Abstract
Introduction: Self-amplifying mRNA vaccines are being developed as a platform technology with potential to be used for a broad range of targets. The synthetic production methods for their manufacture, combined with the modern tools of bioinformatics and synthetic biology, enable these vaccines to be produced rapidly from an electronic gene sequence. Preclinical proof of concept has so far been achieved for influenza, respiratory syncytial virus, rabies, Ebola, cytomegalovirus, human immunodeficiency virus and malaria.
Areas covered: This editorial highlights the key milestones in the discovery and development of self-amplifying mRNA vaccines, and reviews how they might be used as a rapid response platform. The paper points out how future improvements in RNA vector design and non-viral delivery may lead to decreases in effective dose and increases in production capacity.
Expert opinion: The prospects for non-viral delivery of self-amplifying mRNA vaccines are very promising. Like other types of nucleic acid vaccines, these vaccines have the potential to draw on the positive attributes of live-attenuated vaccines while obviating many potential safety limitations. Hence, this approach could enable the concept of vaccines on demand as a rapid response to a real threat rather than the deployment of strategic stockpiles based on epidemiological predictions for possible threats.
Keywords:
1. Introduction
In 2010, President Obama called for the launch of a new initiative to develop our ability to respond faster and more effectively to bioterrorism and infectious disease threats at home and to strengthen public health abroad Citation[1]. In particular, the aim was to develop a nimble, flexible capacity to produce medical countermeasures rapidly in the face of any public health attack or threat, known or unknown Citation[2]. One potential solution would be to produce preventive or therapeutic vaccines ‘on demand,’ though sufficient pace and scale have not been feasible so far. Self-amplifying mRNA vaccines Citation[3,4], currently in development at Novartis Vaccines, are produced using a generic manufacturing platform utilizing completely synthetic methods and have potential to meet this aim.
Conventional egg and cell culture-based approaches for vaccine production are not suited to the rapid response requirements for effectively countering emerging pathogens and biodefense threats, as illustrated by the delayed response to the 2009 (H1N1) influenza pandemic. The virus first emerged in 2009 in Mexico and California, and subsequently quickly spread globally. It was antigenically distant from recently circulating seasonal influenza strains and on June 11 the World Health Organization announced community level outbreaks of the new virus in multiple regions of the world, indicating that an influenza pandemic was underway. Three months later, several manufacturers had completed vaccine development, received regulatory authorization, scaled up production and begun to supply vaccines Citation[5]. Despite the unprecedented speed of the response to this pandemic, substantial quantities of vaccine were only available in November 2009, well after the second pandemic wave had peaked Citation[6]. These events provided a strong impetus for the development of next-generation vaccine technologies more amenable to a rapid response. These include mammalian cell culture-based vaccines, novel influenza virus synthetic seed production techniques, VLP-based vaccines expressed from insect cells and plants, a recombinant subunit vaccine expressed from insect cells, a live-attenuated vaccine, mRNA and plasmid (pDNA) vaccines Citation[7]. Current Novartis Vaccine strategies in support of US and global efforts to facilitate a rapid response to newly emerging pandemic influenza threats involve: i) the use of licensed flu cell culture manufacturing technology to replace older manufacturing methods and increase surge capacity in a state-of-the-art, licensed facility; ii) the stockpiling of vaccines prior to an outbreak; and iii) constructing new vaccine variants with synthetic genes, as implemented for the H7N9 influenza response in 2013 Citation[8].
The recent H7N9 outbreak in China, though not yet designated as a pandemic because of the limited human-to-human spread, has raised considerable concerns due to its pathogenicity Citation[9]. Several of the new technologies listed above have been tested in clinical trials for immunological potency, and some have been added to the strategic national stockpile as a countermeasure against this virus should the situation evolve into a pandemic. In addition, the escalating Ebola crisis in West Africa has accentuated the need for medical countermeasures, such as vaccines to complement global health security activities focused on biosurveillance, and traditional public health strategies, such as containment through contact tracing. In this editorial, we propose an alternative strategy where vaccines could be produced ‘on demand’ when needed rather than stockpiled prior to a threat that may never occur.
