Abstract
Objective: To evaluate serum anti-C1q antibodies as a biomarker of systemic lupus erythematosus (SLE) flare and as a proposed noninvasive alternative to renal biopsy which is still the “gold standard” to determine renal activity in SLE.
Methods: Serum anti-C1q antibodies were measured in our patients (all were females), they were followed at the nephrology and pediatric nephrology units at the Faculties of Medicine of Cairo University and Misr University for science and technology (MUST). Our study included 120 patients in the pediatric and adolescent age group and they were categorized into three groups with (mean ± SD of 16.7 ± 3, 16.1 ± 2, 15.9 ± 3) respectively: Group 1 including 40 patients with SLE and active lupus nephritis; Group 2 including 40 patients with SLE and without active lupus nephritis, but with some extra renal activity mainly arthritis; and Group 3 including 40 healthy subjects.
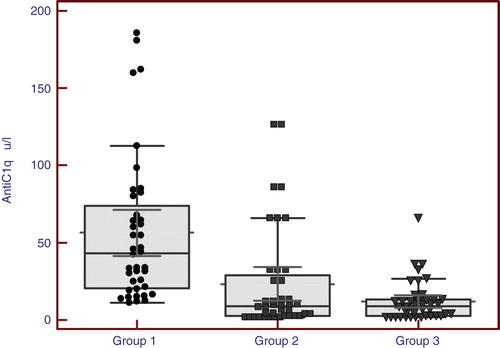
Results: Anti-C1q antibodies were found to be significantly higher in patients with active lupus nephritis than those without active nephritis than control individuals with a median (range) of [27.5 (14 – 83), 9 (2.5 – 30), 7 (2 – 13)] respectively. In those with active lupus nephritis, anti-C1q was found to correlate significantly with other parameters assessing lupus nephritis activity like C3 (r = -0.33, p < 0.04), C4 (r = -0.32, p < 0.044), daily urinary protein excretion (r = 0.32, p < 0.036), renal SLEDAI (r = 0.64, p < 0.001), and activity index (r = 0.71, p < 0.001).
Conclusions: Anti-C1q antibodies can be used as a considerable marker for LN activity in that age group with 97.5% sensitivity and 65% specificity with the cutoff level 12 U/l. These levels are clearly higher than those for traditional markers of disease activity such as C3/C4 consumption and anti-dsDNA.
1. Introduction
Despite aggressive immunosuppression, the ability to control severe systemic lupus erythematosus (SLE) glomerulonephritis (GN) remains unsatisfactory. Treatment with intravenous or oral cyclophosphamide followed by maintenance immunosuppression with azathioprine or mycophenolate mofetil (MMF) yields complete and partial remissions in less than 50% of patients after 6 months Citation[1]. Similar outcomes are seen when mycophenolate mofetil (MMF) is used for both induction and maintenance therapy Citation[2].
Because kidney biopsies are not repeated at every flare, a noninvasive predictor of renal pathology would be very useful in choosing therapy. This approach to treatment represents a fundamental change from a reactive to a proactive paradigm and will require biomarkers that accurately predict SLE nephritis activity, pathology, and prognosis to guide therapeutic decisions Citation[3].
A biomarker that can forecast lupus nephritis flare well before thresholds of proteinuria, renal function, and urine sediment that signal clinical flare are reached would be a valuable tool. Such a predictive biomarker could be followed serially, and based on biomarker levels, treatment could be initiated before the development of significant inflammatory injury in the kidney. This is likely to result in more complete remissions and less chronic kidney damage than waiting to treat until the flare is fully developed Citation[4].
Early treatment may also permit a decrease in the total exposure to immunosuppressive medications, thereby reducing toxicity Citation[5].
The traditional clinical biomarkers for SLE, including complement components 3 and 4 (C3, C4) and anti-double-stranded DNA antibodies (ADNA) have low sensitivity (49 – 79%) and specificity (51 – 74%) for concurrent renal flare and do not reliably predict impending flare when measured serially, with sensitivities and specificities around 50 and 70%, respectively Citation[6].
