Abstract
Neurodegenerative diseases occur when neuronal cells in the brain or spinal cord progressively lose function and eventually die. Pathological analysis of these tissues reveals changes that include the loss of synapses, tangles of misfolded protein and immune cell activation, even during very early stages of disease well before debilitating clinical signs are apparent. This suggests that if neurodegeneration is treated early enough, drugs designed to delay the progress of these diseases by either repairing the early damage and loss of neurons, or protecting neuron functionality from further insult, may be efficacious. MicroRNAs (miRNAs) are small non-coding RNAs that can post-transcriptionally regulate gene expression. They are particularly numerous within neurons where many are expressed with high specificity, which suggests that they have important roles in the healthy brain. Indeed, miRNAs are essential for the post-mitotic survival of neurons, implying a crucial role in survival and neuroprotection. This has focused attention on exploring the use of miRNA-based drugs as a means to correct cellular abnormalities and maintain neuronal function in neurodegenerative diseases. These efforts are spurred on by the rapid progress to clinical trials for a number of miRNA-based therapies for other diseases such as cardiovascular diseases, fibrosis and cancer.
1. Introduction
MicroRNAs (miRNA) are an abundant family of endogenous non-coding RNAs that regulate gene expression in fundamental cellular processes. These small, ∼ 21 nucleotide long RNA molecules, typically bind regions of partial complementarity in the 3′-untranslated region of messenger RNA and are sequestered in the miRNA-induced silencing complex (miRISC) to prevent translation and/or to increase degradation Citation[1]. An abundance of miRNAs specifically expressed within neurons implies their importance, and numerous studies have uncovered crucial roles for miRNAs in fundamental processes such as neuronal differentiation, development, plasticity and survival Citation[2]. Indeed, knocking out miRNAs altogether, by manipulating the processing machinery that is required to produce functional miRNAs, leads to neurodegenerative pathologies in a number of animal models. Additionally, evidence from human tissues suggests that the dysregulation of miRNA expression plays a role in the development of neurodegenerative disorders. Based on these findings it is reasonable to hypothesize that the manipulation of miRNA levels in the brain may prove to be beneficial for the maintenance of functioning neurons, thereby slowing or halting neurodegeneration ().
Figure 1. Methods of miRNA manipulation in neurodegeneration. (A) Upon loss of expression of neuroprotective miRNA, miRNA mimics or synthetic miRNAs can be utilized for functional replacement. (B) Loss of expression of neuroprotective mRNA during disease due to repression by miRNA favoring neurodegeneration can be ameliorated by using anti-miRs (antisense inhibitors), multiple-target anti-miRNAs or miRNA sponges.
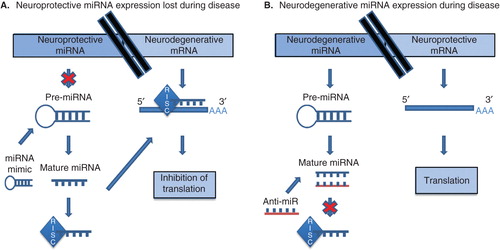
Figure 2. miRNA implicated in neurodegenerative disease. (A) Use of an miR-128 mimic in Parkinson’s-like mice resulted in improved motor function and downregulation of genes involved in the ERK1/2 signaling network which is implicated in excitotoxicity as well as downregulation of ion channels Citation[7]. (B) miR-29a is upregulated in SOD1G93A transgenic mice, a familial model of ALS. Amelioration of upregulation using anti-miR-29a resulted in alleviation of repression of target genes, and increased lifespan Citation[9]. (C) In a mouse model of Huntington’s disease, upregulation of miR-196a is protective against neurodegeneration and suppressed expression of HTT in the brain. Studies using patient-derived induced pluripotent stem cells overexpressing miR-196a also showed reduction of HTT levels as well as improvement in pathological aggregates Citation[14].
