Abstract
There is a large unmet need for helping rare disease patient groups and Findacure aims to empower these groups to become effective campaigners for change. Through its scientific meetings, Findacure also aims to gain the support of the scientific community in recognizing the importance of ‘fundamental diseases’ (conditions that manifest themselves as extreme and rare genetic disorders that offer a unique opportunity to better understand other diseases, including many common ones) and help to create a receptive research environment. Since it may often be commercially unattractive to develop treatments for many fundamental diseases, Findacure helps facilitate patient groups to themselves drive the development of treatments for fundamental diseases, using multi-stakeholder collaborative models.
1. A scientific odyssey
It is a common story. Parents find out that their child has a rare disease. Desperate to do something, they set up a patient group and try to raise funds for a treatment. They search all over the internet, connect with parents of other patients and go to conferences to meet scientists and clinicians. They stay up late at night reading scientific papers, trying to understand the technical jargon.
They go from despair, to hope and back to despair again. It is an unending cycle. They meet a scientist who seems to have some answers, only then to meet another one who says the opposite. They put some of their funds into a research project. The researcher goes away for a few years, publishes his findings and moves on to something else. In some cases, the researcher disappears with the money – preying on the desperate parents’ goodwill.
And yet, out of all this, some patient groups thrive thanks to a mix of dogged perseverance, relentless networking, and vision. Take the muscular dystrophy groups, for instance. They are among the most organized and well resourced of rare disease patient groups. Through a combination of ambitious fundraising, powerful lobbying and savvy business skills, the muscular dystrophy groups have been at the heart of much of the legislation on rare diseases, the most exciting fundraising campaigns and the most ground-breaking patient–industry partnerships for clinical trials.
Or consider Alström Syndrome UK Citation[1]. Thanks to her law experience and being driven by a passion for justice, Chief Executive officer Kay Parkinson took on the UK’s Department of Health – which was refusing to recognize Alström Syndrome’s relationship with its centers of excellence – and won. She paved the way for other rare disease patient groups to create partnerships with hospitals to set up centers of excellence.
Then there is Lysogene Citation[2]; founded by Karen Aiach, the mother of a child with an ultra-rare neurological disorder called Sanfilippo Syndrome (Mucopolysaccharidosis III). Lysogene is a biotech company that has successfully taken an innovative gene therapy from research to clinical trials in just a few years.
What can we learn from these success stories? What can other patient groups do to mimic their success?
When we were setting up the Alkaptonuria (AKU) Society Citation[3] 12 years ago to find a treatment for the ultra-rare disease AKU, we looked for advice on what to do. There was not much out there: the internet was still in its early days and umbrella groups such as European Rare Diseases Organisation (EURORDIS) Citation[4] were just starting off.
We had to learn things the hard way: through trial and error. We were fortunate: we had the support of committed clinicians and scientists who made it their life’s mission to find a treatment for AKU. But we had no idea how to run clinical trials, how to navigate the regulatory maze or how to work with industry. For that, we had to go out of our way to meet people who knew about these issues and were willing to help.
From speaking to other patient groups, the situation is similar for many of them. Even though our diseases are all very different, we have similar challenges: access to funding, experience in running clinical trials and research projects and how to put together networks of scientists, clinicians and industry who share the same vision of developing treatments.
There is a huge unmet need for helping patient groups. With > 7000 rare diseases now identified, touching between 6 and 8% of the population, no single organization will ever manage to help all of them.
2. Findacure: the fundamental diseases partnership
Inspired by the example of EURORDIS, we set up Findacure: The Fundamental Diseases Partnership Citation[5] to provide the training, mentoring and capacity building for patient groups to help themselves. We believe that patient groups should be active agents in the quest for treatments and cures – it is a quest that is too big to be left solely to scientists and industry. Patient groups live with their disease each day. They understand it intimately. They are driven by a desire to succeed. But often, they do not know where to start.
Findacure has three primary aims:
empowering patient groups to become effective campaigners for change;
facilitating patient groups to drive the development of treatments for fundamental diseases;
campaigning for a receptive research environment that recognizes the pivotal importance of fundamental diseases.