C1q is the first component of the classical pathway of complement activation and its main function is to clear immune complexes from tissues and self-antigens generated during apoptosis Citation[7]. Hereditary deficiency of this component is a known risk factor for the development of SLE Citation[8].
In 1984, antibodies directed to C1q (anti-C1q) were reported in the serum of patients with SLE, with a prevalence ranging from 34% to 47%, and in patients with hypocomplementemic urticarial vasculitis syndrome with a prevalence of 100% Citation[9].
Recently, it has been suggested that the presence of anti-C1q is a required condition for the development of lupus nephritis. Some investigators have also proposed that monitoring anti-C1q might be valuable for the clinical management of SLE patients as a noninvasive biological marker of renal disease Citation[10].
The complement system plays an important role in SLE pathogenesis because tissue damage is strongly mediated by immune complex deposition Citation[11]. Paradoxically, hereditary classical complement component deficiencies are frequently associated with SLE development as a result of an impairment in the clearance of apoptotic debris Citation[12]. Homozygous C1q deficiency is the strongest genetic risk factor related to SLE, and 93% of C1q-deficient patients develop SLE or lupus-like manifestations However, the majority of SLE patients frequently have secondary complement deficiency caused by the presence of anti-C1q antibodies Citation[13].
Of note, anti-C1q antibodies have been detected in up to 65% of adult SLE patients and associated with disease activity, especially with lupus nephritis Citation[14]. However, there are only two studies that evaluated anti-C1q antibodies in juvenile SLE (JSLE), and these studies had discordant results and did not have a comparative healthy control group. In 1997, Ravelli et al. Citation[15] found serum anti-C1q antibody in 17 of 29 (59%) JSLE patients and this was not associated with active nephritis. More recently, Kozyro et al. Citation[16] reported the presence of anti-C1q antibody in 7 of 12 (58%) lupus nephritis patients. Six of these seven patients were in renal flare.
1.1 Aim of the work
The aim of this study was to evaluate serum anti-C1q antibodies as a biomarker of SLE flare and as a proposed noninvasive alternative to renal biopsy which is still the “gold standard” to determine renal activity in SLE.
2. Subjects and methods
The present study was carried out in accordance with the ethical standards of the updated Declaration of Helsinki which was approved by the Medical Ethics Committee at the Faculties of Medicine of Cairo University and Misr University for Science and Technology (MUST) during the period from January 2011 till January 2012. An informed consent was taken from the parents of all subjects.
All patients (all females) were followed at the nephrology and pediatric nephrology units at the Faculties of Medicine of Cairo University and Misr University for Science and Technology (MUST). Our study included 120 patients in the pediatric and adolescent age group and they were categorized into three groups with mean ± SD of 16.7 ± 3, 16.1 ± 2, and 15.9 ± 3 respectively:
Group 1 including 40 patients with SLE and active lupus nephritis.
Group 2 including 40 patients with SLE and without active lupus nephritis, but with some extra renal activity mainly arthritis.
Group 3 including 40 healthy subjects.
All the patients were subject to thorough clinical examination including assessment of the vital signs (heart rate, blood pressure), body weight, lower limb edema, and signs of uremia.
SLE disease activity was measured in all patients, using the SLE disease activity index renal (SLEDAI) Citation[17]. Lupus activity was defined arbitrarily as renal SLEDAI > 4. The diagnosis of nephritis was established according to the presence of proteinuria > 0.5 g/24 h, presence of cellular casts, persistent hematuria > 10 red blood cells under high-power field, and renal failure. The mean renal SLEDAI for group 1 was 12 while the mean renal SLEDAI for group 2 was 4. The renal histopathology was described according to the World Health Organization (WHO) classification Citation[18].
