Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of liver disease and nowadays it is recognized as one of main leading cause of liver fibrosis worldwide. Because of the high risk to develop cirrhosis and hepatocellular carcinoma, the early assessment of liver fibrosis is an important part of the management of NAFLD patients. To date, histological evaluation of liver biopsy represents the cornerstone for staging and grading liver fibrosis. However, due to the several drawbacks of this approach, during the last decade clinicians and researchers are dedicating their efforts to the identification of novel, safe and effective non-invasive tools to assess liver fibrosis. As due to their accuracy degree, transient elastography (TE) and serum biomarkers seem to be able to replace liver biopsy to determine at least the presence of significant liver fibrosis. The combination of these tools may greatly enhance their diagnostic power. Nevertheless, investigations of new imaging techniques and the molecular pathogenesis of NAFLD are necessary to develop reliable non-invasive alternative approaches to liver biopsy. Here, the authors discuss the salient aspects of diagnostic performance of TE and serum biomarkers available for detecting hepatic fibrosis in NAFLD and provide suggestions as to how these non-invasive techniques can be incorporated into diagnostic management of patients affected by this disease.
1. Two-step screening methods for liver fibrosis in NAFLD
Non-alcoholic fatty liver disease (NAFLD) has become the most frequent obesity-associated chronic liver disease in industrialized countries. NAFLD includes a constellation of liver disorders ranging from simple fatty liver to more severe non-alcoholic steatohepatitis (NASH). NASH is histologically characterized by the presence of steatosis, necro-inflammation, ballooning and possible centrilobular fibrosis with a high risk of progression to cirrhosis and hepatocellular carcinoma Citation[1]. As the NAFLD prevalence has risen substantially, this disease is now recognized as one of the leading causes of liver fibrosis worldwide Citation[2].
Histological assessment is crucial in the diagnosis and management of NAFLD, in fact, currently liver biopsy represents the ‘gold standard' to carefully evaluate and discriminate among the different histological features which characterize NAFLD. However, the use of liver biopsy as standard diagnostic technique in NAFLD patients may be limited by the occurrence of clinical complications that encompass pain and hypotension as most frequent effects, and intraperitoneal bleeding and biliary system injury as most serious consequences with a low, but not negligible, risk of morbidity and mortality. Furthermore, diagnostic power of liver biopsy may be conditioned by several drawbacks including high cost, sampling errors, lack of controls and criticisms in terms of reproducibility due to intra- and inter-observer discrepancies Citation[3]. Finally, histological evaluation of liver biopsy is static and it does not provide any information about the progression rate of fibrotic damage. For these reasons, the diagnostic utility of liver biopsy in NAFLD is becoming very questionable and nowadays the major efforts of clinicians and researchers are devoted to the identification of safe and effective non-invasive tools for the assessment of liver fibrosis.
The optimal characteristics for a non-invasive test should include broad availability and reproducibility, a very good cost/benefit ratio, ease of use and the capacity to assess changes in fibrosis over time. However, the rigorous validation versus liver biopsy remains a crucial step for evaluating the effectiveness of novel non-invasive diagnostic methods Citation[4]. The area under the receiver operator characteristic curve (AUROC) provides a measure of this efficiency and allows to determine both the sensitivity and the specificity of a test. Assuming for liver biopsy sensitivity and specificity > 90% and a prevalence of significant fibrosis approximately of 40%, an efficient non-invasive diagnostic tool would only reach an AUROC of 0.90 Citation[5]. Therefore, values of sensitivity and specificity near to 85% may be considered sufficient due to the lack of relevant clinical consequences resulting from false-positive and false-negative cases. A further advantage of a non-invasive test is that it can be repeated several times with no risk for the patient and combined with other non-invasive test becoming even more efficient.
Currently, two types of non-invasive diagnostic approaches match a large number of these requirements: transient elastography (TE) and serum biomarkers Citation[6,7].
In a recent review published in the present issue of this journal Citation[8], the authors provide an overview of these two approaches for the non-invasive assessment of liver fibrosis in NAFLD. Boursier et al. 8 carefully discussed characteristics, strengths and weaknesses of TE with respect to serum biomarkers.
