ABSTRACT
Gemtuzumab ozogamicin, an anti-tumour antibiotic linked to an anti-CD33 antibody (Mylotarg®), has been well studied in AML in adults but to a lesser extent in children. No review has yet been published on the dose-related toxicity and efficacy of gemtuzumab ozogamicin in pediatric AML patients. Here we looked at 14 studies then scatterplots and linear regressions were used to estimate the relationship between the dose of gemtuzumab and its toxicity and efficacy. A non-significant increase in bilirubin level and in incidence of veno-occlusive disease was seen with higher doses of gemtuzumab ozogamicin when used as single-agent. In terms of efficacy, even a low dose of 3 mg/m2 of gemtuzumab ozogamicin can have antileukemic effect, but available data do not allow conclusions on its dose-dependency. Data indicate that higher doses of gemtuzumab ozogamicin account for more adverse events. The data do not show that a high dose is required for anti-leukemic efficacy of gemtuzumab ozogamicin. This study also indicates that there seems to be a role for gemtuzumab ozogamicin in the treatment of pediatric AML and further studies are required to assess its optimal dose, schedule and balance between efficacy and side-effects.
Introduction
The prognosis of acute myeloid leukemia (AML) in children has improved tremendously during the last decades with current survival rates approximating 70% [Citation1]. Unfortunately, one-third of the patients eventually relapse [Citation1–Citation8]. Over the past years, new therapies have been designed to improve the prognosis in AML including the development of antigen-targeted therapies. Gemtuzumab ozogamicin (GO), also known as Mylotarg® (Wyeth-Ayerst Pharmaceuticals, Radnor, PA, USA), consists of a humanized monoclonal IgG4 antibody (hP67.6) against the CD33 antigen linked to a derivative of calicheamicin (N-acetyl-γ calicheamicin 1,2-dimethyl hydrazine dichloride), an antitumor antibiotic [Citation9]. The CD33 surface antigen is present in approximately 90% of AML cells [Citation10].
GO was first studied in adults before it was used in children. The first study in 1999 in 40 adults, a phase I dose-escalation study, showed that maximum tolerated dose of GO as a single agent was considered to be 9 mg/m2 and resulted in a reduction in bone marrow leukemic blast count to <5% in eight patients (20%). Most reported toxicities were infusion-related chills, fever and myelosuppression. Dose-limiting toxicity was defined as severe marrow hypoplasia of ≤6 weeks duration after a single dose of GO, one grade IV study drug-related toxic effect or two grade IV toxic conditions of ambiguous relationship to GO [Citation11].
Based on the results of this study, an open-label multicenter phase II trial with GO at 9 mg/m2 was started in ≤200 adults with AML. The overall response rate was 30%. The most frequent side effect was severe myelosuppression, and about 30% of the patients developed grade 3 or 4 treatment-related infections. Furthermore, some patients developed an increase of bilirubin (23%) and transaminases (17%). Abnormalities of liver function were generally transient and reversible. Veno-occlusive disease (VOD) occurred in seven patients (3%), of whom two patients deceased [Citation12]. VOD is the most severe adverse event of GO. Because of the concerns in adults regarding liver toxicity using higher doses of GO in combination with other cytotoxic agents, subsequent pediatric and adult trials have reduced the dose of GO and it was advised to have an interval between GO and stem cell transplantation of at least 3.5 months [Citation13]. Larson et al. reported in 2005 that 0.9% of 277 patients in their study developed VOD [Citation14].The single-agent use of GO resulted in a 26% remission rate with no major side effects. McKoy et al. reported that of adult AML patients who received GO in clinical trials, in total 3% experienced VOD when given GO ≤ 6 mg/m2, administered as monotherapy or in combination with nonhepatotoxic agents. On the other hand, when GO was combined with thioguanine, 28% experienced VOD [Citation14,Citation15].
GO was approved by the US FDA in 2000 for its use in patients with relapsed AML being 60 years or older, but withdrawn in 2010 by Pfizer (previously Wyeth) because of adverse events and doubts regarding its effectiveness in a pivotal study in which mortality with gemtuzumab was 5.7% compared to 1.4% without the agent [Citation16]. Since then, studies in newly diagnosed AML in adults have regenerated interest in the compound [Citation17–Citation20].
In 2011, Burnett et al. reported from the Medical Research Council AML 15 trial that a significant proportion of younger patients, especially patients with favorable cytogenetics, with AML had improved survival rate with the addition of GO to induction chemotherapy [Citation17]. Another study from Burnett et al. reported in 2012 shows similar data in another group of patients, that is, elderly patients with AML. The authors concluded that adding GO at a dose of 3 mg/m2 to induction chemotherapy reduces relapse risk and improves chances of survival with little additional toxicity [Citation18].
In 2012, Castaigne et al. reported on a randomized, open-label, phase 3 study. They investigated whether the addition of five doses of intravenous GO (3 mg/m2) to the standard frontline chemotherapy would improve the outcome of patients with AML without causing excessive toxicity. After 2 years, significant improvement was found in relapse-free survival, event-free survival (EFS) and overall survival. Hematological toxicity, especially prolonged thrombocytopenia, was significantly more common in the GO group than in the control group [Citation19].