![Figure 2. miRNA implicated in neurodegenerative disease. (A) Use of an miR-128 mimic in Parkinson’s-like mice resulted in improved motor function and downregulation of genes involved in the ERK1/2 signaling network which is implicated in excitotoxicity as well as downregulation of ion channels Citation[7]. (B) miR-29a is upregulated in SOD1G93A transgenic mice, a familial model of ALS. Amelioration of upregulation using anti-miR-29a resulted in alleviation of repression of target genes, and increased lifespan Citation[9]. (C) In a mouse model of Huntington’s disease, upregulation of miR-196a is protective against neurodegeneration and suppressed expression of HTT in the brain. Studies using patient-derived induced pluripotent stem cells overexpressing miR-196a also showed reduction of HTT levels as well as improvement in pathological aggregates Citation[14].](/cms/asset/ee2524b0-bc73-4d0d-89ee-42647c9a3791/iedc_a_981254_f0002_oc.jpg)
Neurodegenerative diseases are etiologically diverse, manifesting a variety of clinical symptoms that depend on the particular subset of neurons affected. Diseases of this nature include Alzheimer’s disease (AD), Frontotemporal degeneration (FTD), Parkinson’s disease (PD), Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS) and prion disease. Nonetheless these diseases share common sub-cellular features, the most obvious of which is a strong association with the accumulation of misfolded, aggregated and insoluble forms of normal host proteins in the brains of patients. However, drugs designed to reduce the levels of these proteins in tissues have had little success to date in clinical trials. Current treatments for neurodegenerative diseases are limited to a small number of drugs that control some of the symptoms of early disease, but do not mitigate the damage to the neurons themselves. Remarkably, neurodegenerative diseases also appear to share the earliest detectable pathophysiological events of neurodegeneration, namely synaptic dysfunction and loss Citation[3]. Indeed, it was recently shown that this early damage is reversible in prion-infected mice by conditional knockdown of the normal cellular form of the prion protein (PrPC) Citation[4]. Undoubtedly, major advances in our understanding of the molecular underpinnings of disease pathogenesis are of paramount importance to identify the genes, proteins, pathways, and perhaps miRNAs, that should be the targets for the next wave of therapeutic development.
Global transcript screening has identified the widespread and profound dysregulation of specific miRNAs in numerous neurodegenerative diseases Citation[5,6]. As master regulators of cellular homeostasis, identifying the networks in which these disease-related miRNAs act will provide information on the pathways that are most crucial for neuronal function and survival. Second, identifying the proteins whose levels are modulated by these miRNAs may suggest new targets for drugs, either by manipulating miRNA levels themselves, or by the use of more conventional drugs such as receptor agonists/antagonists, kinase inhibitors, and so on. Third, disease-related miRNAs and their targets may be biomarkers for disease risk or prognosis.
2. MicroRNA regulators in neurodegenerative diseases
A survey of the literature reveals numerous miRNAs whose dysregulation is known to be associated with neurodegenerative diseases Citation[5,6]. Perhaps unsurprisingly these miRNAs have been mostly studied for their ability to alter the expression of proteins whose misfolding and accumulation is characteristic of particular neurodegenerative disease pathologies, such as beta-amyloid in AD, the mutant huntingtin protein in HD or proteawe resistant prion protein (PrPSc) in prion diseases. However, given that numerous drugs have already been identified for their ability to block the misfolding and aggregation of these proteins in cells, and all have proved to be unsuccessful in preventing clinical disease in animals, miRNAs that target the expression of this type of disease-associated protein may prove not to be therapeutic.
MiRNAs that target pathways that lead either to neuronal damage or to neuroprotection and survival are perhaps the most interesting in terms of understanding the progression of neurodegenerative disease. The identity of the targets of the vast majority of miRNAs dysregulated in disease, and their biological functions in degenerating brain tissue, has yet to be elucidated. Even fewer studies validate a pathophysiological role for specific miRNAs in animal models of neurodegenerative disease or human patients. Nonetheless several noteworthy miRNA contenders are currently under investigation as potential drug targets (). Notably, nearly all of these miRNAs are relatively abundant in healthy neurons. For example, in a recent study abnormal motor activities in a chemically induced PD model were found to be ameliorated by the overexpression of miR-128, an abundant neuron-enriched miRNA Citation[7]. Very recently, the level of miR-128 was shown to be decreased in the frontal cortex of transgenic HD non-human primates and correspondingly in striatum samples analyzed from both pre-symptomatic and symptomatic human patients Citation[8].
