Abstract
Introduction: Hutchinson–Gilford progeria syndrome (Progeria) is an ultra-rare premature aging syndrome that affects children worldwide and causes death at an average age of 14.6 years due to an accelerated form of athersclerosis leading to heart attacks and strokes.
Areas Covered: Like many rare disease research and patient advocacy organizations, The Progeria Research Foundation (PRF) was formed by an affected child’s parents, who saw the complete lack of attention to the disease and refused to accept that there was nothing they could do for their child. Fast forward 15 years, and that lack of attention has been transformed into worldwide recognition of Progeria, a first-ever treatment that improves vascular stiffness and estimated patient survival, and Progeria seated in the forefront of scientific efforts to discover additional treatments and a cure. Much of this extraordinary progress is due to PRF’s comprehensive programs and services, all of which are steadfastly mission-focused.
Expert Opinion: It is our hope that the description of the PRF programs and services that follows, along with an account of how they are helping PRF accomplish its mission to save children with Progeria, will assist and inspire others to take similar action for the many rare disease populations that need immediate attention.
1. Progeria’s First Century
Today we understand that Hutchinson–Gilford progeria syndrome (Progeria or HGPS) is a disease caused by a mutation in the LMNA gene, which produces a protein found in the nuclear membrane that is integral to the normal function of most cell types in the body (for current scientific and clinical reviews, see Citation[1,2]). The original 1904 article assigning the syndrome name ‘Progeria’ based on two patients treated by Johnathan Hutchinson and Hastings Gilford gave an excellent clinical picture as a syndrome resembling aging in many ways, with onset sometime in the first year after birth, including growth failure, loss of fat and hair, skin changes, bone and joint disease, and atherosclerosis Citation[3]. Over 100 years passed with almost no progress in understanding its biological or genetic basis, and no progress toward improving morbidity and mortality.
2. Jumpstarting progress: The Progeria Research Foundation’s history and mission
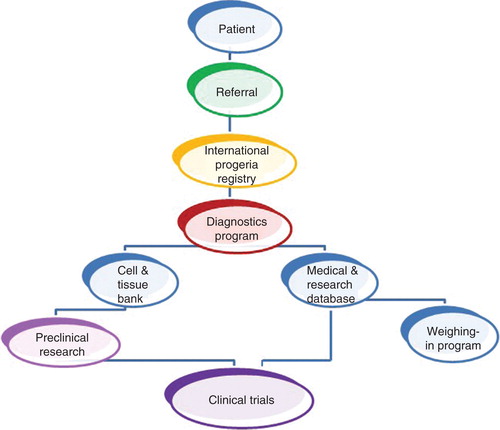
The Progeria Research Foundation (PRF) was founded in 1999 after Sam Berns, the son of Massachusetts physicians Drs. Leslie Gordon and Scott Berns, was diagnosed with Progeria or HGPS just before his second birthday. As with many rare disease advocacy organizations, PRF’s creation was in response to an extreme absence of information, resources and research on the disease: no known cause, no funding for Progeria research, no treatments, and no organization advocating for the children. Dr. Gordon serves as Medical Director, Dr. Berns is Board Chair and Audrey Gordon, Sam’s aunt, left her law practice to become PRF’s Executive Director. PRF’s original mission was to find the cause, treatments and cure for Progeria. This changed in 2003 when the first goal, cause, was achieved Citation[4,5]. It is now the only organization in the world solely dedicated to discovering treatments and the cure for Progeria. A steadfast focus on the mission has been crucial to success; constantly revisiting the mission helps organization members to decide on which new grants to fund and which new programs to launch ().
Figure 1. PRF’s original mission included cause, treatments and cure for Progeria. Cause is achieved, and now the mission is focused on treatments and cure. Multipronged collaborations, all keenly focused on the mission, are key to success.
Organizationally, PRF began with an executive director, board of directors, board of advisors, medical research committee, development committee and public awareness committee, all volunteers and all composed of friends, family and colleagues. In addition to these founding groups, PRF now has 7 fulltime employees, 8 chapters throughout the United States, hundreds of volunteer translators and fundraisers, pro bono providers of legal and public relations services, and many professional and organizational partners – all devoted to finding a cure for children with Progeria. Keys to successful growth have included worldwide outreach through website tools and social media to educate the public about Progeria, as well as educational and empowerment tools for volunteers. In 2013 alone, PRF’s social media followers increased by 50%, and approximately 650 million people were reached through top-tier media outlets reporting on Progeria and PRF’s work.
