Abstract
In June 2013, US FDA finalized changes to the Orphan Drug Regulation, clarifying the potential of using a single drug for multiple indications that would cause the total patient population to be > 200,000, but maintaining orphan drug status for a ‘subset’ of a disease where each patient population is < 200,000. In this editorial, an example is presented where drug candidates targeting biological pathways with the potential for multiple indications could capitalize on orphan indications, while maintaining a broader development strategy. The mammalian target of rapamycin pathway is highlighted with potential orphan indications in ocular diseases, tuberous sclerosis complex-related diseases and rare cancers.
1. Existing orphan drug business models
Pariser et al. Citation[1] recently reviewed the expedited development programs supported by US FDA, which included fast-track designations for drugs designed to treat a serious disease and fill an unmet medical need. As part of these programs, the designation of orphan drug status, available since 1983 when the Orphan Drug Act was passed, pertains to any disease affecting < 200,000 individuals in any given year Citation[2]. The European Union (EU) passed similar legislation for ‘orphan medicinal products’ which also covers certain tropical diseases; orphan drug legislation also exists in other countries, including Japan and Australia. A common application but separate approval process has been established between the USA and EU as an added incentive for companies to move orphan drug pursuit into the mainstream of drug development strategies, possibly offering substantial bottom-line profitability. This increased value would be based on the availability of a competition-free market space allowing more freedom in product pricing, focused marketing with smaller budgets, smaller clinical trials and fast-track approvals.
However, over the past several years, questions of strategy have arisen within the drug development community on how best to pursue these rare disease indications. The typical business model involves the use of an existing approved drug commonly referred to as ‘repurposing’. This strategy allows direct entry into clinical trials for rare diseases, but also presents the conundrum of potentially having to create new dosage forms, possibly establishing new dosing regimens for optimal treatment of a new disease and the consideration of different pricing for a new indication. It also places the orphan disease on the ‘back side’ of the development process which is a disadvantage to patients and orphan disease advocacy groups hoping for acceleration on the emergence of new treatments. Placing the orphan drug indication on the ‘front side’ of the development process, or the first indication for approval, presents a theoretical business model problem for large companies, because of the standard use of profitability calculations for portfolio analysis that sets the first-approved indication as the rationale for all future revenue projection. Typically, this presents a negative bottom-line value based on real and projected costs versus projected revenues from an initial rare disease approval. Because of these issues, this ‘front side’ business model is usually pursued by smaller biopharmaceutical companies where a blockbuster market prediction model is less influential in driving portfolio decisions. For either type of business venture, the definition of an ‘orphan subset’ has caused concern from both sides of the regulatory process. For instance, can a drug given to a subset of patient population of < 200,000 in an existing disease be given orphan drug status thereby offering incentives to developers, even though the drug is (or could be) used to treat multiple indications with potentially large patient populations? In June 2013, the US FDA clarified this when it issued final changes to the Orphan Drug Act and these were published in the Federal Register Citation[2]. Explaining such changes with examples, the clarification states that, if the subset of a disease or disease-related conditions is distinct, then it qualifies – the example was pneumonia in cystic fibrosis as orphan without having to consider the larger population of community-acquired pneumonia. It was also stated that some new dosage forms may prove to be clinically superior to previously approved dosage forms and, therefore, potentially eligible for orphan exclusivity.