Through Findacure, we aim to empower the patient sector to take control of their disease area and help patient groups meet the challenges of medical research and drug development. These groups rarely have the knowledge or expertise to initiate basic and/or clinical research for their disease and Findacure aims to provide tools and advice on matters from how to raise funding and how to engage with industry, through to orchestrating a clinical development program.
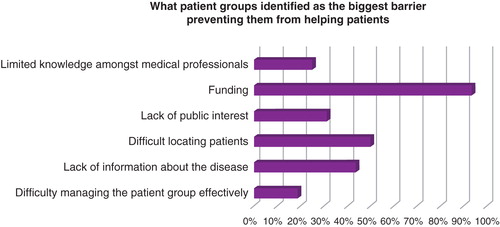
To identify the key areas patient groups most needed help, we conducted a survey with 54 patient advocates in the UK. As part of this survey, advocates were asked what they viewed as the biggest barriers preventing them from helping patients. The results are displayed in .
We have used this research to focus our work in supporting rare disease patient advocates. We mentor marginalized patients in setting up entrepreneurial patient organizations so that they can build networks with the scientific, medical and pharmaceutical sectors, and become involved in developing treatments. We offer one-to-one assistance to nascent and small patient groups, providing guidance tailored to their needs.
For example, we have supported two sisters living with Ehlers-Danlos Syndrome in setting up an organization for others with chronic conditions. Over the course of several meetings, we advised them on the legal form the organization should take, potential funding sources and the format in which they could offer support. They have since established a social enterprise that offers programs to support families affected by chronic conditions. We also launched a pilot peer mentoring scheme in late September, in which nascent and struggling patient groups will be matched with experts in the field in a year-long project to improve their patient support and involvement in research. Through this type of mentoring, Findacure empowers patient groups to grow and to offer greater care to rare disease patients.
3. The needs of patient groups
Findacure also organizes free training workshops for patient groups, covering pertinent issues and developing key skills relating to the barriers shown in . The workshops feature expert speakers and case studies from patient groups offering specialized knowledge. Four such workshops have taken place throughout 2014, covering the topics of fundraising on a budget, applying to the European Commission to fund international research, developing clinical trials, and good governance for patient groups. In total, 124 patients and advocates attended the workshops, representing over 75,900 patients living with rare diseases in England and Wales.
In the feedback surveys following the events, 92% of respondents agreed or strongly agreed that the workshop content was relevant to their needs and that it was comprehensively presented. With regards to improving knowledge and skills, overall 90% stated that the workshops were useful in increasing their knowledge and skills. Future workshops will continue to assess and address key barriers as identified by patient groups to develop their role within medical research and patient support.
In order to develop these workshops, Findacure launched a crowdfunding campaign Citation[6]. Crowdfunding is the practice of funding a project by raising money from a large number of people – the crowd – typically via the internet. The campaign aimed not only to fundraise for Findacure's patient advocate projects, but also to raise international awareness around the issue of rare diseases and the isolation that patients experience. Over seven weeks, $29,000 was raised in donations from 15 different countries and the campaign was features in numerous blogs, radio shows, and newspapers. In addition, Findacure is developing a book, which will provide a practical guide on the many issues patient groups may face, from how to set up a patient charity to engaging with academia, working with industry and raising finance, through to designing a clinical trial program.
Findacure not only addresses patient groups’ internal barriers, but also the external barriers to their work. To address the barrier of limited knowledge among medical professionals, we are campaigning for a receptive research environment. We run annual scientific workshops to engage the scientific community around the importance of rare disease research. These workshops emphasize the message that rare disease research unlocks new insights and discovers potential treatments not only for the disease being researched, but for more common conditions as well. This helps to show that rare diseases are relevant for everyone.
Our first scientific conference took place in Cambridge in March 2014. The conference was attended by 36 people representing 4 universities, 9 research teams, 3 pharmaceutical companies, 4 biotechnology companies and 9 medical charities. The workshop highlighted key research being done, and aimed to motivate researchers and the pharmaceutical industry to push further. In the survey we conducted following the workshop, 70% of those who responded agreed that the workshop helped them realize the importance of studying rare diseases, with another 10% stating that they were already aware of the importance. It is our aim that this increased exposure among the medical profession will benefit patients, through the long-term increased focus on their conditions, leading to better treatments and hopefully also to cures.