2. The mRNA vaccine revolution
In 1990, Wolff et al. Citation[10] demonstrated that direct injection of mRNA or pDNA into skeletal muscle of a mouse resulted in expression of the encoded protein. However, the feasibility of developing a prophylactic mRNA vaccine was initially uncertain given concerns about RNA instability and large-scale manufacturing. Hence, DNA became the focus of nucleic acid vaccine research and development for the subsequent 20 years. However, due to technological advancements in RNA biology and chemistry, these issues are no longer perceived as barriers to the wide-spread implementation of the technology. The critical discoveries and events referenced in highlight the progress made in the field of RNA-based vaccines and on recent developments with self-amplifying mRNA vaccines. The discovery that lipid-based formulations could be used to deliver self-amplifying mRNA vaccines has the hallmarks of a disruptive innovation, as it brings a completely new approach to vaccine production utilizing a simple, synthetic, rapid, generic and cell-free process, and could enable many new products in the future Citation[11].
Table 1. Time-line of critical discoveries and developments that led to the recent progress on the non-viral delivery of self-amplifying mRNA vaccines.
3. Self-amplifying mRNA vaccines
This vaccine platform is based on a synthetic, self-amplifying mRNA Citation[12], delivered by a synthetic lipid nanoparticle (LNP) Citation[13] or cationic nanoemuslion Citation[14]. The mRNA can be produced in a few hours by a cell-free enzymatic transcription reaction, which avoids the use of cell culture, and enables simple downstream purification with very rapid and cost-effective manufacturing methods Citation[4]. The self-amplifying mRNA is based on an engineered alphavirus genome containing the genes encoding the alphavirus RNA replication machinery Citation[12], but lacking the genes encoding the viral structural proteins required to produce an infectious alphavirus particle. Hence, upon delivery of the RNA replicon, a transduced cell undergoes a single round of RNA replication and amplification of mRNA encoding the vaccine antigen but does not produce a new viral particle. Initial preclinical testing of self-amplifying mRNA vaccines has shown non-viral delivery to be capable of producing potent and robust innate and adaptive immune responses in various animal species, including non-human primates (NHPs) Citation[4,13-16]. If the encouraging preclinical data on the potency and safety of self-amplifying mRNA vaccines are matched in human trials, this platform could establish RNA vaccines as a versatile new tool for human immunization.
4. Proof of concept for a rapid response using self-amplifying mRNA vaccines
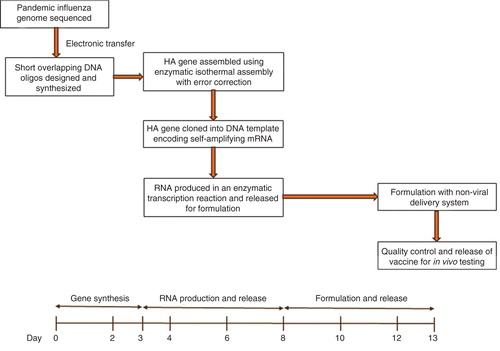
On 31 March 2013, the China Center for Disease Control and prevention (China CDC) announced a human influenza outbreak with an H7N9 avian influenza strain and posted hemagglutinin (HA) and neuraminidase gene coding sequences on the Global Initiative for Sharing All Influenza Data system. Cell-free gene synthesis and enzymatic error correction were used to make a synthetic HA gene based on this information Citation[15]. This gene was then cloned into the mRNA vaccine DNA template and, using an enzymatic in vitro transcription reaction, the self-amplifying mRNA was produced within 8 days following publication of the HA gene sequence. The RNA was then formulated with an LNP delivery system, mice were vaccinated and the vaccine was shown to be immunogenic at levels considered protective Citation[15]. highlights the steps and timeline involved in making the vaccine. While this was conducted as a research project at laboratory bench scale, it demonstrates the potential capability of this technology for rapid response purposes. Under ideal conditions of readiness, it is anticipated that this timeline could be reduced to 5 days.
5. Vaccines on demand: Attributes of self-amplifying mRNA vaccines
The platform has four attributes that make it an attractive rapid response platform for pandemic influenza and other newly emerging pathogens or biodefense threats:
Raw materials that can be stockpiled and production equipment that can be co-located within a single facility
A synthetic and scalable process that is amenable to automation and rapid manufacture of drug product, in the absence of biological systems
A robust, generic means to manufacture vaccines against many pathogen targets
A small manufacturing footprint with standard, disposable equipment.