2. New business models
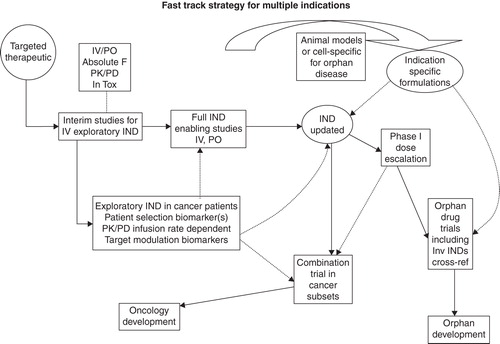
These clarifications give rise to several potential new business models for developing orphan drugs by theoretically moving the orphan disease process to the ‘front side’ of the development process to attain the first-approved indication. One hypothetical model which is referred to in this editorial as ‘[f]ast-tracking an orphan drug indication within a broader development project’ involves targeting certain biological pathways with the potential for multiple indications in a new drug candidate as shown in . The broader indication is designated as Oncology Development and the IND works like a ‘Master File’ by updating new data both non-clinically and clinically. This ‘Master File’ IND is available for cross-referencing and, therefore, can be used in initiating exploratory, investigator-sponsored and multiple indication trials. As shown in the figure, the ideal approach is a pharmacokinetic/pharmacodynamic (PK/PD), biomarker-driven process which could be used in a translational science approach to support multiple indications. If one were to choose an ideal target, it would be a multifunction biological target/pathway with prior experience showing that target modulation does yield a desired biological effect. Ideally, there would be known biomarkers to quantify target modulation and/or biomarkers useful for patient selection. These points would present an opportunity for proof-of-concept clinical trials in different indications for accelerated approval. In the ideal situation, there would be patient samples available corresponding to the desired patient populations for proof-of-concept research for new drug candidates. The ideal drug candidate molecule would provide the opportunity for multiple formulations for different systemic and local delivery, which would give flexibility needed for specific indications. A typical starting point in this approach would be to access a list of potentially qualifying diseases from the National Center for Advancing Translational Sciences, Office of Rare Disease Research Citation[3]. As an example, in this database, there are lists of 501 rare cancers, 102 rare lung diseases and 481 rare eye diseases. Diseases and gene-related targets can be connected through GeneCards (The Human Gene Compendium) Citation[4] and MalaCards (The Human Malady Compendium) Citation[5].
Figure 1. IND strategy for multiple indications is shown. A full IND that supports i.v. and p.o. studies acts as an overall Master File. An exploratory i.v. IND with PK/PD and biomarkers provides early proof-of-concept target modulation in patients. The Master File IND supports orphan drug trials including investigator-sponsored INDs (Inv IND).
3. Multiple development approach with mammalian target of rapamycin example
The mammalian target of rapamycin (mTOR) pathway has been selected as the potential target for this discussion Citation[6], as mTOR plays a well-known major role in cell growth and proliferation, protein synthesis and autophagy, and the aberrant regulation of mTOR has been implicated in several diseases Citation[7]. Accordingly, there is precedence for target modulation in cancer Citation[8,9], inflammatory disorders such as transplant rejection Citation[10], obesity and diabetes Citation[6], eye diseases Citation[11,12], neurodegenerative disorders and cognitive dysfunction Citation[6,10]. mTOR signals through two functionally distinct macromolecular complexes, mTORC1 (raptor) complex and mTORC2 (rictor complex) Citation[13]. The mTORC1 complex (rapamycin-dependent) is negatively regulated by the tuberous sclerosis complex 1 (TSC1)/TSC2 tumor suppressor complex and mutations in these two tumor suppressor genes are associated with TSC, an autosomal-dominant disease Citation[10]. This biological phenomenon makes rare diseases associated with TSC potential orphan indications that would be potential targets for mTOR modulation Citation[13]. Several mTOR inhibitors have been approved and additional candidates are currently in pipelines, which also include modulators of targets both upstream and downstream of mTOR. Examples of approved drugs include sirolimus for immunosuppression; everolimus for neuroendocrine tumors of pancreatic origin, renal cell carcinoma, renal angiomyolipoma and TSC and temsirolimus for renal cell carcinoma. All three drugs are substrates of cytochrome P450 3A4 (Cyp 3A4) and, therefore, subject to drug–drug interactions when used in combination therapy or by patients taking certain medicines unrelated to the treatment. In addition, sirolimus is an inhibitor of Cyp 3A4 as is temsirolimus since it is metabolized to sirolimus. Everolimus