TE is a novel ultrasound-based technique that permits to measure liver stiffness. The TE measurement is performed by the FibroScan device (EchoSens, Paris, France) that consists of an ultrasound transducer that transmits a vibration of mild amplitude and low frequency to the liver producing an elastic shear wave within the tissue Citation[9]. Then, a pulse-echo ultrasound acquisition system follows the share wave and computes its velocity, directly related to liver stiffness, in kilopascals (kPa). As shown by Boursier et al. Citation[8], FibroScan AUROC in NAFLD patients for diagnosis of significant fibrosis (F ≥ 2) ranges from 0.73 to 1.00 among the different studies with a best diagnostic cut-off 7.0 kPa. Despite a few drawbacks of TE by FibroScan, it is a rapid, non-invasive method that is highly reproducible, with a very low risk of sampling error that discriminates significant fibrosis from different etiologies.
Close to TE, magnetic resonance elastography (MRE) also represents a promising imaging technique for the assessment of NAFLD-related fibrosis. Even though the high cost remains a major limit of MRE, its strengths over TE are: the possibility of scanning whole liver avoiding sampling errors and to acquire contemporaneously conventional magnetic resonance imaging (MRI), and no effect of body mass Citation[10].
Figure 1. Integration of transient elastography (TE) and serum biomarkers in a diagnostic algorithm for diagnosis of non-alcoholic fatty liver disease (NAFLD)-related fibrosis.
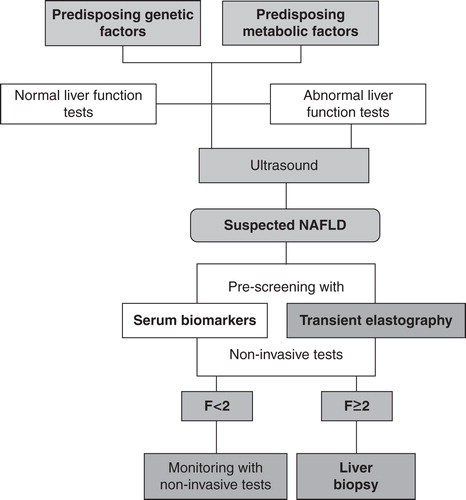
Recently, a novel imaging technique has been developed that combines conventional hepatic ultrasonography with evaluation liver stiffness in the region of interest (ROI): the acoustic radiation force impulse (ARFI) by Siemens (Acuson S2000 Virtual Touch Tissue QuantificationTM, Mountain View, CA; USA) Citation[11]. Short-duration acoustic pulses are generated close to the designated ROI resulting in shear waves away from the site of excitation. The speed of these waves (m/s) is then measured within ROI by ultrasound tracking beams. ARFI AUROC for the diagnosis of significant fibrosis in NAFLD is 0.90 and its comparison with FibroScan AUROC showed that both methods are similarly accurate for the widespread non-invasive diagnosis of liver fibrosis Citation[12].
Over the past two decades, a significant consideration has been directed to the identification of serum fibrosis biomarkers that may offer an attractive cost-effective option for diagnosis of fibrosis in NAFLD patients. Two classes of these markers are currently incorporated in the clinical routine of several analysis laboratories: indirect and direct biomarkers Citation[13]. Indirect biomarkers combine one or more serum parameters to generate composite scores by relatively simple or more complicated formulas. Boursier et al. Citation[8] reported some of the most used serum indirect biomarkers for NAFLD-related severe fibrosis: i) the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) which is calculated as (AST/upper limit of normal range)/platelet count (109/l) × 100; ii) the BARD score which is composed of three variables including AST/ALT ratio, presence of diabetes and body mass index (BMI); iii) the FibroTest that comprises α-2-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT); iv) the NAFLD fibrosis score which is generated by the analysis of six parameters including age, hyperglycemia, BMI, platelet count, albumin and AST/ALT ratio; v) the FIB-4 test which includes age, the platelet count, ALT and AST; vi); FibroTest/FibroSure includes γ-2-macroglobulin, γ-2-globulin, γ-globulin, apolipoprotein A1, GGT, total bilirubin; vii) and finally the FibroMeter that combines seven variables including age, weight, fasting glucose, AST, ALT, ferritin and the platelet count.