These studies warrant reassessment of GO as frontline therapy for AML in adults and suggest the usefulness of this drug in pediatric AML as well. Currently, at least four trials on GO, (also) enrolling children, are still active ().
Table 1. Overview of ongoing clinical trials of gemtuzumab ozogamicin in AML, also enrolling children.
In 2003, the first data on the use of GO in children were published by Zwaan et al. They treated 15 children on a compassionate-use basis with different doses of GO, ranging from 4 to 9 mg/m2 [Citation21]. Several studies about the use of GO in children have been reported since then. {Gamis, 2014 112 /id;de Vries, 2012 18 /id;Loke, 2015 116 /id} In pediatric trials, different schedules have been used to diminish the risk of complications, but an optimal dose and schedule has yet to be established. No review has been published so far on the dose-related toxicity and efficacy of GO in pediatric AML. We, therefore, reviewed and compared all eligible and published studies regarding the use of GO in children in relationship to its dose-related toxicity and efficacy.
Search strategy
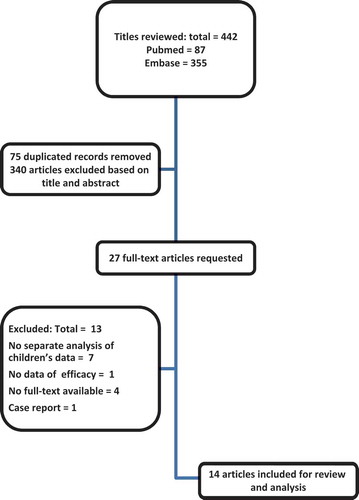
We have used PubMed and Embase electronic databases, with no restrictions on date of publication, to identify studies about the dose-related toxicity and efficacy of GO in children. The keywords combination we have applied is shown in . To broaden our search strategy, we applied not only GO, but also Mylotarg. No limits were used to broaden our search strategy. All articles until 24 February 2015 were included in the search. Also, bibliographies of the obtained articles were screened for relevant publications. In total, 442 abstracts from two databases were screened, 87 from PubMed and 355 from Embase.Twenty-seven articles were included based on their abstracts. After reading full-text articles, 14 studies were included in this study [Citation4,Citation21–Citation33].
Table 2. Search terms electronic databases.
Selection criteria
Studies were first selected based on their title. After initial selection, inclusion criteria were children from 0 to 18 years old and studies about the use of GO in AML. Exclusion criteria were no separate data on GO, no separate data on children, case reports, no English full-text available, studies on other diseases than AML, and no toxicity or efficacy data available. shows the selection process. In total, 340 articles were excluded based on title or abstract; 29 articles were excluded because of lack of clinical data, 21 articles showed no separate data on children, 3 articles were not published in English, 214 articles had no separate data on GO, 15 articles contained only data about VOD, 52 articles were about other diseases than AML and 6 studies had no efficacy data.
Statistical analysis and definitions
All extracted data were analyzed with SPSS Statistics 20 [Citation34]. Complete remission (CR) was defined as bone marrow blasts <5%, absence of blasts with Auer rods, absence of extramedullary disease, absolute neutrophil count ≤1.0 × 109/L (1000/µL), platelet count ≤100 × 109/L (100,000/µL) and independence of red cell transfusions. CR with incomplete blood count recovery (CRi) was defined as all CR criteria except for residual neutropenia (<1.0 × 109/L (1000/µL)) and/or thrombocytopenia (<100 × 109/L (100,000/µL))[Citation35].
Hyperbilirubinemia was graded according to the grading system of the NCI, the Common Toxicity Criteria for Adverse Events, version 4.0: CTCAEv4. VOD was classified according to the Seattle criteria [Citation36,Citation37]. From previous studies, it is known that VOD is the most severe side effect of GO. Therefore, a special focus was given to VOD as toxicity. To determine which variables are likely to be confounders or effect modifiers of the incidence of VOD, we carried out univariate linear regression analyses to determine which variables were most significantly associated with toxicity of GO. First of all, we determined with Spearman’s rho whether the total single and cumulative dose of GO had a causal relationship with the incidence of VOD and bilirubin elevation.
Scatterplots were used to identify any relationship between the dose of GO and its toxicity and efficacy. Also, linear regressions were estimated to establish which variables had an impact on the toxicity and efficacy of GO. Furthermore, toxicity and efficacy were separated between single-agent therapy and combination therapy. Combination therapy is defined as other chemotherapeutics used in the same regimen as GO. Other chemotherapeutics could have been used previously or after GO administration, defined as not in the same course with other chemotherapy.
In all tests, a two-sided p-value of <0.05 is considered statistically significant.
Results
We evaluated all studies on the use of GO in pediatric AML. In total, 14 articles met our eligibility criteria. Because of heterogeneity between the studies, data could not be pooled. In total, data from 640 patients were analyzed. We evaluated both the toxicity and the efficacy of GO. In and , an overview of the most important variables of all eligible studies is listed.