3. Progeria and PRF’s landmark accomplishments
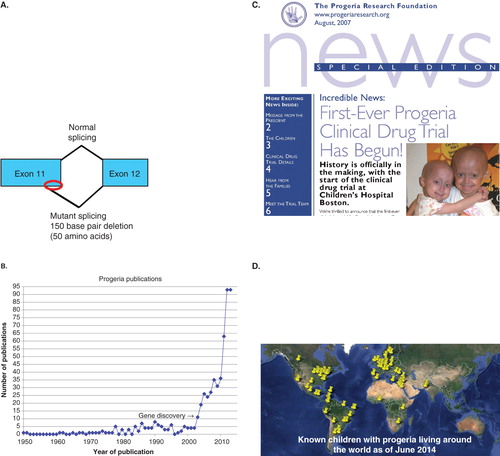
In just 15 years, the PRF community has achieved some historic milestones (). Key advancements include the Progeria gene mutation discovery in 2003 Citation[4,5], a 2000% increase in the number of annual research publications on Progeria Citation[6], a first-ever clinical drug trial initiated in 2007, the 2012 publication of the first treatment, and a nearly 300% increase in the number of children identified with Progeria. These and other advancements are due in large part to PRF’s creation of a comprehensive network of research-related programs and services that foster communication with the children and their families, scientists, clinicians, academic institutions, pharmaceutical companies, volunteers of many types, and the general public. Continuing this pattern of program-building and communication will undoubtedly be pivotal for discovering additional treatments and cure for children with Progeria.
Figure 2. Landmark accomplishments since PRF’s inception. A. A single base substitution optimizes an internal splice site within exon 11 of the LMNA gene, and produces a 150 base pair (red oval) and hence 50 amino acid deletion in the protein product, called progerin; B. A 2000% increase in scientific publications; C. The 2007 PRF Newsletter cover announcing the first-ever Progeria clinical trial; D. Pin map showing a 300% increase in identified children.
4. How did we know what to build? We asked
To achieve our goals, our initial steps were to identify the tools and resources essential to pushing the field forward. We knew that reaching the patient population would be key, because we could not help the children if we did not first find them. The question ‘How do we reach the patient population?’ precipitated the creation of the PRF International Progeria Registry and Translation Programs to communicate with the non-English-speaking families. We also looked to the literature, and found a handful of researchers that had done some work in the field of Progeria or its related progeroid syndromes, and asked them ‘Why aren’t you working on Progeria anymore?’ Each answer led to the identification of a need PRF had to fill. ‘There’s no funding’ led to the creation of the PRF Medical Research Grant Program; ‘There are no biological tools’ led to the creation of the PRF Cell and Tissue Bank. These programs foster work toward understanding the basic biology that underlies Progeria. ‘A detailed understanding of the natural history of disease in Progeria is sorely lacking.’ To deal with this, we created the PRF Medical & Research Database. ‘Discovering the genetic cause of Progeria (if indeed it turns out to be genetic) would catapult the field forward.’ PRF created a basic research funding program and the PRF Genetics Consortium, whose goal was to find the Progeria gene mutation. ‘There is no opportunity to gather scientists and brainstorm ideas and share data’ led to PRF’s international scientific conferences. Finally, we heard time and again, ‘What is Progeria?’. It was evident that the need to raise awareness and educate the general public was equally vital to PRF’s mission.
As the accomplishments progressed, these established programs have grown and others added such as diagnostics testing made possible after the Progeria gene mutation discovery, and first-ever clinical drug trials after the gene defect was intensely studied and potential treatments identified. With each program, we gain collaborating partners and the forces supporting children with Progeria grow.
5. Details on PRF’s programs and how they are playing a key role in Progeria research
From the beginning, the need for a centralized system to maintain basic information on children and families living with Progeria was apparent. PRF’s International Progeria Registry assures rapid distribution of new information to both parents and the children’s physicians that may benefit the children. This is a small, widespread population: prevalence is 1 in 18 million; thus, there are an estimated 300 – 350 children living with Progeria worldwide today Citation[6]. The Registry has identified children in 48 countries and every continent. One example of the Registry’s importance involves clinical trial enrollment. Trial leaders were able to complete enrollment in the first drug trial in just 6 months, and expansion of subsequent trials is possible as more children are identified and enrolled in the registry. By combining Registry data with treatment trial data, researchers were able to show that farnesylation inhibitors result in an extension of estimated lifespan for children with Progeria () Citation[7].
