Abstract
Some rare disease treatments come from drug repurposing. The biopharma industry focuses on commercial rare disease drug repurposing, and disease-specific non-profits and governments focus on philanthropic rare disease drug repurposing. In the future, it is likely that both groups will increase their use of drug repurposing as a means of developing treatments for rare diseases, but for different reasons.
There are currently over 7000 of these unsolved rare diseases that affect more than 300 million people worldwide.[Citation1] The biopharma industry has tackled some rare disease issues using drug repurposing or compound repositioning [Citation2] that can provide a commercial profit, such as the repurposing of thalidomide for the blood cancer multiple myeloma,[Citation3] the repositioning of sildenafil for erectile dysfunction and then repurposing for pulmonary arterial hypertension,[4] and the repurposing of dimethyl fumarate for MS.[Citation5] The biopharma de novo drug development model is getting more expensive and time-consuming, pushing industry to look for new commercial routes to treatment creation and profitability, including repurposing and repositioning. Government programs, embedded in legislation like the 21st Century Cures Act, may soon provide additional economic benefits to industry to repurpose their patent protected drugs.
Philanthropy has also been involved in the repurposing of drugs to create rare disease solutions. Non-profits have aided the commercial development of repurposed drugs by providing early-stage grants to academic researchers to create the preclinical and early clinical data that moved the drug repurposing approval process forward. A number of non-profits supported early research in the repurposing of thalidomide, including millions of dollars of research grants from sources such as Goldman Philanthropic Partnerships, the Multiple Myeloma Research Foundation, and the Leukemia and Lymphoma Society.[Citation6] This research supported to the work that Celgene did to receive FDA approval of thalidomide.
Non-profits have also financed drug repurposing in rare diseases that have led to off-label use of generic drugs as a rare disease solution. Dr. David Teachey at Children’s Hospital of Philadelphia used non-profit funding to build a mouse model of the rare pediatric disease autoimmune lymphoproliferative syndrome (ALPS) and showed that the drug sirolimus might be a disease-modifying solution. Additional philanthropic support created a small proof of concept clinical trial that proved that sirolimus could ameliorate most of the symptoms of ALPS.[Citation7] While there was no economic incentive to pursue FDA or other regulatory approval for this very rare disease, the clinical response was enough to support adoption of off-label sirolimus for this rare disease. And the success of this repurposing for ALPS has led to the clinical testing of sirolimus in a number of other rare pediatric autoimmune diseases, including pediatric lupus, CVID, IPT, Evan’s disease, and autoimmune hemolytic anemia.[Citation8]
Philanthropy has also supported the repurposing of nutriceuticals, or herbal medicines, for rare diseases. One remarkable series of nutriceutical discoveries come from the laboratory of Berish Rubin and Sylvia Anderson at Fordham University. Over the course of 8 years, they discovered a series of nutriceuticals that acted with great biologic efficacy through a series of well-documented mechanisms to reduce or eliminate many of the most serious symptoms of the rare disease familial dysautonomia.[Citation9,Citation10]
There are many rare disease drug repurposing initiatives in progress as of the writing of this article. One started serendipitously at Johns Hopkins Medical Center. Dr. Gregory Riggins and his team at Hopkins were doing mouse model brain cancer research. One group of mice in the Riggins’ lab that should have developed brain tumors did not. The researchers discovered that the mice had been treated with the veterinary antiparasitic drug fenbendazole. There was information in the literature that fenbendazole inhibited cancer growth. The team then tested the related human-approved drug mebendazole and found it could inhibit glioblastoma. The scientists believe the drug blocks tumors by inhibiting formation of proteins needed by cancer cells to grow.
Mebendazole is a 60-year-old drug that is no longer manufactured and sold in the US. The Riggins’ team found a company in India to manufacture a slightly altered version of the drug that provides enhanced penetration into the brain with a reduced side effect profile. A clinical trial started in 2015,[Citation11] and the Riggins’ lab is providing mebendazole under compassionate use to oncologists treating pediatric patients.
Another philanthropic repurposing involves the drug nitisinone, being repurposed for the rare disease alkaptonuria (AKU). AKU is caused by the lack of the enzyme homogentisic dioxygenase. This leads to a condition in which patients cannot fully break down a toxic acid called homogentisic acid or HGA. Although some HGA is eliminated in urine, HGA builds up in the body at 2,000 times the normal rate, causing discoloration of bone and cartilage in a process called ochronosis, that ultimately leads to the breakdown of the cartilage and bone.