The following laboratory investigations were done to the subjects of the study:
Complete blood count,
Erythrocyte sedimentation rate,
Serum creatinine, blood urea, serum sodium, and serum potassium
Serum C3, C4
Anti-dsDNA (Qualitative)
Urine analysis
Twenty-four hours urinary proteins
Serum anti-C1q by ELISA technique
Renal biopsy for all the 80 SLE patients in group 1 and group 2 (renal biopsy was performed in group 2 when they had active disease in the past). The renal histopathology in both groups ranged between class IV and class V (diffuse proliferative glomerulonephritis and membranous glomerulonephritis according to the WHO classification). The renal biopsy and blood sampling for detection of anti-C1q antibodies in group 1 patients were done simultaneously at the same time. Group 1 patients were on active treatment during this time also.
Anti-C1q antibodies were detected by ELISA (ORGENTEC Diagnostika GmbH, Mainz, Germany) with duplicated samples. Tests were performed strictly according to manufacturer protocols, including the suggested cutoff values of 20 U.
2.1 Sample collection and treatment
2.1.1 Specimen
Three milliliters of blood was drawn by venipuncture, left to clot and serum was separated by centrifugation at 2500 × g for 10 min. Hemolyzed and lipemic sera were avoided.
2.1.2 Specimen storage
Specimens were capped and stored at -20°C for few months until the time of the assay. Repeated thawing and freezing were avoided.
2.2 Immunoassay of the test
The Anti-C1q ELISA kit (ORGENTEC Diagnostika GmbH, Mainz, Germany) – Highly purified human C1q is bound to microwells. Antibodies against this antigen, if present in diluted serum, bind to the respective antigen. Washing of the microwells removes unspecific serum and plasma components. Horseradish peroxidase (HRP)-conjugated antihuman IgG immunologically detects the bound patient antibodies forming a conjugate/antibody/antigen complex. Washing of the microwells removes unbound conjugate. An enzyme substrate in the presence of bound conjugate hydrolyzes to form a blue color. The addition of an acid stops the reaction forming a yellow end-product. The intensity of this yellow color is measured photometrically at 450 nm. The amount of color is directly proportional to the concentration of IgG antibodies present in the original sample.
2.3 Statistical analysis of the data collected
Analysis of data was done by IBM computer using SPSS (statistical program for social science version 15.0) (SPSS, Inc., Chicago, IL, USA) for Windows®. as follows:
Description of quantitative variables as mean, SD, and range
Description of qualitative variables as number and percentage
Chi-square test was used to compare qualitative variables between groups. Fisher exact test was used instead of chi-square when one expected cell or more are less than 5. Unpaired t-test was used to compare two groups as regard quantitative variable. Paired t-test was used to compare quantitative variables in the same group.
One-way ANOVA test (analysis of variance) was used to compare more than two groups as regards quantitative variable. Kruskal–Wallis test was used instead of one-way ANOVA in nonparametric data. Spearman correlation test was used to rank different variables positively or inversely. ROC (receiver operator characteristic) curve was used to find out the best cut of value and validity of certain variable. p value > 0.05 was considered as insignificant, p < 0.05 significant, and p < 0.01 highly significant Citation[19].
3. Results
Serum anti-C1q antibodies were measured in our patients (all were females), they were followed at the nephrology and pediatric nephrology units at the Faculties of Medicine of Cairo University and Misr University for Science and Technology (MUST). Our study included 120 patients in the pediatric and adolescent age group and they were categorized into three groups with mean ± SD of 16.7 ± 3, 16.1 ± 2, and 15.9 ± 3 respectively: Groups 1, 2 and 3.
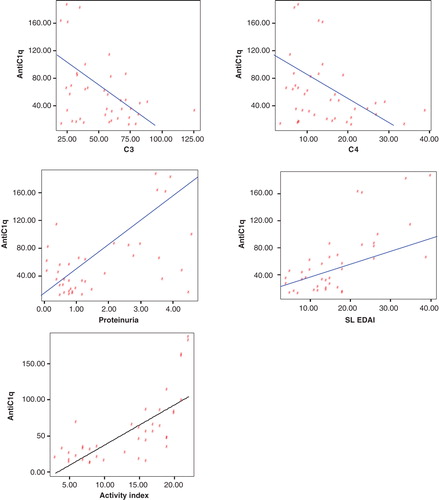
Anti-C1q antibodies were found to be significantly higher in patients with active lupus nephritis than those without active nephritis than control individuals with a median (range) of [27.5 (14 – 83), 9 (2.5 – 30), 7 (2 – 13)] respectively. In those with active lupus nephritis anti-C1q was found to be significantly correlating with other parameters assessing lupus nephritis activity like C3 (r = -0.33, p < 0.04), C4 (r = -0.32, p < 0.044), daily urinary protein excretion (r = 0.32, p < 0.036), renal SLEDAI (r = 0.64, p < 0.001), and activity index (r = 0.71, p < 0.001).
shows that there was no statistically significant difference between the studied groups with respect to age.