MiR-29a, a member of the miR-29 family, is another miRNA that has been implicated in multiple neurodegenerative diseases. In one study miR-29a was found to be specifically increased in the brain and spinal cord of SOD1 (G93A) transgenic mice, a model of familial ALS. Intracerebroventricular injection of an miRNA inhibitor specific to miR-29a did not bring about measurable changes in disease progression or motor function; however, mice did appear to have an increased lifespan Citation[9]. Interestingly, miR-29 is also upregulated in AD Citation[10] and prion disease Citation[11], downregulated in HD, and has shown to alter the expression of β-secretase, an enzyme involved in the generation of the amyloid-β peptide Citation[12] and progranulin, which is involved in FTD Citation[13]. In other studies, increased cellular levels of miR-196a have been reported to ameliorate neurodegenerative phenotypes of Huntington disease in a mouse model Citation[14]. Additionally, human-induced pluripotent stem cells derived from one individual with HD (HD-iPSCs) infected with a lentivirus expressing miR-196a had decreased levels of the huntingtin protein, huntingtin, as well as reduced pathological aggregates Citation[14]. In this case, the use of a double transgenic mouse overexpressing the miRNA of interest and a disease-related mutation, alongside the evidence of therapeutic modulation of the mutant HD-iPSCs, presents compelling evidence for the direct involvement of miR-196a in HD disease and advocates the potential benefits of an miRNA-based therapeutic.
3. Current progress in microRNA therapeutics
Given that the regulatory potential of miRNAs has only been recognized for a little over a decade, progress towards the developing the first miRNA-based drugs has been rapid. MiRNA mimics/mimetics, synthetic miRNAs or complementary molecules called anti-miRs that act as antisense inhibitors are used to modulate the endogenous levels of individual miRNAs. Whole families of miRNA can be inhibited using ‘miRNA sponges’ Citation[15] and ‘multiple-target anti-miRNAs’ Citation[16]. miRNA inhibitors have been the most extensively studied as potential drug candidates. This is understandable as they can be selectively designed to target a specific miRNA, thus lessening potential ‘off-target’ interactions. A number of innovative companies, most notably Santaris Pharma Citation[17], miRagen Therapeutics Citation[18], Mirna Therapeutics Citation[19] and Regulus Therapeutics Citation[20,] have trialed miRNA inhibitors that target infections, cardiovascular diseases, fibrosis and cancer amongst others. The first miRNA-targeted therapeutic to enter Phase II clinical trials is miravirsen, a locked nucleic acid (LNA) modified DNA phosphorothioate antisense oligonucleotide developed by Santaris Pharma that specifically binds to miR-122 and effectively decreases hepatitis C virus replication Citation[21]. Encouragingly, the miRNA inhibitors tested to date have shown high affinity, specificity, stability and pharmacokinetic properties in human and animal trials Citation[22].
The ability of a single miRNA to target multiple proteins, perhaps within the same regulatory network, is theoretically one of the biggest attractions for the therapeutic use of an miRNA mimic. It can be envisaged that this may well induce a profound physiological change in a cell through modulation of a normal cellular pathway. For example, miR-146a can simultaneously inhibit a number of key signaling intermediates in the toll-like receptor signaling pathway so that its overexpression can very effectively block the expression of inflammatory cytokines Citation[23]. One mimic in the first phase of clinical trials is miR-34a, a tumor suppressor miRNA that is downregulated in various types of cancer. Mirna Therapeutics has developed an oligonucleotide mimic of miR-34a that can be delivered to cancer patients as a replacement therapy to restore it to functional levels Citation[24]. Notably, miR-34a has been shown to inhibit proliferation of human lung cancers in mice, prostate cancer stem cells and metastasis Citation[25]. Nonetheless, as alluded to previously, the therapeutic use of an miRNA mimic has one obvious caveat; its intrinsic ability to bind multiple targets may induce unpredicted ‘off-target’ effects. These may be particularly apparent in unaffected tissues or those in which the miRNA is not normally expressed. Interestingly, a synthetic miRNA designed to knockdown PrPC was tested in vitro and surprisingly a scrambled sequence control miRNA was able to knockdown PrPC almost as well as the synthetic miRNA itself Citation[26]. A better understanding of how small RNAs interact with their targets within cells is obviously an area that requires investigation so that ‘off-target’ effects can be accurately predicted by computational analysis.