4. Expert opinion: why fundamental diseases matter
One of the biggest problems with research into rare diseases is that, until recently, they have been largely ignored by the scientific community and the pharmaceutical industry. This is exemplified by the very term ‘orphan drug,’ which was coined in 1968 to describe those drugs that are ‘homeless’ or ‘orphan’ and abandoned by society Citation[7].
The most likely reason for this is that their importance has not been recognized. By referring to these diseases as rare, it has been very difficult to gain support from the general public, scientists and the commercial sector. However, rare diseases can often have much wider relevance than may be initially obvious. This concept was already recognized as far back as the 17th century by the great British physician William Harvey, who wrote:
“Nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows tracings of her workings apart from the beaten paths; nor is there is any better way to advance the proper practice of medicine than to give our minds to the discovery of the usual law of nature by the careful investigation of cases of rarer forms of disease Citation[8]”.
It is true of many rare diseases that their study is fundamental to the understanding of human biology and we have therefore coined a new term ‘fundamental disease’ to describe this relationship. Fundamental diseases may be defined as those that manifest themselves as extreme and rare genetic disorders that offer a unique opportunity to better understand other diseases, including many common ones.
This link between the rare and the common was more recently expressed by Frederick Kaplan, Professor of Orthopaedic Molecular Medicine at The University of Pennsylvania:
“Nature does not use different genes, molecules and pathways for common conditions than it does for rare ones. Rather, it is often the rare disease that actually reveals which gene, molecules or pathways nature hijacks in its common infirmities Citation[9]”.
The main features of a fundamental disease are that they are caused by a specific (usually single) gene defect (and are therefore rare), such that it is in theory possible to fully understand the disease at the molecular level and develop a highly selective treatment Citation[10]. The knowledge gained may then be used for other conditions.
For example, the study of the rare genetic disease familial hypercholesterolemia led to the discovery of the low-density lipoprotein (LDL) receptor and an understanding of its role in the regulatory mechanism by which the LDL receptor controls cholesterol synthesis by a negative feedback mechanism on 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This work also demonstrated how the LDL receptor itself is controlled and how drugs such as statins, which inhibit cholesterol synthesis by inhibiting HMG-CoA reductase, could selectively lower LDL in the blood. Statins are now used to treat millions of people with raised cholesterol.
Even where there is not a direct relation between the fundamental disease and a common disease, the knowledge gained by studying the rare disease can have wider implications. For example, an enzyme replacement therapy (ERT) developed for a specific rare may well generate techniques to develop ERTs for several other diseases. Similarly, a gene therapy for one genetic disease may pave the way for the development of other gene therapies using the same technology.
Notwithstanding the clear potential benefit of studying and developing treatments for fundamental diseases, one of the biggest issues for research is that they are often commercially uninteresting when one considers the huge investment and risk involved in developing a new therapy and bringing it to market.
5. New models of collaborative drug development
In recent years, the pharmaceutical industry has begun to recognize the importance of a collaborative approach and has established collaborations with academia, for example, GlaxoSmithKlein’s Discovery Partnerships with Academia Citation[11] or Johnson & Johnson’s Innovation Centers Citation[12]. However, it may still be difficult for many rare diseases to attract the necessary interest and investment to take a product into development. Although there are a range of incentives to help improve the situation for rare diseases, for example, the orphan drug legislation provides financial benefits during the development process and several years of market exclusivity following approval, in many cases there is simply no business case to be made, even with the possibility of a high price for the orphan drug once it is on the market.
Patient groups have been funding research for years, but this is often done in a ‘fund and forget’ fashion, in which no specific deliverables are agreed and the funded party is not held accountable. Sometimes there is follow-up in which certain deliverables, such as publications, may be specified and occasionally there may be a more detailed ‘venture philanthropy’ approach in which the funder receives a return for the investment, such as royalties on future sales. This is exemplified by the collaboration between the Cystic Fibrosis Foundation and the company Vertex, which resulted in the development of ivacaftor (Kalydeco). However, the funder needs to be acutely aware of the potential for conflicts of interest, as discussed in a recent BMJ article Citation[13].
It may also be possible to create multi-stakeholder consortia, funded with public grants, statutory investment or venture philanthropy and drive forward development, which would otherwise have been abandoned. For example, the European Union Seventh Framework (FP7) grant program recently funded a patient group-led consortium comprising hospitals, universities, small and medium-sized enterprises and a pharmaceutical company to join together in the development of a treatment for a rare metabolic disease, AKU Citation[14].