Table 1. Comparison between mean age of patients in all groups.
and show that anti-C1q antibodies were highest among cases compared to the other two groups with statistically significant difference in-between by using Kruskal–Wallis test.
Table 2. Comparison between the studied groups as regard laboratory data.
and show statistically significant positive correlations between anti-C1q antibodies versus 24 h proteins and renal SLEDAI and Activity index, while they show negative correlation between anti-C1q antibodies versus C3 and C4 by using Spearman correlation test. On the other hand, there is no significant correlation as regard other variables.
Table 3. Correlation between anti-C1q antibodies versus all variables among cases (group 1).
Figure 2. Correlation between anti-C1q antibodies versus C3, C4, Proteinuria, and renal SLEDAI and Activity index in cases (group 1).
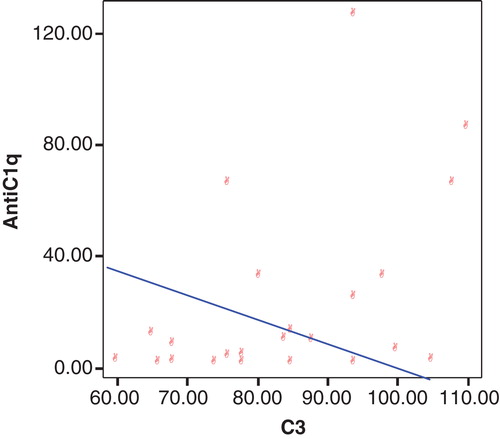
and show statistically significant negative correlation between anti-C1q antibodies versus C3 in group 2 by using Spearman correlation test. On the other hand, there is no significant correlation as regard other variables.
Table 4. Correlation between anti-C1q antibodies versus all variables among group without active lupus nephritis (group 2).
Figure 3. Correlation between anti-C1q antibodies and C3 among group without active lupus nephritis (group 2).
shows no statistically significant correlation between anti-C1q antibodies versus different variables by using Spearman correlation test.
Table 5. Correlation between anti-C1q antibodies versus all variables among controls (group 3).
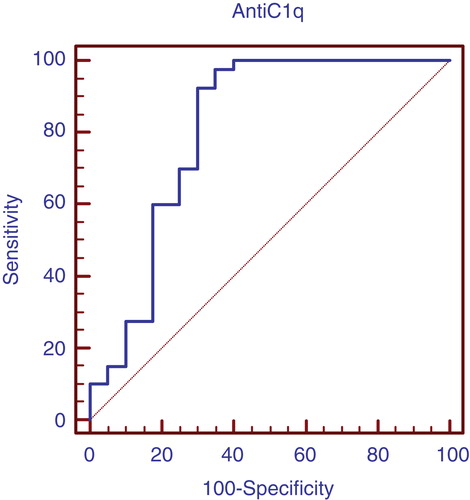
and show that anti-C1q antibodies is considered better positive marker than negative in case of lupus nephritis with higher sensitivity.
Table 6. Validity of anti-C1q antibodies in prediction of lupus nephritis in group 1 patients.