is an inhibitor of mTORC1 but not mTORC2 Citation[14]. When considering this hypothetical opportunity, the ideal candidate for this example would be a dual inhibitor of mTOR (both mTORC1 and 2) which may suppress feedback activation of Akt and would be designed away from Cyp 3A4 to eliminate major drug–drug interactions. Another advantage of the mTOR pathway in this example is that biomarkers of target modulation have been established, which include ribosomal protein S6 kinase (S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), and phosphatase and tensin homolg (PTEN) tumor suppressor deletions have been used as patient selection criteria Citation[6]. Other biomarkers that confirm a positive clinical outcome, depending on the disease being treated, have been employed in clinical trials and correlated with PK/PD to the biomarkers of target modulation. Ocular diseases potentially responding to mTOR inhibition offer an interesting opportunity. For treatments of potential eye diseases, including orphan drug qualifying conditions, ideally the drug candidate could be formulated for both intravitreal injections as well as for topical application. Broad diseases could include age-related macular degeneration, diabetic retinopathy, proliferative vitreoretinopathy, dry eye syndrome and uveitis Citation[11,12]. Potential orphan indications might include chronic noninfectious posterior uveitis. Therefore, a broad strategy might include an accelerated approval strategy for proliferative vitreoretinopathy and an orphan drug strategy for posterior uveitis which would fulfill an unmet need for steroid-sparing locally administered therapies. It is also possible that these indications could be associated or piggy-backed with a broad oncology indication through different formulations and established clinical end points based on known target modulation criteria.
Several cancers have been treated with mTOR inhibitors Citation[6] and as such for this example, oncology development has been selected as the broad target with subsets of rare cancers potentially responsive to mTOR inhibition as the orphan indications Citation[9]. For example, signaling through the epidermal growth factor receptor family is an important stimulator for breast cancer growth and downstream signaling is transduced via two main pathways: PI3K/Akt/mTOR and RAS-Raf-MAPK. Recent evidence indicates that stimulation (hyperactivation) of the PI3K/Akt/mTOR pathway may play a role in resisting current endocrine and HER2-targeted therapies Citation[7,15]. Breast cancer is then highlighted in as a subset disease with a limited population (resistance to certain therapeutics) within a broader oncology development project to stimulate accelerated approval. In addition, inflammatory breast cancer would qualify as a rare disease as would Hodgkin's lymphoma. Mark et al. studied mTOR activity with immunohistochemistry on tissue biopsy microarrays where an association with mTOR activity was correlated with clinical outcome. The results suggest that Hodgkin's lymphoma could be a rare cancer possibly susceptible to mTOR inhibition Citation[16]. This could be coupled with other orphan qualifying diseases, including ocular or lymphangioleiomyomatosis (LAM) Citation[13].
Figure 2. Specific example of multiple indication strategy is shown. An investigative mTOR inhibitor is highlighted with both patient selection and PK/PD biomarkers. The Master File IND supports trials in oncology subsets and ocular and/or LAM disease with the aid of specific animal models and patient cells. Oncology subsets include rare breast cancers and Hodgkin's lymphoma.
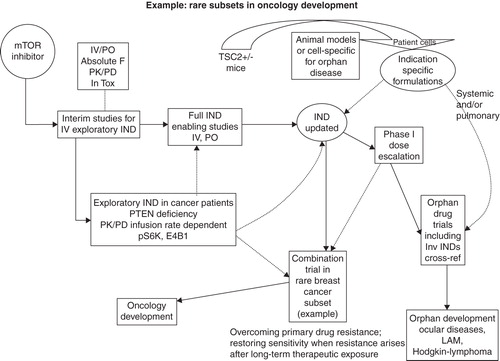
LAM is a rare lung disease primarily affecting women in their mid-30s and 40s. Clinically, abnormal, muscle-like cells (LAM cells) tend to grow uncontrollably in certain tissues and organs. This is particularly true in the lungs, lymph nodes and kidneys. Chronically, LAM cells can grow throughout lung tissue and eventually destroy normal lung function, which also has a major effect on oxygen supply to other organs Citation[10,12,13]. LAM is associated with TSC. Approved mTOR inhibitors have been used in LAM clinical trials Citation[17-19], which makes the disease amenable to new mTOR inhibitor approaches. In addition to mTOR-related biomarkers, LAM outcome has been associated with circulating VEGF-D (a family member of vascular endothelial growth factor) Citation[20]. From a pharmacology standpoint, TSC2+/− mouse models have been developed for research. In addition, biopsy specimens from LAM patients have been biobanked for research purposes which could aid in targeted therapeutic discovery Citation[19].
