapy for months to over 1 year post surgery, depending upon the surgical procedure employed Citation[9,55,56]. Indeed, caution has been sounded regarding inappropriate cessation of T2D medications post surgery Citation[57].
Healthcare and surgical resources are considered inadequate for applying metabolic/bariatric surgery as a primary treatment modality for obesity, let alone obesity-related comorbidities such as T2D. Metabolic/bariatric surgery is capable of treating a very limited percentage of obese individuals who qualify for the procedure, especially given the projected surges in global diabesity incidence Citation[10,12]. As a specific example, in the USA, metabolic/bariatric surgery is utilized for < 1% of currently eligible patients, thereby excluding some 99% of qualified individuals Citation[58]. Thus, metabolic/bariatric surgery alone cannot be relied upon to manage the diabesity epidemic, whereas the applicability of synthetic T2D drugs is intrinsically much more inclusive across the overall T2D patient population.
4. New avenues for pharmaco-surgical T2D treatment synergies
Among the advantageous humoral responses to RYGB favoring glycemic control, the exaggerated postprandial glucagon-like peptide-1 (GLP-1) secretion observed acutely and up to 10 years post procedure has been considered of paramount importance, perhaps reflecting GLP-1's abilities to promote pancreatic β-cell regeneration and help ensure euglycemia by increasing insulin biosynthesis and secretion after meal ingestion Citation[59,60]. In diet-induced obese rats, Habegger et al. recently reported that a GLP-1 receptor agonist enhances the surgically induced decreases in body weight and adiposity after AGB, whereas pharmacological attenuation of orexigenic drive through the endocannabinoid biosignaling system with a cannabinoid receptor-1 antagonist/inverse agonist (rimonabant) did not improve experimental AGB outcome Citation[61]. These laboratory results suggest that GLP-1 receptor agonists might find new clinical utility as the pharmaceutical component of a pharmaco-surgical approach for optimizing AGB (). In this manner, AGB might attain the levels of weight reduction and metabolic improvement associated with the more invasive RYGB, although procedure-related complications have led to a decline in the use of AGB Citation[12], and currently available GLP-1-based therapies have been associated with potential risks of pancreatitis and pancreatic cancer Citation[62]. Extrapolation of any such preclinical efficacy studies to the clinic must also be tempered by our incomplete understanding of the factors that impact upon T2D diabetes resolution after AGB or RYGB Citation[26-29]. Nonetheless, experimental findings of this type do suggest practical avenues of synergy between pharmacotherapy and metabolic/bariatric surgery potentially applicable to humans.
A recent clinical study has demonstrated that obese (BMI ≥ 35 kg/m2) patients with insulin-treated T2D who were managed after RYGB surgery with a personalized titration schedule for the insulin analog glargine and the synthetic anti-hyperglycemic agent metformin reported no symptomatic hypoglycemia and achieved complete T2D remission at 1-year follow-up with a rate several-fold greater (i.e., 50 vs 6.1%) than a matched cohort whose postoperative care did not include a protocol-driven drug treatment Citation[63]. Although this result needs confirmation in a large-scale, randomized, controlled clinical trial, the data suggest that even the T2D-related benefits of RYGB, the most efficacious metabolic/bariatric-surgery approach Citation[9,13,18], might be enhanced with pharmacological glycemic management ().
5. Expert opinion
Despite metabolic/bariatric surgery's unique ability to effect long-term T2D remission in some – but not all – diabesity patients, surgical T2D management is unlikely to become a population-based therapeutic modality with the degrees of practicality, applicability and versatility already attained by T2D synthetic drugs. Consequently, known and emerging T2D synthetic agents will be required not only to manage those diabetics disqualified from metabolic/bariatric surgery, but also to control T2D in post-surgery diabesity patients with residual or re-emergent T2D who have not achieved an acceptable level of metabolic homeostasis following surgery. This line of thinking argues for the potential of active glycemic management with synthetic T2D drugs to become an integral component of more personalized post-surgical glucose control.
As complex disease states, obesity and T2D are promoted and sustained by several directionally variable, interacting information pathways inherently resistant to interventional perturbation of a single process/molecular component and subject to influence by multiple (epi)genetic and environmental factors Citation[64]. This systems-biology perspective may help explain why the obesity and T2D therapeutic spaces are replete with drug failures, market withdrawals and adverse-event cautions, despite the fact that most pharmacotherapeutics for these indications have been developed as agents directed to apparently pathologically important, mechanism-based disease targets Citation[6,65]. As applied to T2D patients in the contexts of moderate and severe obesity, both known and emerging T2D drugs may themselves shed much-needed, therapeutically relevant light on the molecular dialog underpinning diabesity so as to facilitate identification of etiological drivers whose pharmaco-surgical modulation would have a more comprehensive salutary effect on T2D in the obesity setting than current best-care approaches. Such insight might also remove some of the mechanistic mystery surrounding the responses of glycemic status to metabolic/bariatric surgery Citation[66].