4. Challenges for the development of microRNA-based drugs for neurodegenerative diseases
Current understanding of the molecular basis of neurodegeneration and the roles that individual miRNAs play in disease onset and progression is sparse, thus precluding choosing miRNA-based drug candidates by prior knowledge. Determination of candidate miRNAs generally proceeds as follows. First, miRNAs that exhibit altered expression levels during disease are identified, followed by reproduction of these altered levels in a disease-related model in an attempt to determine their functions. This process is undoubtedly slow and laborious and there are a number of challenges, including a lack of information regarding temporal miRNA expression during the progression of neurodegenerative disease in patients, as well as tissue- and cell type-specific datasets. This has undoubtedly led to the numerous contradictory reports in the literature of the levels of specific miRNAs being increased, decreased or unchanged in a particular disease. Given the slow development of clinical signs in most neurodegenerative diseases, often spanning decades, and the difficulties in obtaining directly correlative tissue samples, this is not surprising. In our own studies we have found that the expression of specific miRNAs changes temporally throughout the preclinical and clinical stages of prion disease in isolated hippocampal cells with some miRNAs increasing in abundance early in disease that resume basal levels or are decreased later on in the disease course Citation[11]. In this respect greater screening of patients for conserved changes in miRNA expression during disease is essential both for the identification of biomarkers to improve diagnostic techniques as well as to identify miRNA of therapeutic interest Citation[27].
Once candidate miRNAs are identified it is important to determine both their biological function(s) and gene targets. Although targets and miRNAs interact by base-pairing, perfect complementarity is not required and the rules governing binding are complex. Current protocols require both bioinformatics as well as experimental validation using functional genomics and comparative quantitative analysis of transcripts and proteins. More frequently this is accompanied by immunoprecipitation of the miRISC complex from the biological samples of interest from which the mRNAs and miRNAs that are bound within can be sequenced. Developing innovative technologies to characterize miRNA targets and function, such as the use of morpholinos to downregulate targets in zebrafish, Citation[28], is very important.
Ultimately the development of high-throughput phenotypic screens is perhaps the most promising strategy for major advances in the microRNA-based drug discovery pipeline. These would require the use of a cell model system that recapitulates a disease-related phenotype so that multiple miRNA mimics or inhibitors can be rapidly assessed for bioactivity. However, the phenotype of choice for screens pertinent to neurodegeneration is particularly challenging as the driving causes behind disease progression are unknown, and neuronal cells are particularly difficult to culture and manipulate. To date, most drug screens pertaining to neurodegenerative disease have concentrated on the removal of the mutant or aggregated proteins that are the hallmark of many diseases; however none of these have translated to bioactivity in vivo or the clinic. Alternative phenotypic screens could be aimed at the determination of miRNAs that can promote neuronal survival or regeneration, or perhaps the modulation of extrinsic factors that may contribute to neuronal death such as neuroinflammation or oxidative damage. The loss of dendritic spine density and synapses is a proven early and consistent phenotype of degenerating neurons and the use of high-throughput image analysis to systematically explore miRNA function in this regard could speed up the drug discovery pipeline Citation[29,30].
Key to validating the efficacy of miRNA drugs is the availability of appropriate animal models. Unfortunately most models fall short in replicating one or another aspect of human disease. The recent development of pluripotent stem cells derived from patients is also a promising strategy for use in the drug discovery process and many such cell lines are currently under development, such as dopaminergic neurons as a model of neurodegeneration in PD Citation[31]. Similarly somatic cell reprogramming has been used to produce human-induced pluripotent stem cells (hiPSCs) from AD and FTD patient skin fibroblasts Citation[32,33]. A second alternative strategy to narrow down those miRNAs particularly important to the development of neurodegenerative diseases in patients is to map genetic risk in a population that is associated with single nucleotide polymorphisms (SNPs) in miRNA binding sites. Interestingly, in one of our own studies we found that SNPs in miRNA binding sites in the untranslated regions of messenger RNAs are enriched in genes whose variation in expression has already been associated with the development of neurodegenerative disorders Citation[34]. A number of studies have been performed in small groups of patients; however, in a number of cases follow-up studies have reported contradictory conclusions. For robust analysis, it is vital that the scale of current studies be increased from tens of patients, to tens of thousands of patients along the lines of those currently underway to map genetic risk in neurodegenerative disease based on SNPs in protein-coding genes.