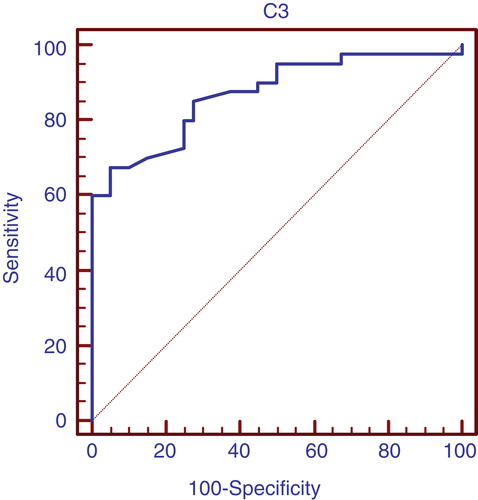
and show that C3 is considered better positive marker than negative in case of lupus nephritis with high sensitivity.
Table 7. Validity of C3 in prediction of lupus nephritis in group 1 patients.
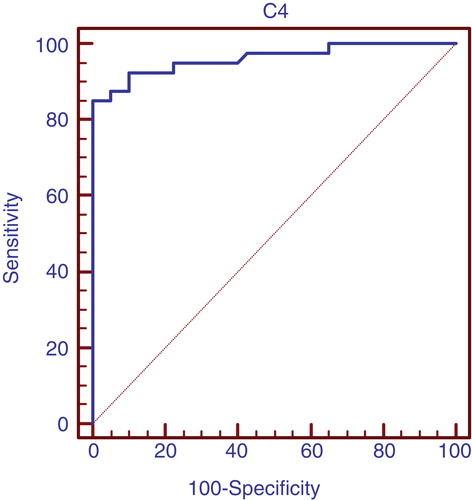
and show C4 is considered better positive marker than negative in case of lupus nephritis with high sensitivity.
Table 8. Validity of C4 in prediction of lupus nephritis in group 1 patients.
4. Discussion
Systemic lupus erythematosus is a systemic autoimmune disease characterized immunologically by a variety of autoantibodies, B-cell hyperactivity, and immune complex (IC) formation Citation[20]. Complement, especially C1q, the first component of the classical pathway of complement, is considered to be involved in the pathogenesis of SLE. This is based on the following: First, almost all patients with C1q deficiency develop a lupus-like syndrome, with homozygous C1q deficiency being the strongest disease susceptibility gene for the development of SLE Citation[21]. Second, a substantial number of patients with SLE develop hypocomplementemia with depletion of C1q and other components of the classical pathway of complement Citation[22]; and C1q is deposited in affected tissues Citation[23].
Glomerulonephritis is a frequent and often severe feature of SLE and is one of the major determinants of poor outcome. Reliable markers for diagnosing and monitoring lupus nephritis are therefore critically important.
The association of anti-Clq antibodies with lupus nephritis has been demonstrated by the discovery of anti-Clq antibodies in lupus nephritis kidneys Citation[24].
The clinical relevance of anti-Clq antibodies in adult patients with active SLE nephritis was raised in several clinical studies Citation[25-28,28-31], however a lower frequency of anti-C1q antibodies with high specificity was found in patients with juvenile onset SLE, in the study done by Jesus et al. Citation[32], but remarkably in this study, there was no definite segregation in number between the groups of juvenile SLE patients with and without lupus nephritis activity, the authors did not clarify how many patients had nephritis activity and how many were without at the time of sampling, and also the patients and controls were not equal in number.
The current cross-sectional study was designed to evaluate the diagnostic performance of anti-C1q antibodies in a cohort of pediatric and adolescent SLE patients with and without LN, and to correlate findings with other disease variables, and standard laboratory investigations used to assess renal function, SLE nephritis and activity indices.
In our study, anti-C1q antibodies were found to be significantly higher in patients with active lupus nephritis than those without active nephritis than control individuals with a median (range) of [27.5 (14 – 83), 9 (2.5 – 30), 7 (2 – 13)] (, ) respectively. In those with active lupus nephritis anti-C1q antibodies were found to be significantly correlating with other parameters assessing lupus nephritis activity like C3 (r = -0.33, p < 0.04), C4 (r = -0.32, p < 0.044), daily urinary protein excretion (r = 0.33, p < 0.036), and renal SLEDAI (r = 0.65, p < 0.001), and activity index (r = 0.71, p < 0.001). That refers to anti-C1q as a considerable marker for LN activity in that age group with 97.5% sensitivity and 65% specificity with the cutoff level 12 U/l and positive predictive value of 74% and negative predictive value of 96% (, ). These levels are clearly higher than those for traditional markers of disease activity such as C3/C4 consumption and anti-dsDNA. When we compared the validity of anti-C1q antibodies in prediction of lupus nephritis in group 1 patients, with C3 and C4, anti-C1q antibodies had the highest sensitivity with 97.5% and standard error of prediction of 0.052 (, ), compared to C3 with sensitivity of 67.5%, standard error of prediction of 0.041 (, ) and C4 with sensitivity of 85%, standard error of prediction of 0.021 (, ).