Although historic and more recent data suggest that some synthetic T2D drugs can have beneficial effects on cardiovascular risk factors and increase life expectancy in obese individuals with cardiovascular disease and T2D Citation[67], robust demonstrations from large-scale prospective clinical trials of a decisive effect of metabolic/bariatric surgery on long-term cardiovascular outcomes in moderately or morbidly obese individuals with T2D have been elusive Citation[6,11,30,68]. Utilization of synthetic T2D drugs in conjunction with metabolic/bariatric surgery may make it possible to realize decisively at least one of two prominent ‘holy grails’ that would enhance surgical outcomes: i) increase post-surgical T2D remission rates with pharmacotherapeutics efficacious in improving β-cell function prior to surgery, since T2D non-remission/recurrence has been correlated with the starting degree of β-cell dysfunction Citation[69,70]; and/or ii) militate cardiovascular risk sufficiently to decrease long-term mortality and improve overall survival of individuals with diabesity, occlusive macrovascular disease leading to myocardial infarction or ischemic stroke constituting the leading underlying cause of T2D-related deaths Citation[3].
While these scenarios at present belong more to the realm of possibility than prescription, connectivity between medical and surgical approaches to T2D treatment has already spurred active drug-discovery efforts that attempt to modulate pharmacologically novel T2D-related molecular mechanisms so as to mimic specific beneficial effects of metabolic/bariatric surgery (). For example, observed increases in bile flow and plasma bile acids associated with the immediate post-RYGB period and believed to stimulate β-cell function by potentiating incretin-hormone (i.e., GLP-1) signaling via activation of the G-protein-coupled bile acid receptor (TGR5) have prompted discovery campaigns to design proprietary, orally active TGR5 agonists as antidiabetic agents Citation[71,72]. Likewise, the critical role of the high-affinity sodium-glucose cotransporter (SGLT-1) in both glucose transport by the proximal intestine and consequent nutrient-sensing information pathways, the SGLT-1 overexpression observed in experimental and clinical T2D, and the reduced glucose-transport capacity of the proximal intestine following RYGB have led to the development of novel SGLT-1 inhibitors with preclinical antidiabetic efficacy Citation[73-75]. Such findings suggest that increased cross-talk between bariatric surgeons and T2D drug-discovery practitioners will not only augment our understanding of the mechanistic basis of surgical T2D remission, but will also generate as-yet unanticipated avenues for novel synthetic T2D drugs in managing and optimizing glycemic control and overall cardiometabolic status in the pre- and post-surgical diabesity patient.
Declaration of interest
The author declares no conflict of interest and has received no payment in the preparation of this manuscript.
Notes
Bibliography
- Safavi M, Foroumadi A, Abdollahi M. The importance of synthetic drugs for type 2 diabetes drug discovery. Expert Opin Drug Discov 2013;8:1339-63
- Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Adv Exp Med Biol 2012;771:340-55
- Bardini G, Rotella CM, Giannini S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet Stud 2012;9:82-93
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant 2011;26:28-35
- Nadeau DA. Physiologic and weight-focused treatment strategies for managing type 2 diabetes mellitus: the metformin, glucagon-like peptide-1 receptor agonist, and insulin (MGI) approach. Postgrad Med 2013;125:112-26
- Mehanna A. Antidiabetic agents: past, present and future. Future Med Chem 2013;5:411-30
- Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145-54
- Stolar MW. Defining and achieving treatment success in patients with type 2 diabetes mellitus. Mayo Clin Proc 2010;85:S50-9
- Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol 2013;33:75-89
- Azizi F. Bariatric surgery for obesity and diabetes. Arch Iran Med 2013;16:182-6
- Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes. A systematic review. JAMA 2013;309:2250-61
- Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23:427-36
- Batchelder AJ, Williams R, Sutton C, et al. The evolution of minimally invasive bariatric surgery. J Surg Res 2013;183:559-66
- Klein S, Fabbrini E, Patterson BW, et al. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity (Silver Spring) 2012;20:1266-72
- Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:628-37
- Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 2012;143:897-912
- Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med 2008;121:885-93
- Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254:410-20
- Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013;341:406-10
- Astiarraga B, Gastaldelli A, Muscelli E, et al. Biliopancreatic diversion in nonobese patients with type 2 diabetes: impact and mechanisms. J Clin Endocrinol Metab 2013;98:2765-73
- Messiah SE, Lopez-Mitnik G, Winegar D, et al. Changes in weight and co-morbidities among adolescents undergoing bariatric surgery: 1-year results from the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2013;9:503-13
- Samaras K. Bariatric surgery for type 2 diabetes: to whom and when? Minerva Endocrinol 2013;38:47-58
- D'Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 2011;34:S161-5
- Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420-8
- Lakdawala M, Shaikh S, Bandukwala S, et al. Roux-en-Y gastric bypass stands the test of time: 5-year results in low body mass index (30-35 kg/m2) Indian patients with type 2 diabetes mellitus. Surg Obes Relat Dis 2013;9:370-8
- Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery. Diabetes Care 2013;36:5287-91
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56
- Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890-901
- Papamargaritis D, Panteliou E, Miras AD, et al. Mechanisms of weight loss, diabetes control and changes in food choices after gastrointestinal surgery. Curr Atheroscler Rep 2012;14:616-23
- Sjöström L. Review of key results from the Swedish Obesity Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34
- Van Gaal LF, De Block CEM. Bariatric surgery to treat type 2 diabetes: what is the recent evidence? Curr Opin Endocrinol Diabetes Obes 2012;19:352-8
- Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-85
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76
- Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes. Diabetes Care 2013;36:2175-82
- Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50-6
- Ikramuddin S, Kroner J, Lee W-J, et al. Roux-en-Y gastric bypass vs. intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia. JAMA 2013;309:2240-9
- Lebovitz HE. Metabolic surgery for type 2 diabetes: appraisal of clinical evidence and review of randomized controlled clinical trials comparing surgery with medical therapy. Curr Atheroscler Rep 2013;15(12):376
- Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934
- Buchwald H, Scopinaro N. An invitation to our medical colleagues: work with us. Obes Surg 2010;20:1465-7
- Pournaras DJ, le Roux CW. Preventing type 2 diabetes, CVD, and mortality: surgical versus non-surgical weight loss strategies. Curr Atheroscler Rep 2013;15:367
- Cuny T, Guerci ZB, Cariou B. New avenues for the pharmacological management of type 2 diabetes: an update. Ann Endocrinol (Paris) 2012;73:459-68
- Gregg EW, Cheng YJ, Venkat KM, et al. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976-2004. Prev Med 2007;45:348-52
- Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes 2013;6:327-38
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467-84
- Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695-704
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56
- DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6:249-53
- Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93-102
- Yamaguchi CM, Faintuch J, Hayashi SY, et al. Refractory and new-onset diabetes more than 5 years after gastric bypass for morbid obesity. Surg Endosc 2012;26:2843-7
- Deitel M. Update: why diabetes does not resolve in some patients after bariatric surgery. Obes Surg 2011;21:794-6
- Blackstone R, Bunt JC, Cortés MC, et al. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis 2012;8:548-55
- Brakoniecki K, Ren-Fielding C, Laferrère B. A closer look at diabetes remission after gastric bypass surgery: a case study. Surg Obes Relat Dis 2013;9:e53-5
- Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009;32:2133-5
- Thorell A, Hagström-Toft E. Treatment of diabetes prior to and after bariatric surgery. J Diabetes Sci Technol 2012;6:1226-32
- Chhabra L, Liti B, Kuraganti G, et al. Challenges in the management of type 2 diabetes mellitus and cardiovascular risk factors in obese subjects: what is the evidence and what are the myths? Int J Endocrinol 2013: published online June 2013, doi:10.1155/2013/856793
- Buchwald H, Ikramuddin S, Dorman RB, et al. Management of the metabolic/bariatric surgery patient. Am J Med 2011;124:1099-105
- Martin M, Beekley A, Kjorstad R, et al. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis 2010;6:8-15
- Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013;62:3044-52
- Dar MS, Chapman WH III, Pender JR, et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg 2012;22:1077-83
- Habegger KM, Kirchner H, Yi CX, et al. GLP-1R agonism enhances adjustable gastric banding in diet-induced obese rats. Diabetes 2013;62:3261-7
- Ryder REJ. The potential risks of pancreatitis and pancreatic cancer with GLP-1-based therapies are far outweighed by the proven and potential (cardiovascular) benefits. Diabet Med 2013;30:1148-55
- Fenske WK, Pournaras DJ, Aasheim ET, et al. Can a protocol for glycemic control improve type 2 diabetes outcomes after gastric bypass? Obes Surg 2012;22:90-6
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363-74
- Janero DR, Lindsley L, Vemuri VK, et al. Cannabinoid 1 G protein-coupled receptor (periphero-) neutral antagonists: emerging therapeutics for treating obesity-driven metabolic disease and reducing cardiovascular risk. Expert Opin Drug Discov 2011;6:995-1025
- Laferrère B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine 2011;40:162-7
- Ovalle F. Cardiovascular implications of antihyperglycemic therapies for type 2 diabetes. Clin Ther 2011;33:393-407
- Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122-31
- Nannipieri M, Mari A, Anslemino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372-9
- Kashyap SR, Schauer P. Clinical considerations for the management of residual diabetes following bariatric surgery. Diabetes Obes Metab 2012;14:773-9
- Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glyceamic control. Endocrinology 2012;153:3613-19
- Duan H, Ninh M, Chen X, et al. Design, synthesis, and antidiabetic activity of 4-phenoxynicotinamide and 4-phenoxypyrimidine-5-carboxamide derivatives as potent and orally efficacious TGR5 agonists. J Med Chem 2012;55:10475-89
- Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol 2009;297:G950-7
- Nguyen CA, Akiba Y, Kaunitz JD. Resent advances in gut nutrient sensing. Curr Med Chem 2012;19:28-34
- Shibazaki T, Tomae M, Ishikawa-Takemura Y, et al. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 2012;342:288-96