A universal challenge for any drug aimed at targeting diseases of the CNS is delivery across the blood–brain barrier (BBB), an endothelial cell layer that isolates and protects the brain, in a pharmacologically active form. MiRNAs or their inhibitors, akin to silencing RNAs, face significant challenges for delivery due to their negative charge and ∼ 15 kDa size. In the past, modification of these molecules was approached through utilization of molecular mimetics, which increased stability but ultimately had no measurable effect on BBB penetration Citation[35]. Mechanisms to increase the permeability of the BBB can be used, such as using adenosine receptor nanoagonists or ultrasound, or stimulation of receptor-mediated endocytosis to promote drug uptake. Direct intracerebral/intracerebroventricular delivery of drugs and colloidal drug carriers are also strategies that are being tested. MiRNA-based drugs must also exhibit minimal off-target effects to ensure safety, be resistant to enzymatic degradation in biological fluids and tissues, and be able to cross cell membranes and enter the cytoplasm of degenerating neurons. LNA inhibitors, short oligonucleotides with a phosphorothioate-modified backbone, are the most utilised format for modulating miRNA function in live animals. MiRNA mimics must also be functionally active once inside the target cell by being correctly incorporated into the miRNA processing machinery (RISC complex). Some promising approaches for packaging and delivery of miRNA-based drugs include nanoparticles, polymers, exosomes and virus-based approaches and are summarized in . In addition, high-speed screening of drug-delivery vehicles, with applications to deliver miRNA, has been developed in zebrafish and yielded results directly translatable to rats Citation[36]. This technology has the potential to be utilized to identify drug-delivery vehicles that successfully penetrate the BBB Citation[36].
Table 1. Delivery methods for miRNA administration to the CNS.
Although alike in the difficulties of administration, miRNAs have a number of unique and attractive features over other nucleic acid-based drugs such as silencing RNAs. For example, they are endogenous to the cell and they are often expressed in a highly tissue specific way so that activity of inhibitors can be selectively targeted, or designed to be expressed in, diseased tissues. As miRNAs bind imperfectly to targets, they create shorter RNA duplexes when bound than do corresponding siRNAs or shRNAs, and thus are unique in functioning without inducing the innate immune antiviral response in the cell Citation[37]. Use of shRNA can also be cytotoxic due to their high expression overloading the RISC machinery and preventing the processing of endogenous miRNA Citation[38]. In addition, although shRNA are more potent it has been shown that miRNA can produce the same gene-silencing effect when expressed at high levels while not causing any toxicity Citation[38].
5. Conclusion
At this time, research into profiling changes in miRNA in diseased tissues, understanding function/targets of these molecules in healthy tissues, and determining whether pharmacological manipulation of levels of certain miRNA could be therapeutically beneficial are thriving areas of research. Extensive research is required to map miRNA interactions before therapies are produced, especially for the brain as neurodegenerative diseases are largely not well understood. This is not feasible with the current technology and high throughput procedures must be implemented. With that in mind, miRNA therapeutics for neurodegenerative diseases are a fertile ground for future research and represent a promising future for therapy.