Launched in 2002, the PRF Cell & Tissue Bank is an essential resource, providing rare genetic and biological material to scientists worldwide who explore the biology of Progeria, search for treatments and cures, and study biological links between Progeria and aging. PRF has collected 193 cell lines, plus tissue samples from children with Progeria and their families. Biological materials have been sent to 63 teams of researchers at 55 institutions in 14 countries, and the bank has been acknowledged in nearly 40 publications. In 2011, the bank was expanded to include induced pluripotent stem cells, which allow scientists to make Progeria stem cells and create Progeria cardiac and vascular cells not otherwise available for studying heart disease that leads to early death in Progeria.
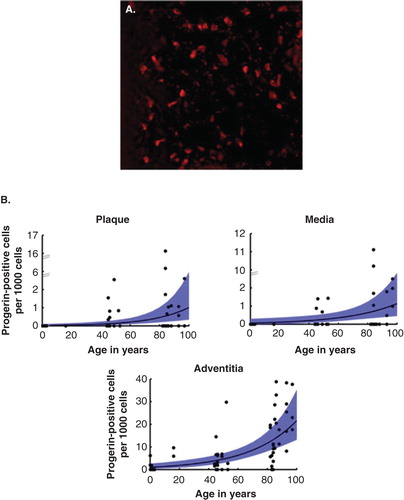
The importance of this program cannot be overstated; science cannot advance without basic scientific discovery, and basic science cannot advance without these biological tools. Cells and tissues from the bank were used for the studies that led to the Progeria gene discovery Citation[5], the identification of progerin in both HGPS and aging human tissues (), Citation[8,9], and many other important discoveries.
Figure 4. PRF Cell and Tissue Bank has contributed to studies showing the presence of progerin in normal aging skin and normal aging vasculature. A. Red stain detects progerin in cells of a skin biopsy from a 90-year old; B. Progerin detection in three layers of the vascular wall increases with age.
Systematic study of the natural history of disease is often difficult for rare disease populations, but is critical for several reasons. Because most medical caretakers have never treated a child with Progeria, there are often questions about how to optimize quality of life through daily care and medical treatment. In addition, in order to advance toward treatment and cure, a thorough understanding of disease characteristics that can be followed objectively and quantitatively is essential. For over 100 years, no one had studied more than a few children with Progeria at a time, and the studies were often limited to a handful of phenotypic observations. PRF’s Medical & Research Database compiles and analyzes a large collection of health records and radiologic scans from children with Progeria in order to understand the commonalities between patients, disease characteristics and disease progression.
In 2010, using information obtained from the database as well as the expertise of the Progeria clinical trial team, PRF published The Progeria Handbook for families and healthcare providers (). From basic health facts to daily care recommendations to extensive treatment guidelines, this 100-page handbook is invaluable for the attending physician to guide the family through the best courses of action. Available in 5 languages, 452 handbooks have been distributed, mainly to doctors and relatives directly caring for children with Progeria.
Figure 5. The Progeria clinical handbook is a 100-page healthcare guide for families and caretakers of children with Progeria.

The database was also responsible for determining the primary clinical outcome parameter (rate of weight gain) for the first Progeria clinical drug trial Citation[10] and continues to collect longitudinal data through its Weighing-in Program. Such pre-therapy measurements are vital for determining a drug’s success, and this program made it possible to do so. Subsequent to this, PRF worked with the National Institutes of Health (NIH) to conduct a natural history study of Progeria with patients examined on site at the NIH Clinical Center Citation[11], and currently includes natural history studies as part of every clinical treatment trial conducted at Boston Children’s Hospital.
An essential element to progress toward the PRF mission is a drive to not only increase the number of basic science publications, but also to fund science that pushes the field toward treatments and cure through its Research Grant Program. Members of The PRF Medical Research Committee are constantly debating each proposal’s worth toward the mission, and as knowledge and technology advance, so do these discussions. To date, PRF has invested over $5.5 million to fund Progeria-related research projects performed in 11 countries.
Perhaps the most dramatic illustration of how PRF’s grant and other programs have driven advancements in the field is the exponential increase in publications on Progeria. Both clinical and basic scientists have utilized the PRF grants, cells and tissues, medical and research database, and clinical trial results to advance discoveries and redefine the natural history of disease; their findings are published in well-known scientific journals read by researchers worldwide. Overall, from 2003 to 2013, 458 scientific publications have appeared, compared to <2 per year for the previous 50 years. This represents over a 2000% average annual increase since the Progeria gene discovery ().