These results support the study done by Moroni et al., which showed 87% sensitivity and 92% specificity for anti-C1q in predicting SLE nephritis activity Citation[27]. It also agrees with the study by Sinico et al. which showed a strong association of anti-C1q with active SLE nephritis. Anti-C1q in the latter study had a better predictive value for active nephritis than other parameters such as C3/C4 consumption and anti-dsDNA Citation[31].
The wide variation in clinical presentation of SLE in pediatric and adolescent patients, together with the myriad of associated autoantibodies, calls for a trend toward determining “organ-specific” autoantibodies for diagnosis, prognosis and possibly specific therapeutic intervention Citation[33]. Albeit unlikely that anti-C1q as a noninvasive biomarker can replace gold standard renal biopsies, it may help to avoid unnecessary invasive biopsies in this particular age group, especially in the context of treatment follow-up. The relatively high sensitivity shown in our study renders anti-C1q antibodies an optimal screening tool for SLE active nephritis. The importance of avoiding potentially toxic drugs with special concern for future fertility in adolescents cannot be overemphasized Citation[34].
In view of relatively limited number of involved patients, further evaluation of such findings in large cohorts of SLE patients with different disease severity is recommended to validate the clinical value of anti-C1q in diagnosis and prognosis of renal disease in SLE. We also recommend in further similar bigger studies to involve study of correlation between anti-Clq antibodies level and anti-dsDNA titer as we measured the anti-dsDNA in qualitative method only. Observations done in our study, in addition to previous studies in different age groups, give cumulative evidence for the important role of anti-C1q in pathogenesis of active SLE nephritis. This in turn opens the way to advancing specific therapeutic targets in C1q-mediated renal injury in SLE.
5. Conclusion
Anti-Clq autoantibodies correlate with renal disease activity and with renal flare-ups like other standard parameters, such as proteinuria and complement levels and renal SLEDAI. Anti-C1q antibodies can be considered a reliable sensitive and specific biomarker to diagnose nephritis flare in pediatric and adolescent Egyptian females with SLE, in addition to, and possibly replacing, other confirmed disease activity indices.
Article highlights.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
This box summarizes key points contained in the article.
Bibliography
- Appel GB, Contreras G, Dooley MA, Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20(5):1103-12
- Bao H, Liu ZH, Xie HL, Successful treatment of class V + IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008;19(10):2001-10
- Bowness P, Davies KA, Norsworthy PJ, Athanassiou P. Hereditary C1q deficiency and systemic lupus erythematosus. Q J Med 1994;87:455-64
- Rovin BH, Zhang X. Biomarkers for lupus nephritis: the quest continues. Clin J Am Soc Nephrol 2009;4:1858-65
- Fiehn C, Hajjar Y, Mueller K. Improved outcome of lupus nephritis during the past decade: importance of diagnosis and treatment. Ann Rheum Dis 2003;62:439
- Gunnarsson I, Ronnelid J, Huang YH, Association between ongoing anti-C1q antibody production in peripheral blood and proliferative nephritis in patients with active systemic lupus erythematosus. Br J Rheumatol 1997;36:32-7
- Houssiau FA, Vasconcelos C, D'Cruz D, Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum 2004;50(12):3940
- Moroni G, Radice A, Giammarresi G, Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009;68(2):234-7