Although the effective dose of self-amplifying RNA for humans remains to be determined, two recent studies in NHPs demonstrated that doses < 100 µg of RNA were immunogenic Citation[14,16]. Moreover, the magnitude of the immune responses elicited by the RNA vaccines was equivalent to or better than benchmark vaccines based on a viral vector or adjuvanted subunit protein previously shown to be immunogenic in humans. With further improvements in RNA vector design and non-viral delivery, effective immune responses in humans could be achieved with RNA doses in the range 1 – 10 μg. While this view is only speculative, it is supported by similar achievements in the siRNA field, where novel nucleic acid chemistry and delivery system optimization have resulted in the median effective dose for hepatic factor VII gene silencing after intravenous administration in mouse being reduced from 1 to 0.005 mg/kg (200 fold) Citation[17].
6. Conclusions
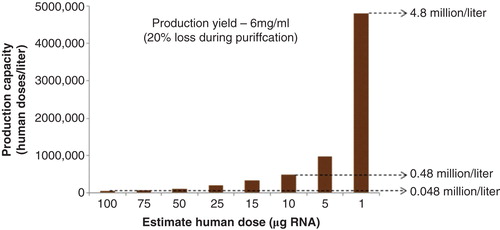
If this vaccine platform proves safe, potent and well-tolerated in humans, fully synthetic self-amplifying mRNA vaccines could provide unparalleled speed of response to influenza outbreaks, potentially generating vaccine candidates within days after the discovery of a new virus strain. Two key elements of a rapid vaccine response are speed and manufacturing capacity. The graph above () models the expected production capacity from an enzymatic in vitro transcription reaction, based on conservative assumptions of a 6 mg/ml yield of RNA Citation[18] and a 20% loss during purification. Depending on the effective human dose, the model predicts that up to millions of doses of vaccine could be produced from a 1L enzymatic transcription reaction in a few hours.
Figure 2. Projected production capacity from a 1-l transcription reaction (human doses/liter). The model assumes an RNA yield of 6 mg/ml, with a 20% loss during purification. A human dose from the current NHP data is predicted to be 100 µg RNA, but with additional improvements in vector design and delivery it could be reduced to between 1 and 10 µg.
In addition, because one only needs to know the sequence of the target antigen, this vaccine platform could also offer a potential solution for a wide range of additional pathogens, due to the versatility of the manufacturing processes and the broad spectrum of immune responses elicited by the vaccine.
7. Expert opinion
The prospects for self-amplifying mRNA vaccines are excellent and, like other types of nucleic acid vaccines, they have the potential to combine the positive attributes of live-attenuated vaccines while avoiding some of the inherent potential safety limitations. In addition, the synthetic, cell-free manufacturing approach to vaccine production might enable the concept of vaccines on demand to immediate threats rather than reliance of strategic stockpiles that may not match the outbreak and may need to be replenished over time. While data from preclinical testing are encouraging, self-amplifying mRNA vaccines are far from a commercial product. The processes and procedures to produce and characterize the vaccine for human trials need to be fully developed. Initially, large-scale production of mRNA was viewed as an insurmountable barrier to commercialization, but this perspective has changed and it is now viewed as one of the greatest assets of this vaccine technology. Development of non-amplifying mRNA-based immunotherapies and vaccines are now more advanced Citation[19,20]. Cost projections for these vaccines produced at an industrial scale are predicted to be competitive, even for the seasonal influenza market Citation[19], and extended stability in solution or after lyophilzation has already been demonstrated Citation[19,20]. The last decade has seen new regulatory initiatives such as Process Analytical Technology, Quality by Design and Real-time Release Citation[21]. If these practices become widely accepted for vaccine production and are applied to the production of self-amplifying mRNA vaccines, a new paradigm could immerge: ‘Vaccines on demand.’ In this scenario, a pathogen is isolated in the field, the genome is sequenced, the gene sequence for the antigen is electronically transferred to the production facility, and the vaccine is produced and released within weeks of detection. The main steps in a rapid vaccine response to a newly emerging pathogen are design, evaluation, production, release and clinical testing. For certain types of pathogens, design can be straightforward, such as for pandemic influenza and certain other viruses where the surface glycoproteins are known to be the main target of protective neutralizing antibodies. For other pathogens, bioinformatics and computational approaches may be necessary to rapidly design antigens as targets for protective T-cell responses Citation[22].