Nitisinone is currently licensed to treat another rare disease called hereditary tyrosinemia type 1. Although nitisinone is not currently licensed for use in AKU, previous animal experiments and research studies have shown that nitisinone could be effective in treating it. Clinical research in the US showed that nitisinone reduced HGA levels by up to 95%. Two clinical trials testing nitisinone in AKU are currently underway in Europe, supported by more than $8M of research funding achieved through crowdfunding and government grants.[Citation12]
Repurposing based on new disease understanding is poised to help the rare disease late infantile neuronal ceroid lipofuscinosis (LINCL). LINCL is a neurodegenerative lysosomal storage disorder characterized by progressive mental deterioration, cognitive impairment, visual failures, seizures, and deteriorating motor function that typically produces symptoms at the age of 2–4 years, progresses rapidly, and ends in death between ages 8 and 15. LINCL is associated with mutations in the Cln2 gene that encodes tripeptidyl-tripeptidase I (TPP-I), a protein that functions in the lysosomal compartment to remove tripeptides. This mutation leads to accumulation of ceroid-lipofuscin, which causes brain cell destruction.
There are different variants of Cln2 mutations, and some LINCL patients have residual TPP-I activity. Thus, one approach for treatment may be to find ways to enhance the levels and residual activity of the TPP1 protein. Dr. Kali Pahan at Rush University Medical Center in Chicago is repurposing a combination of two drugs, gemfibrozil and ATRA, to help patients with LINCL.[Citation13] This repurposed drug combination, in both cell lines and animal models, upregulates the gene that makes the enzyme TPP1 via the PPARα/RXRα pathway. A clinical trial is planned to begin soon, supported by the Noah’s Hope and Hope for Bridget Foundation.
One last example of philanthropic drug repurposing is based almost exclusively on literature searches combined with scientific and clinical reasoning. An international group of scientists and clinicians spent the better part of two years researching the literature and evaluating clinical observations to create the CUSP9* treatment protocol for recurrent glioblastoma. CUSP9* drugs—aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, sertraline, and ritonavir—are all drugs approved for non-cancer indications. Each drug inhibits one or more important pathways used by glioblastoma. By blocking survival pathways, the aim is to render the current standard cytotoxic drug used in primary glioblastoma treatment, temozolomide, more effective.
Many of the CUSP9* drugs have been used together in compassionate use clinical cases of glioblastoma with good tolerability. According to the research protocol, “This group of drugs blocks signaling at, or the activity of, AKT phosphorylation, aldehyde dehydrogenase, angiotensin converting enzyme, carbonic anhydrase −2,- 9, −12, cyclooxygenase-1 and −2, cathepsin B, Hedgehog, interleukin-6, 5-lipoxygenase, matrix metalloproteinase −2 and −9, mammalian target of rapamycin, neurokinin-1, p-gp efflux pump, thioredoxin reductase, tissue factor, 20 kDa translationally controlled tumor protein, and vascular endothelial growth factor.” The researchers believe that given the current prognosis after a glioblastoma has recurred, a trial of CUSP9* is warranted.[Citation14] This clinical trial, supported by the AntiCancer Fund and other philanthropies, is scheduled to begin in 2016.
Expert opinion
There is a bright future for repurposing and repositioning in both the commercial and philanthropic worlds. On the commercial side, knowledge of diseases and drugs increases at a faster pace. Quicker screening of compounds, in silico design, new tools like CRIPSR/Cas, and more efficient and effective animal and artificial models will help researchers more easily piece together which drugs can help which diseases. There are many rare disease non-profits eager to fund drug discovery research, but they do not have any current drug candidates because so little is known about the disease. They will benefit as these tools continue to develop, and their early funded discoveries can lead to biopharma commercialization.
Government incentives focused on rare diseases will fuel this industry repurposing revolution. The 21st Century Cures legislation,[Citation15] passed by the US House of Representatives in 2015, is designed to jumpstart medical innovation. One provision in the bill would allow the FDA to grant an extra 6 months of exclusive marketing rights to a company if an existing patent-protected drug is repurposed and approved to treat a rare disease. For a drug earning $500 M US per year, a 6 month extension is worth $250M, which is likely to be 6–10 times the cost of the repurposing regulatory approval. That is enough leverage for a company to take on the risk of testing for the rare indication.