- Siegert CE, Kazatchkine MD, Sjoholm A, Wurzner R. Autoantibodies against C1q: view on clinical relevance and pathogenic role. Clin Exp Immunol 1999;116:4-8
- Walport MJ. Complement. Part 1. N Engl J Med 2001;344:1058-66
- Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 2007;40:560-6
- Janko C, Schorn C, Grossmayer G, Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE). Autoimmun Rev 2008;8:12
- Trendelenburg M, Lopez-Trascasa M, Potlukova E, High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol Dial Transplant 2006;21:3115-21
- Shoenfeld Y, Szyper-Kravitz M, Witte T, Autoantibodies against protective molecules- C1q, C-reactive protein, serum amyloid P, mannose- binding lectin, and apolipoprotein A1: prevalence in systemic lupus erythematosus. Ann NY Acad Sci 2007;1108:227-39
- Ravelli A, Wisnieski JJ, Ramenghi B, IgG autoantibodies to complement C1q in pediatric-onset SLE. Clin Exp Rheumatol 1997;15:215-19
- Kozyro I, Perahud I, Sadallah S, Clinical value of autoantibodies against C1q in children with glomerulonephritis. Pediatrics 2006;117:1663-8
- Brunner HI, Silverman ED, To T, Sensitivity of the systemic lupus erhytematosus disease activity index, British Isles Lupus Assessement Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood- onset systemic lupus erhytematosus. Arthritis Rheum 1999;42:1360
- Niaudet P. Treatment of lupus nephritis in children. Pediatr Nephrol 2000;14:158-66
- Inflinger JA, Mosteller FM, Thibodeau LA, Ware JH. Biostatistics in clinical medicine. MacGraw-Hill, Inc; New York: 1994
- Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 2001;2:764-6
- Barilla-LaBarca ML, Atkinson JP. Rheumatic syndromes associated with complement deficiency. Curr Opin Rheumatol 2003;15:55-60
- Agnello V. Association of systemic lupus erythematosus and SLE-like syndromes with hereditary and acquired complement deficiency states. Arthritis Rheum 1978;21:S146-52
- Jennette JC, Hipp CG. Immunohistopathologic evaluation of C1q in 800 renal biopsy specimens. Am J Clin Pathol 1985;83:415-20
- Mannik M, Merrjll CE, Stamps LD, Multiple autoantibodies form the glomerular immune deposits in patients with SLE. J Rheum 2003;30:1495-504
- Moroni G, Tredelenburg M, Del Papa N, Anti-C1q antibodies may help diagnosing a renal flare in lupus nephritis. Am J Kidney Dis 2001;37:490-8
- Meyer OC, Nicaise RP, Cadoudal N, Anti−C1q antibodies antedate active glomerulonephritis in patients with SLE. Arthritis Res Ther 2009;11:R87
- Moroni G, Radice A, Giammarresi G, Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009;68:234-7
- Trendelenburg M, Lopez-Trascasa M, Potlukova E, High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol Dial Transplant 2006;21:3115-21
- Mok CC, Ho LY, Leung HW, Wong LG. Performance of anti-C1q, antinucleosome, and and anti-dsDNA antibodies for detecting concurrent disease activity of systemic lupus erythematosus. Transl Res 2010;156:320-5
- Bigler C, Hopfer H, Danner D, Anti-C1q autoantibodies do not correlate with the occurrence or severity of experimental lupus nephritis. Nephrol Dial Transplant 2011;26:1220-8
- Sinico RA, Rimoldi L, Radice A, Anti-C1q autoantibodies in lupus nephritis. Ann NY Acad Sci 2009;1173:47-51
- Jesus A, Silva CA, Carneiro-Sampaio M, Anti-C1q antibodies in juvenile-onset systemic lupus erythematosus. Contemporary challenges in autoimmunity. Ann NY Acad Sci 2009;1173:235-8
- Ho A, Barr SG, Magder LS, Petri M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum 2001;44(10):2350-7
- Ricker DM, Hebert LA, Rohde R, Serum C3 levels are diagnostically more sensitive and specific for systemic lupus erythematosus activity than are serum C4 levels. The Lupus Nephritis Collaborative Study Group. Am J Kidney Dis 1991;18(6):686-8