Findacure is currently developing partnerships with academia for a number of potential gene therapies for which the universities have so far been unable to attract commercial investment. Findacure is helping the academic institutions to write orphan designation applications, design the development programs and will partner with them in consortia to raise funding to take the products into clinical development. If strong early clinical data can be generated through such a collaborative approach, it may then be possible to gain the interest of a commercial party to complete the development.
This kind of proactive approach shows that the process can be driven, at least in the early stages, by patient groups and academia without the need for commercial parties. This should greatly increase the chance of therapies being developed for otherwise ‘orphaned’ fundamental diseases.
6. Conclusion
There are many barriers facing rare disease patient groups, as identified by a mini project undertaken in the UK. Findacure provides practical support for these groups, which, feedback has shown, is relevant and valuable. Feedback from Findacure’s scientific meetings has also been positive with regard to a newly coined term ‘fundamental diseases.’ Findacure is working with a number of organizations not traditionally involved in drug development to create multi-stakeholder collaborations that can drive the development process.
Declaration of interest
AK Hall is co-founder of Findacure, a shareholder at PSR group B. V. and since 18th August 2014 has been Medical Director for Prosensa N.V. N Sireau is co-founder of Findacure and F Raffai is project manager at Findacure. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgments
AK Hall is a medical doctor with 20 years of drug development experience in the pharmaceutical industry, the last 4 as a consultant exclusively in rare diseases (since August 2014, he is working as Medical Director at Prosensa, a company developing treatments for Duchenne muscular dystrophy). He speaks and chairs conferences on orphan drugs and publishes extensively on issues related to orphan drugs, from clinical development strategies to pricing and value issues. He provides this expertise pro bono to Findacure. N Sireau is Chairman and CEO of the AKU Society since 2005 and has raised more than £11 million in funding and taken a treatment into clinical development. He is also Co-founder and Chairman of Findacure. Previously, he founded the charity SolarAid in 2006, which he grew into a multi-million pound award-winning social enterprise. F Raffai is Project Manager at Findacure. As part of this role, she develops new projects, organizes patient group training and scientific conferences, runs Findacure’s online communications and leads on funding applications.
Bibliography
- Alström Syndrome UK. Available from: http://www.alstrom.org.uk [Accessed 16 April 2014]
- Lysogene, a gene therapy biotechnology company. Available from: http://www.lysogene.com/en [Accessed 16 April 2014]
- Alkaptonuria Society. Available from: http://www.akusociety.org [Accessed 16 April 2014]
- EURORDIS, a non-governmental alliance of patient organisations. Available from: http://www.eurordis.org [Accessed 16 April 2014]
- Findacure, a charity builing the fundamental disease community to drive research and develop treatments. Available from: http://www.findacure.org.uk [Accessed 16 April 2014]
- Findacure’s campaign page on a crowdfunding platform. Available from: https://www.indiegogo.com/projects/fundamental-diseases-it-s-time-to-care [active from 28 April 2014 until 17 June 2014]
- Provost G. "Homeless" or "orphan" drugs. Am J Hosp Pharm 1968;25(11):609
- Harvey W. Letter IX, to John Vlackveld, 24 April 1657. In: The circulation of the blood. Cosimo, New York; 2006. p. 200-1
- Kaplan FS. The key to the closet is the key to the kingdom: a common lesson of rare diseases. Orphan Dis Update 2006;24(3):1-9
- Timmis O, Hall AK. Fundamental diseases: treasure your exceptions. Innovations in Pharmaceutical Technology. 2014;49:12-14
- Discovery Partnerships with Academia (DPAc). Available from: http://dpac.gsk.com/ [Accessed 16 April 2014]
- Johnson & Johnson Innovation Centre. Available from: http://www.jnj.com/partners/innovation-centers [Accessed 16 April 2014]
- Cohen D, Raftery J. Paying twice: questions over high cost of cystic fibrosis drug developed with charitable funding. BMJ 2014;348:g1445
- Clinical Development of Nitisinone for Alkaptonuria. Available from: http://cordis.europa.eu/project/rcn/106157_en.html [Accessed 16 April 2014]