Scientific Workshops on Progeria provide a forum for attendees to collaborate, sharing their ideas and contributing their expertise and cutting-edge scientific data to combat this lethal disease. PRF has organized 11 international scientific conferences (7 general workshops and 4 smaller, focused meetings). All have been co-funded by the NIH, primarily through the R13 granting program. The first meeting, held in 2001, was attended by 46 scientists, including leaders from a variety of areas of clinical and research-based expertise, including cardiology and arteriosclerosis, bone metabolism, molecular, cellular and developmental biology, immunology, endocrinology, dentistry, geriatrics and genetics. In those early days, only one attendee was an expert on Progeria, but all were willing to apply their knowledge to the field and try to steer the course of study. The most recent workshop boasted 180 attendees from 18 countries, with a packed agenda and 56 poster presentations. The vast depth and breadth of the meeting’s content reflected remarkable activity in the field Citation[2]. Smaller, focused meetings assemble experts to address a specific problem. For example, in 2002, 21 scientists comprising the PRF Genetics Consortium assembled. The goal was to find the genetic cause of Progeria. In less than a year they accomplished their goal Citation[5]. The discovery of the Progeria gene was a critical step in the ultimate quest for a cure; this historic finding literally flung open the doors of science, as PRF-funded researchers and others excitedly dove into intensely studying this defect. The gene discovery has brought us not only treatments for Progeria, but also has allowed us to study the common pathways between Progeria and diseases of aging such as atherosclerosis.
PRF created the Diagnostics Testing Program in the wake of the gene discovery so that children, their families and medical caretakers could for the first time be given a definitive genetic diagnosis. This has translated into earlier diagnoses, fewer misdiagnoses and early medical intervention to ensure a better quality of life for the children. After registration, diagnosis is the first step in enrolling children into PRF programs and clinical treatment trials.
PRF’s programs logically feed into the development of drugs that will lead to a cure. Beginning in 2007, just 4 years after the Progeria gene discovery, a PRF-sponsored Clinical Drug Trial brought children from around the world for promising treatments that we hoped would improve disease and possibly extend the lives of children with Progeria. This required intense collaboration with the pharmaceutical company that produced a drug named lonafarnib (Schering-Plough, now Merck), a collaboration that continues today in full force. It also required building a clinical research team at Boston Children’s Hospital and Brigham and Women’s Hospital that have become the world’s premier experts for Progeria worldwide. PRF’s supporting programs have culminated in its ability to co-coordinate and fund three clinical trials (see www.clinicaltrials.gov), one of which is currently enrolling patients. To date, children have come to these trials from 24 countries speaking 17 different languages. Most importantly, they facilitate planning for future clinical trials that will test medications that held promise for adding treatment benefit and eventually a cure.
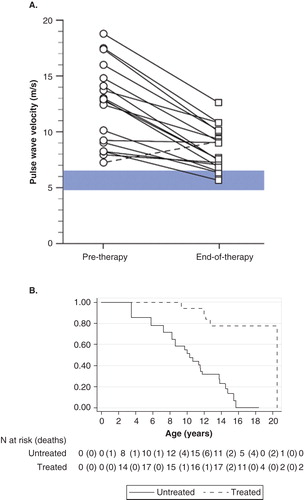
The most valuable discovery in treating Progeria thus far has been the realization that some key aspects of disease can actually be improved. While advancements in treatment are encouraging, improving vascular and bone integrity, neurological status, and modest increase in survival (), many aspects of progeria were not affected Citation[7,12,13]. Children continue to develop disease and need additional treatments to thwart the fatal heart disease that ensues. Each trial builds upon the experience of and knowledge gained from previous trials, primarily through defining new ways to assess disease and changes with treatment.
Figure 6. Results showing influence of FTIs on children with Progeria. A. Vascular pulse wave velocity, a measure of vascular stiffness, decreased with treatment (Gordon et al, PNAS, 2012). Each circle represents the PWV of one subject at trial initiation, and is connected to the PWV of that patient after 2 years of treatment. Blue horizontal bar indicates PWV for normal age-matched population. B. Probability of survival increased with treatment (Gordon et al, Circulation, 2014, reproduced with permission). With Kaplan–Meier survival estimates comparing untreated (solid line) to treated (dashed line) cohorts using matched analysis (unadjusted p < 0.001) where Time 0 on the x-axis (i.e., beginning of patient follow-up) is defined as patient birth and subject becomes at risk at the age of treatment initiation for the treated patient in the matched pair.