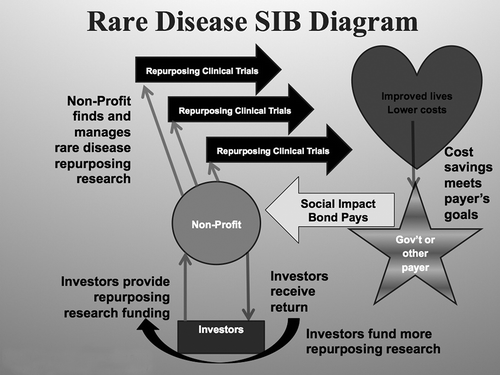
On the philanthropic side, the key to the future is creating new economic engines to support generic drug repurposing. One way to create an incentive for investors to finance repurposing for rare diseases is through a Repurposing Social Impact Bond (RSIB). An RSIB is a financial arrangement in which a government agrees to pay a pre-agreed sum if a repurposing clinical trial proves a new use for a generic drug to help solve a rare disease.
demonstrates how an RSIB uses funds contributed by for-profit or impact investors to pay for the repurposing clinical trials. The investors do this in exchange for a share of the government payments, if performance targets are met. A non-profit or other management company finds, vets, and manages the rare disease repurposing research. Research that proves a new indication is incorporated into the care regimen for the rare disease patients, and a percentage of the cost savings is paid back to the RSIB, which repays the investors and uses the additional funds to create a new portfolio of rare disease repurposing projects.
An RSIB could be the ideal vehicle to further the government’s dual mission to improve the health of its citizenry and reduce healthcare costs. A pilot RSIB is being developed in England, sponsored by the UK non-profit FindaCure, supported by the social impact investing organization Numbers4Good and the US non-profit Cures Within Reach.
A great deal of drug repurposing is happening in the rare disease space, both commercially and philanthropically. Repurposing will continue to grow in the rare disease space as tools become better and funds become scarcer, both for research and for payment of healthcare outcomes.
Declaration of interest
Bruce Bloom is the president and chief science officer of Cures Within Reach. He has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
- RARE List™. Global Genes, Allies in Rare Diseases [Internet]. 2015. [ cited 2015 Nov 11]. Available from: https://globalgenes.org/rarelist/.
- For the purposes of this article, drug repurposing will be defined as finding a new disease indication for an already approved drug. Drug repositioning will be defined as finding a disease indication for a drug-like compound that has been found safe in humans through clinical testing, though it that failed for efficacy or other reasons during development for the initial disease indication(s).
- FDA Approval for Thalidomide. National Cancer Institute [Internet]. 2013. [ cited 2015 Nov 11]. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-thalidomide.
- Barnett C, Machado R. Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag. 2006;2(4):411–422.
- FDA approves new multiple sclerosis treatment: Tecfidera [Internet]. U.S. Department of Health and Human Services; 2013 [ cited 2015 Nov 11]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345528.htm.
- Kumar S, Witzig T, Rajkumar S. Thalidomide: Current Role in the Treatment of Non-Plasma Cell Malignancies. J Clin Oncol. 2004;22(12):2477–2488.
- Teachey DT, Greiner R, Seif A, et.al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145(1):101–106.
- Funding Partner Report March 2015. Cures Within Reach [Internet]. 2015. [ cited 2015 Nov 11]. Available from: http://cureswithinreach.org/images/ProjectReports/LDOGTeachey_Report_March2015.pdf.
- Anderson SL1, Liu B, Qiu J, et al. Nutraceutical-mediated restoration of wild-type levels of IKBKAP-encoded IKAP protein in familial dysautonomia-derived cells. Mol Nutr Food Res. 2012;56(4):570–579.
- FD NOW website. Treatment breakthroughs in FD research from the Fordham Laboratory for familial dysautonomia research [Internet]. 2015. [ cited 2015 Nov 11]. Available from: http://www.fdnow.org/research/treatment-breakthroughs.
- A Phase I Study of Mebendazole for the Treatment of Pediatric Gliomas, Clinicaltrials.gov [Internet]. 2015. [ cited 2015 Nov 11. Available from: https://clinicaltrials.gov/ct2/show/NCT01837862?term=mebendazole+pediatric+glioma&rank=1.
- Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2), Clinicaltrials.gov [Internet]. 2015. [ cited 2015 Nov 11]. Available from: https://clinicaltrials.gov/ct2/show/NCT01916382?term=nitisinone&rank=5.
- Ghosh A, Corbett G, Pahan K, et.al. Gemfibrozil and Fenofibrate, ood and Drug Administration-approved Lipid-lowering Drugs, Up-regulate Tripeptidyl- Tripeptidyl-peptidase 1 in Brain Cells via Peroxisome Proliferator-activated Receptor α. J Biol Chem. 2012;287(46):38922–38935.
- Kast RE, Karpel-Massler G, Halatsch ME. CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget. 2014;5(18):8052–8082.
- H.R.6 - 21st Century Cures Act. Congress.gov, 2015. [ cited 2015 Nov 11]. Available from: https://www.congress.gov/bill/114th-congress/house-bill/6.