6. Expert opinion
Transcriptional profiling and gain-and-loss of function studies have implicated miRNAs in numerous neurodegenerative diseases. The fact that many miRNAs are highly enriched in neurons, and intricately involved in cellular development and phenotypic maintenance, implies a potential for direct, or indirect, association with neuronal dysfunction in neurodegenerative disease. These observations are compelling indicators that miRNAs could be effective drug targets within the neurodegenerative cascade, regulating pathways crucial to neuronal function and survival. Thus numerous investigators have set out to understand the role of miRNAs in neurodegenerative diseases, a daunting task considering our limited understanding of exactly what it is that triggers the neurodegenerative cascade in most instances. Although neurodegenerative diseases are very diverse in origin and symptoms, many pathophysiological characteristics of the diseases are shared, such as reduced synaptic connectivity and neuronal loss, excitotoxicity and neuroinflammation. One possible inroad to speed up studies to identify bioactive miRNAs is to use these traits in phenotypic screens that can be performed at high throughput. Although these strategies are unlikely to cure the underlying cause of neurodegeneration, therapies designed to reprogram miRNA-regulated networks involved in these pathologies constitute an evidence-based strategy that theoretically has applications in numerous diseases. These therapies have the potential to relieve the devastating clinical stage of disease, delaying disease progression long enough for the generally aged sufferers of neurodegenerative diseases to maintain their quality of life. Drugs that have the ability to slow the progression of these processes, either by protecting neuronal functionality for longer, or alternatively promoting regeneration of new neurons that are able to functionally replace damaged cells, could have significant benefit to patients.
Perhaps most enticing to researchers and pharmaceutical companies pursuing this strategy is the ability of miRNAs to act as ‘master regulators’ of entire biochemical pathways and protein interaction networks in the maintenance of cellular homeostasis. Any given miRNA may have hundreds of unique targets so that altering the cellular level of a single miRNA has the potential to regulate multiple cellular pathways simultaneously. This property is typically considered a caveat of miRNA-based therapeutics, and rightly so, but this promiscuity in binding can also be advantageous as a means to modulate disease processes in their entirety and potentially reprogram a diseased cell to revert to its healthy status. This is particularly attractive in terms of multifactorial disorders, such as neurodegenerative disease, given the long and complex disease process.
Nonetheless, the challenges to the development of miRNA-based drugs for neurodegeneration are considerable. Not the least of which apply to all nucleic acid-based therapies, their biological stability, tissue-specific delivery, cellular uptake, potential off-target effects and safety. Other pertinent foci for research are the identification of tools and biomarkers with which to diagnose neurodegeneration early, improved animal models of neurodegenerative disease plus a better understanding of miRNA biogenesis and function, and most importantly is the development of phenotypic screening platforms to identify bioactive miRNA therapeutics as well as rigorous methodologies to identify miRNA targets and to explore their functionality. It is unlikely that we will see a single bullet drug for neurodegeneration, but miRNAs as homeostatic regulators of neuronal health and function, are not only promising targets for therapeutics but are undoubtedly powerful tools in a growing arsenal to uncover the cause of the neurodegenerative cascade as well as critical pathways, proteins and networks that will lead to targeted drug discovery.
It is becoming increasingly clear that the manipulation of human gene expression during disease directly using endogenous cellular agents such as miRNA is the way of the future for therapy development. There is no more direct way of ‘fixing’ a diseased cell than by manipulating it at the genomic level. Therefore miRNA therapeutics based on extensive research into target interactions specific to tissues and cells they encounter holds much promise as future therapies for neurodegenerative diseases.
Declaration of interest
The authors are supported by the Public Health Agency of Canada. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33
- Boudreau R, Jiang P, Gilmore et al. Transcriptome-wide discovery of microRNA binding sites in human brain. Neuron 2014;81(2):294-305
- Jeffrey M, Halliday WG, Bell J, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol 2000;26:41-54