As with any rare disease, public awareness efforts are needed to educate the public and build support for the advocacy organization’s efforts. Prior to PRF’s existence, few knew what Progeria was. Now, through PRF’s outreach efforts, millions know about the disease and its far-reaching implications for the aging population. PRF’s global awareness campaign, Find The Other 150, drives the search for unidentified children with Progeria worldwide. FindTheOther150.org contains educational materials about Progeria and PRF in 21 languages, podcasts from PRF spokespeople and families of children with Progeria, and images and videos of known children with Progeria around the world. It also includes information on spreading the word, and PRF’s contact information for visitors who believe that they know a child with Progeria. PRF partners with Global Health PR to conduct portions of the Find The Other 150 campaign.
In 2012, 14 new children were identified, and print, Internet and broadcast media coverage reached over 206 million people through nearly 400 pieces that aired worldwide, including every major US TV and cable news network and major outlets in 9 other countries. That 206 million figure tripled in 2013. Maintaining the disease in the public eye is vital to every aspect of PRF’s work.
A comprehensive, up-to-date web site and social media presence are PRF’s awareness and education hallmarks. ProgeriaResearch.org provides access to the latest information on Progeria research and support for families, and through Facebook, Twitter and 4 other mediums, PRF’s direct social media reach is nearly 700,000. PRF’s story has appeared on CNN, ABC World News Tonight, The Dr. Oz Show, Primetime, Dateline, The Katie Couric Show and The Today Show, in Time and People magazines, The New York Times, The Wall Street Journal and many other widely read media outlets. The award-winning 2013 HBO film Life According to Sam has also brought awareness to a new level.
PRF eliminates barriers to communication for patients and their families around the world through its Translation Program that has amassed a team of over 300 volunteer translators to assist with a variety of translations – everything from emails to brochures to consent forms. This initiative has succeeded in translating PRF program and medical care materials into 26 different languages. Volunteer translation services save PRF an immense amount of money, while also empowering the people who contribute their language skills to help children with Progeria. PRF now periodically posts needs for specific languages on Facebook, always with positive response.
6. Progeria and aging
From its first published description in 1886 Citation[14], to PRF’s inception in 1999, there was only speculation that the Progeria disease process somehow overlapped with aging. Through PRF’s research efforts, scientists have made definitive connections between Progeria, heart disease and normal aging, primarily through the discovery that progerin, the protein that causes Progeria, is made by everyone’s cells both in vitro and in vivo, and increases with aging (). Thus, finding the cure for one of the rarest diseases on earth may also help millions of adults who suffer from heart disease, as well as the entire aging population. While this finding has helped gain scientific and general public interest, PRF continues to tackle the challenges of securing support for a rare disease.
Understanding the intersections and distinctions between HGPS and normal aging can inform both fields of study. Perhaps the absence of the common cardiovascular disease factors that are prevalent in adult populations, such as smoking, increased cholesterol, and obesity, can help us to cleanly interpret the clinical influence that progerin has after a lifetime of low-level accumulation in aging Citation[8,9,15] versus its intense production in children with HGPS. For example, children with Progeria do not develop Alzheimer’s disease, or cancers common to the elderly. Though there is significant overlap between the cardiovascular disease in Progeria and aging, there are some differences in tissue pathology between the two diseases. Overall, it is likely that the disease manifested in Progeria is an intensified representation of a subset of the factors that influence heart disease in normal aging. Dissecting out these factors, and focusing on improving them, will benefit both children with Progeria and those adults suffering from cardiovascular disease.
7. Partnering for success
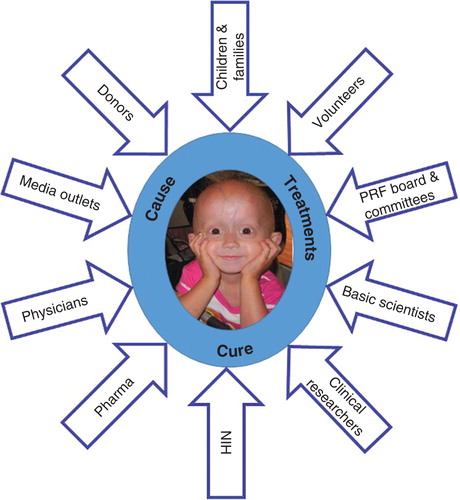
PRF continually works to cultivate and solidify partnerships with experts in a variety of fields, as well as financial supporters, all of whom are key to its accomplishments and goals. This multipronged approach comes with a recognition that there is no way to achieve the mission and save the children unless everyone – the children, their families, volunteers, donors, academic institutions, the pharmaceutical industry, the NIH, preclinical and clinical researchers, clinicians – knows that they are crucial to success ().