Direct biomarkers are drawn from the current knowledge of hepatic fibrogenesis. It is well known that fibrogenesis results from chronic liver injury in conjunction with the activation of different liver-resident cells, which trigger the excessive production/deposition of extracellular matrix (ECM) components, and the production/secretion of inflammatory molecules Citation[14]. During the dynamic process of fibrogenesis, a chronic liver injury promotes hepatic stellate cells (HSCs) activation into myofibroblast-like phenotype with contractile and pro-inflammatory capabilities, as well as a pro-fibrogenic potential Citation[15]. During the terminal phase of the fibrogenesis, activated-myofibroblastoid HSCs are stimulated to secrete more cytokines and other circulating factors, such as tissue inhibitor of metalloproteinases (TIMPs), which can sustain a continuous HSC activation and fibroblastoid differentiation. In this phase, matrix metalloproteinases (MMPs) and TIMPs regulate production/release of several ECM components including various types of collagens, proteoglycans, structural glycoproteins and hyaluronic acid (HA) Citation[15]. Therefore, several of these components, produced during ECM remodeling, have been used alone or in combination as direct fibrosis biomarkers in NAFLD. The most studied direct markers is HA that can be incorporated with age and other different direct markers (TIMP1, and aminoterminal peptide of procollagen III) to generate the ELF (European liver fibrosis), a test for liver fibrosis in NAFLD; or with age, platelet count, γ-2-macroglobulin, AST, prothrombin index and blood urea nitrogen to create FibroMeter.
As reported by Boursier et al. Citation[8], the AUROC of serum biomarkers for diagnosis of severe fibrosis in NAFLD is highly variable, ranging from 0.64 to 0.94, even thought the most high AUROC values have been reported for test integrating multiple indirect and direct markers (i.e., ELF and FibroMeter) Citation[16,17].
2. Expert opinion
Diagnosis of NAFLD-related fibrosis represents a crucial clinical issue to achieve successful management of patients. The identification of fibrosis patterns associated with this disease, suggests that up to now the histological liver biopsy remains the unique method to reach a final diagnosis. However, it is believed that the identification of non-invasive diagnostic approaches may represent a valid alternative. In fact, not only these non-invasive methods may provide an early, accurate and reproducible diagnostic tool to discriminate at-risk patients from those without significant fibrosis, but also an useful mean of monitoring the course of liver disease in response to therapy.
Several non-invasive diagnostic tools have been developed during the last decade, but to date only a few of these, such as TE and serum biomarkers, presents realistic advantages compared with liver biopsy. However, even though the accuracy of these non-invasive methods is near to 90% for diagnosis of severe fibrosis in NAFLD compared with liver biopsy, their complexity and limitations need further investigations both in terms of pathological/diagnostic significance and technological implementation. One of main future goals is to understand how to integrate these non-invasive methods in routine clinical practice. Combining TE and serum biomarkers in the management program of NAFLD patients could be a winning strategy to increase their diagnostic performance and limiting the possible drawbacks. In this scenario, TE and serum biomarkers may become two-step screening methods for diagnosing liver fibrosis before liver biopsy. Indeed, the authors propose a diagnostic flowchart in which these two non-invasive techniques could be integrated as pre-screening tools for reducing the number of patients who really need liver biopsy for staging and grading of fibrosis ().
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010;7:195-203
- De Minicis S, Svegliati-Baroni G. Fibrogenesis in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 2011;5:179-87
- Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut 2010;59:861-6
- Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol 2009;50:1-3
- Martinez SM, Crespo G, Navasa M, Noninvasive assessment of liver fibrosis. Hepatology 2011;53:325-35
- Baranova A, Lal P, Birerdinc A, Non-invasive markers for hepatic fibrosis. BMC Gastroenterol 2011;11:91
- Jaffer OS, Lung PFC, Bosanac D, Is ultrasound elastography of the liver ready to replace biopsy? A critical review of the current techniques. Ultrasound 2012;20:24-32
- Boursier J, Rousselet M-C, Aube C, Cales P. Liver fibrosis in patients with non-alcoholic fatty liver disease: diagnostic options in clinical practice. Expert Opin Med Diagn 2012;6(5):381-394
- Sandrin L, Fourquet B, Hasquenoph JM, Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705-13
- Myers RP. Noninvasive diagnosis of nonalcoholic fatty liver disease. Ann Hepatol 2009;8(Suppl 1):S25-33
- Palmeri ML, Wang MH, Dahl JJ, Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol 2008;34:546-58
- Friedrich-Rust M, Romen D, Vermehren J, Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol 2012;81:e325-31
- Schmeltzer PA, Talwalkar JA. Noninvasive tools to assess hepatic fibrosis: ready for prime time? Gastroenterol Clin North Am 2011;40:507-21
- Castera L. Assessing liver fibrosis. Expert Rev Gastroenterol Hepatol 2008;2:541-52
- Rombouts K, Marra F. Molecular mechanisms of hepatic fibrosis in non-alcoholic steatohepatitis. Dig Dis 2010;28:229-35
- Guha IN, Parkes J, Roderick P, Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008;47:455-60
- Cales P, Laine F, Boursier J, Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009;50:165-73