- Mallucci GR, White MD, Farmer M, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 2007;53:325-35
- Maciotta S, Meregalli M, Torrente Y. The involvement of microRNAs in neurodegenerative diseases. Front. Cell. Neurosci 2013;7:265
- Majer A, Boese A, Booth SA. The role of microRNAs in neurodegenerative diseases: implications for early detection and treatment. In: Mallick B, Ghosh Z, editors. Regulatory RNAs. Springer-Verlag Berlin Heidelberg; Berlin Heidelberg USA; 2012. p. 443-73. doi:10.1007/978-3-642-22517-8_18
- Tan CL, Plotkin JL, Venø MT, et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 2013;342:1254-8
- Kocerha J, Prucha MS, Zhao D, Chan AWS. MicroRNA-128a dysregulation in transgenic Huntington’s disease monkeys. Mol Brain 2014;7:46
- Nolan K, Mitchem MR, Jimenez-Mateos EM, et al. Increased expression of microRNA-29a in ALS mice: functional analysis of its inhibition. J Mol Neurosci 2014;53:231-41
- Shioya M, Obayashi S, Tabunoki H, et al. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol Appl Neurobiol 2010;36(4):320-30
- Majer A, Medina SJ, Niu Y, et al. Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice. PLoS Pathog 2012;8(11):e1003002
- Hébert SS, Horré K, Nicolaï L, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA 2008;105(17):6415-20
- Jiao J, Herl LD, Farese RV, Gao FB. MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PLoS One 2010;5:e10551
- Cheng PH, Li CL, Chang YF, et al. miR-196a ameliorates phenotypes of Huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am J Hum Genet 2013;93:306-12
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: progress and possibilities. Nat Methods 2007;4(9):721-6
- Lu Y, Xiao J, Lin H, et al. A single anti-microRNA antisense oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers an improved approach for microRNA interference. Nucleic Acids Res 2009;37:e24
- Santaris pharma. Available from: www.santaris.com
- MiRagen therapeutics. Available from: http://miragentherapeutics.com/
- Mirna therapeutics. Available from: http://www.mirnarx.com/
- Regulus therapeutics. Available from: http://www.regulusrx.com/
- Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94
- Van Rooij E, Purcell AL, Levin A. Developing microRNA therapeutics. Circ Res 2012;110:496-507
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006;103:12481-610
- Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011;17:211-15
- Shi S, Han L, Gong T, et al. Systemic delivery of microRNA-34a for cancer stem cell therapy. Chem Int Ed Engl 2013;52:3901-5
- Kang S, Roh Y, Lau A, et al. Establishment and characterization of Prnp knockdown neuroblastoma cells using dual microRNA-mediated RNA interference. Prion 2011;5(2):93-102
- Lau P, Figerio CS, De Strooper B. Variance in the identification of microRNAs deregulated in Alzheimer’s disease and possible role of lincRNAs in the pathology: the need of larger datasets. Ageing Res Rev 2014;17:43-53
- Salta E, Lau P, Frigerio CS, et al. A Self-Organizing miR-132/Ctbp2 Circuit Regulates Bimodal Notch Signals and Glial Progenitor Fate Choice during Spinal Cord Maturation. Dev Cell 2014;30:1-14
- Ofengeim D, Shi P, Miao B, et al. Identification of small molecule inhibitors of neurite loss induced by Aβ peptide using high content screening. J Biol Chem 2012;287(12):8714-23
- Zhang M, Luo G, Zhou Y, et al. Phenotypic screens targeting neurodegenerative diseases. J Biomol Screen 2014;19(1):1-16
- Watmuff B, Hartley BJ, Hunt CP, et al. Pluripotent stem cell-derived dopaminergic neurons as models of neurodegeneration. Future Neurol 2013;8:649-61
- Doege CA, Abeliovich A. Dementia in a dish. Biol Psychiatry 2014;75:558-64
- Boxer AL, Gold M, Huey E, et al. Frontotemporal degeneration, the next therapeutic frontier: molecules and animal models for frontotemporal degeneration drug development. Alzheimers Dement 2013;9:176-88
- Saba R, Medina SJ, Booth SA. A functional SNP catalog of overlapping miRNA-binding sites in genes implicated in prion disease and other neurodegenerative disorders. Hum Mutat 2014;35(10):1233-48
- Boudreau RL, Rodriguez-Lebron E and Davidson BL. RNAi medicine for the brain: progresses and challenges. Hum Mol Genet 2011;20(1):21-7
- Chang T, Shi P, Steinmeyer JD, et al. Organ-targeted high-throughput in vivo biologics screen identifies materials for RNA delivery. Integr Biol 2014;6:926
- Kleinman ME, Yamada K, Takeda A, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 2008;452:591-8
- Boudreau RL, Martins I, Davidson BL. Artificial MicroRNAs as siRNA Shuttles: improved safety as compared to shrnas in vitro and in vivo. Mol Therapy 2009;17(1):169-75
- Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011;29(4):341-5
- O’Mahony AM, Godinho B MDC, Ogier J, et al. Click-modified cyclodextrins as nonviral vectors for neuronal siRNA delivery. ACS Chem Neurosci 2012;3:744-52