4. Expert opinion
The example presented highlights an opportunity to use a combined IND approach to develop both broad indications with those that would qualify for orphan drug status within the same project. Fast-tracking the orphan drug indication by essentially moving it to the ‘front side’ of the development process could be of significant benefit to patients suffering from rare diseases. Using a Master File style IND with a PK/PD biomarker-driven approach can preserve larger indications for portfolio evaluation, while using the smaller, orphan indications as target validation, proof-of-concept trials for accelerated approval, thereby adding multiple value inflections during development and favorable for portfolio analyses The mTOR pathway has been used as an example of a potential multifunctional target; however, there are several other biological pathways that would also be appropriate candidates. Newer methods involving system pharmacological modeling to investigate biological systems as they relate to specific diseases and to design new therapeutics may be used in the future to identify multiple indications, both broad and rare, for drug discovery and development Citation[21].
Declaration of interest
The author of the paper was previously involved in mTOR drug discovery with Emiliem, Inc.
Notes
Bibliography
- Pariser AR, Robb M, Sherman RE. Expedited programs for drug development and approval. Expert Opin Orphan Drugs 2013;1(7):507-10
- Kux L. Final rule: orphan drug regulations. 21 CFR Part 316 [Docket No. FDA-2011-N-0583] RIN 0910-AG72 Food and Drug Administration. Federal Register 2013;78(113):35117-35
- Genetic and Rare Diseases Information Center (GARD) National Center for Advancing Translational Sciences, Office of Rare Disease Research. Available from: http://rarediseases.info.nih.gov
- GeneCards. Human Gene Database. Weizmann Institute of Science. 2013. Available from: http://www.genecards.org/
- MalaCards. Human Maladies and their annotations. Weizmann Institute of Science. 2013. Available from: http://www.malacards.org/
- Sudarsanam S, Johnson DE. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel 2010;13(1):31-40
- Gough NR. Focus issue: TOR signaling, a tale of two complexes. Sci Signal 2012;5(217):eg4
- Kelsey I, Manning BD. mTORC1 status dictates tumor response to targeted therapeutics. Sci Signal 2013;6(294):pe31
- Azab SS. Targeting mTOR signaling pathways in breast cancer: more than the rapalogs. J Biochem Pharmacol Res 2013;1(2):75-83
- Kwan P, Tse MT. mTOR and its physiological impacts – part I: an overview. J Biochem Pharmacol Res 2013;1(2):124-37
- Shanmuganathan VA, Casely EM, Raj D, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol 2005;89(6):666-9
- Zhou P, Zhao MW, Li XX, et al. siRNA targeting mammalian target of rapamycin (mTOR) attenuates experimental proliferative vitreoretinopathy. Curr Eye Res 2007;32(11):973-84
- Kristof AS. mTOR signaling in lymphangioleiomyomatosis. Lymphat Res Biol 2010;8(1):33-42
- Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res 2011;39:D1035-41. Available from: http://www.drugbank.ca/
- Margariti N, Fox SB, Bottini A, et al. Overcoming breast cancer drug resistance with mTOR inhibitors. Could it be a myth or a real possibility in the short term future? Breast Cancer Res Treat 2010;128(3):599-606
- Mark A, Hajdu M, Varadi Z, et al. Characteristic mTOR activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease – a combined tissue microarray, in vitro and in vivo study. BMC Cancer 2013;13:250-61
- Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14-26
- Davies DM, de Vries PJ, Johnson SR, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 2011;17(12):4071-81
- McComack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008;133(2):507-16
- Young LR, Lee H-S, Inoue Y, et al. Serum VEGF-D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med 2013;1:445-52
- Vinci P, van der Graaf PH. Systems pharmacology for drug discovery and development: paradigm shift or flash in the pan. Clin Pharmacol Ther 2013;93(5):379-81