These relationships did not happen overnight – they grew over time and PRF has made sure all are duly recognized and thanked for their tremendous efforts on behalf of children with Progeria. There are many people willing to donate their time and talents – they just need to be asked. One of the shining examples of a successful collaboration is with the children and their parents who travel from around the world to come to the Boston trials. The families always come in pairs (). This is an absolutely essential part of their ability to emotionally withstand the rigors of a clinical trial schedule; giving the children and parents the opportunity to bond with another Progeria family provides a positive atmosphere during an otherwise stressful time. They represent PRF’s core belief and practice: Working together is exponentially better than working alone.
PRF’s challenges are tackled with thoughtful planning and staunch determination – two vital ingredients for any non-profit organization. Fostering collaboration and addressing all mission-based needs provide one version of a roadmap for how to optimize the chances of tackling a disease – a map that can be replicated to create a successful translational research organization, moving from the lab to treatments and a future promise of cure.
Declaration of interest
L Gordon is The Progeria Research Foundation’s (PRF) Medical Director and Principal Investigator for PRF’s research-related programs. A Gordon is the President and Executive Director of PRF. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgment
We are tremendously grateful to all children with Progeria and their families for their participation in the PRF programs. We also gratefully acknowledge Scott D. Berns, MD, MPH, co-founder of The Progeria Research Foundation and Chair of its Board of Directors, for his organizational leadership that has significantly contributed to PRF’s journey detailed in this article.
Notes
Bibliography
- Kieran MW, Gordon LB, Kleinman ME. The role of the farnesyltransferase inhibitor lonafarnib in the treatment of progeria. Exp Opin Orphan Drugs 2014;2:95-105
- Gordon LB, Rothman FG, López-Otín C, Misteli T. Progeria: a paradigm for translational medicine. Cell 2014;156:400-7
- Gilford H. Progeria: a form of senilism. The Practitioner; 1904. 188-217
- De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in hutchinson-gilford progeria. Science 2003;300:2055
- Eriksson M, Brown WT, Gordon L, et al. Recurrent de novo point mutations in lamin a cause hutchinson-gilford progeria syndrome. Nature 2003;423:293-7
- Gordon LB. PRF by the numbers. Available from: www.progeriaresearch.org [Accessed 7 August 2014]
- Gordon LB, Massaro J, D’Agostino RB, et al. Impact of farnesylation inhibitors on survival in hutchinson-gilford progeria syndrome. Circulation 2014
- McClintock D, Ratner D, Lokuge M, et al. The mutant form of lamin a that causes hutchinson-gilford progeria is a biomarker of cellular aging in human skin. PloS One 2007;2:1-9
- Olive M, Harten I, Mitchell R, et al. Cardiovascular pathology in hutchinson-gilford progeria: Correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol 2010;30:2301-9
- Gordon LB, McCarten KM, Giobbie-Hurder A, et al. Disease progression in hutchinson-gilford progeria syndrome: Impact on growth and development. Pediatrics 2007;120:824-33
- Merideth MA, Gordon L, Clauss S, et al. Phenotype and course of hutchinson-gilford progeria syndrome. N Eng J Med 2008;358:592-604
- Ullrich NJ, Kieran MW, Miller DT, et al. Neurologic features of hutchinson-gilford progeria syndrome after lonafarnib treatment. Neurology 2013;81:427-30
- Gordon LB, Kleinman ME, Miller DT, et al. Clinical trial of a farnesyltransferase inhibitor in children with hutchinson-gilford progeria syndrome. Proc Natl Acad Sci USA 2012;109:16666-71
- Hutchinson J. Congenital absence of hair and mammary glands with atrophic condition of the skin and its appendages in a boy whose mother had been almost wholly bald from alopecia areata from the age of six. Med Chir Trans 1886;69:473-7
- Scaffidi P, Misteli T. Lamin a-dependent nuclear defects in human aging. Science 2006;312:1059-63