In our opinion, the future and continued success of the RNA vaccine technology lies in the optimization of vaccine potency. For example, based on animal testing, ∼ 1 × 104 to 1 × 105 times as much RNA by non-viral delivery is required to achieve equivalent potency compared to viral delivery Citation[3]. Therefore, if needed, there is much room for improvement to increase the efficiency of non-viral RNA delivery. Two key areas for enhancement can be found in engineering of the RNA replicon and formulation to facilitate delivery.
Declaration of interest
The authors are all employees of Novartis Vaccines Inc. A Geall is also the Principal Investigator on a Defense Advanced Research Project Agency agreement (HR0011-12-3-001), which partially funded the development of the self-amplifying mRNA vaccines at Novartis Vaccines Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
- Available from: http://www.whitehouse.gov/the-press-office/remarks-president-state-union-address
- Available from: http://www.phe.gov/Preparedness/mcm/enterprisereview/Pages/default.aspx
- Deering RP, Kommareddy S, Ulmer JB, et al. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin Drug Deliv 2014;11(6):885-99
- Geall AJ, Mandl CW, Ulmer JB. RNA: the new revolution in nucleic acid vaccines. Semin Immunol 2013;25(2):152-9
- Abelin A, Colegate T, Gardner S, et al. Lessons from pandemic influenza A(H1N1): the research-based vaccine industry’s perspective. Vaccine 2011;29(6):1135-8
- Rappuoli R, Dormitzer PR. Influenza: options to improve pandemic preparation. Science 2012;336(6088):1531-3
- Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother 2012;8(1):45-58
- Dormitzer PR, Suphaphiphat P, Gibson DG, et al. Synthetic generation of influenza vaccine viruses for rapid response to pandemics. Sci Transl Med 2013;5(185):185ra68
- Al-Tawfiq JA, Zumla A, Gautret P, et al. Surveillance for emerging respiratory viruses. Lancet Infect Dis 2014;14(10):992-1000
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science 1990;247(4949 Pt 1):1465-8
- Kaslow DC. A potential disruptive technology in vaccine development: gene-based vaccines and their application to infectious diseases. Trans R Soc Trop Med Hyg 2004;98(10):593-601
- Smerdou C, Liljestrom P. Non-viral amplification systems for gene transfer: vectors based on alphaviruses. Curr Opin Mol Ther 1999;1(2):244-51
- Geall AJ, Verma A, Otten GR, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA 2012;109(36):14604-9
- Brito LA, Chan M, Shaw CA, et al. A Cationic Nanoemulsion for the Delivery of Next-generation RNA Vaccines. Mol ther 2014;22(12):2118-29
- Hekele A, Bertholet S, Archer J, et al. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emer Microbes Infect 2013;2:8
- Bogers WM, Oostermeijer H, Mooij P, et al. Potent immune responses in rhesus macaques induced by non-viral delivery of a self-amplifying RNA vaccine expressing HIV-1 envelope with a cationic nanoemulsion. J Infect Dis 2014. [ Epub ahead of print]
- Jayaraman M, Ansell SM, Mui BL, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 2012;51(34):8529-33
- Pokrovskaya ID, Gurevich VV. In vitro transcription: preparative RNA yields in analytical scale reactions. Anal Biochem 1994;220(2):420-3
- Kallen KJ, Thess A. A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide-based vaccines and drugs. Ther Adv Vaccines 2014;2(1):10-31
- Sahin U, Kariko K, Tureci O. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov 2014;13(10):759-80
- Josefsberg JO, Buckland B. Vaccine process technology. Biotechnol Bioeng 2012;109(6):1443-60
- De Groot AS, Einck L, Moise L, et al. Making vaccines "on demand": a potential solution for emerging pathogens and biodefense? Hum Vaccin Immunother 2013;9(9):1877-84
- Martinon F, Krishnan S, Lenzen G, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol 1993;23(7):1719-22
- Zhou X, Berglund P, Rhodes G, et al. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 1994;12(16):1510-14
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009;8(2):129-38
- Hoerr I, Obst R, Rammensee HG, et al. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol 2000;30(1):1-7