Table 3. Overview of the most important variables of all eligible studies in pediatric AML, single-agent therapy.
Table 4. Overview of the most important variables of all eligible studies in pediatric AML, combination therapy.
Toxicity
Bilirubin elevation
Eleven studies mentioned the incidence of bilirubin elevation [Citation4,Citation20,Citation22,Citation25,Citation26,Citation28–Citation31,Citation38,Citation39]. First, single-agent therapy of GO will be discussed, followed by the use of GO in combination with other chemotherapeutics.
Single-agent therapy
With increasing single dose of GO increases, bilirubin level remains more or less similar (r2 = 0.002, p = 0.934). This pattern is also seen for cumulative dose of gemtuzumab (r2 = 0.002, p = 0.934).
Combination therapy
With GO in combination with other chemotherapy, absolute single dose of GO does not significantly influence bilirubin level (r2 = 0.365, p = 0.113) nor does cumulative dose (r2 = 0.416, p = 0.084).
There were no studies that used >12 mg/m2 of GO as a cumulative dose in combination with other chemotherapeutics.
Veno-occlusive disease
All 14 eligible studies contained data on the incidence of VOD. Incidence of VOD ranged from 0% to 24% in the selected studies. In total, 39 out of 640 (6.1%) patients developed VOD. First, single-agent therapy of GO will be discussed, followed by the use of GO in combination chemotherapy.
Single-agent therapy
As a single agent, GO has been used with a dose ranging from 2 to 9 mg/m2 and with different schedules.
When absolute single dose of GO increases, incidence of VOD also increases (r2 = 0.476, p = 0.040) (). This pattern is also seen when cumulative dose of gemtuzumab is higher (r2 = 0.476, p = 0.040) (). VOD incidence in the high single-dose group (arbitrarily defined as ≤6 mg/m2) was not significantly higher than in the low single-dose group (U = 3.000, p = 0.090).
Figure 2. Scatterplot for the single dose of gemtuzumab ozogamicin in relation to incidence of VOD. Single-agent therapy.
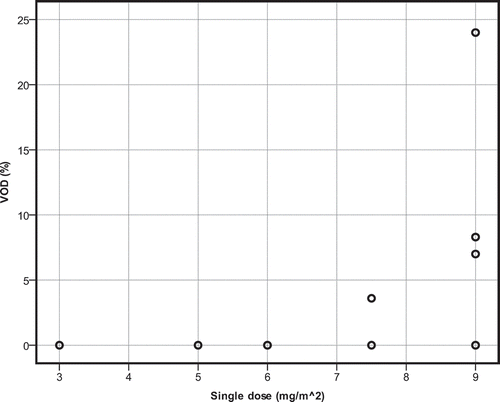
Figure 3. Scatterplot for the cumulative dose of gemtuzumab ozogamicin in relation to incidence of VOD. Single-agent therapy.
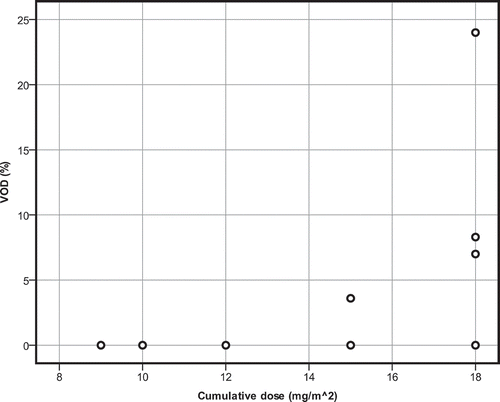
High cumulative dose (arbitrarily defined as a dose ≤12 mg/m2) did not result in higher VOD incidence than in the low-dose group (U = 3,000; p = 0.090).
Combination therapy
GO is occasionally combined with other chemotherapeutics, such as fludarabine, anthracyclines, cyclophosphamide, busulfan and cytarabine [Citation22,Citation23,Citation30]. When single dose of GO as part of combination therapy increases, incidence of VOD does not change significantly (r2 = 0.023, p = 0.675) () also not for total dose of gemtuzumab (r2 = 0.159, p = 0.253) ().
Figure 4. Scatterplot for the single dose of gemtuzumab ozogamicin in relation to incidence of VOD. Combination therapy.
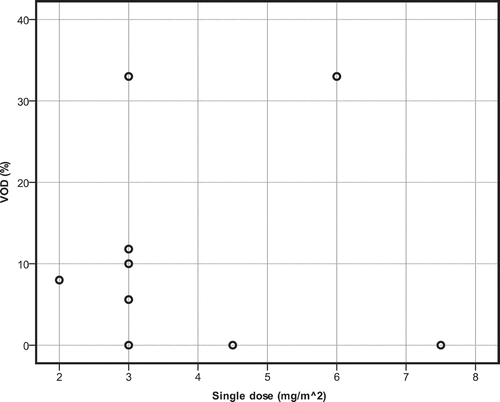
Figure 5. Scatterplot for the cumulative dose of gemtuzumab ozogamicin in relation to incidence of VOD. Combination therapy.
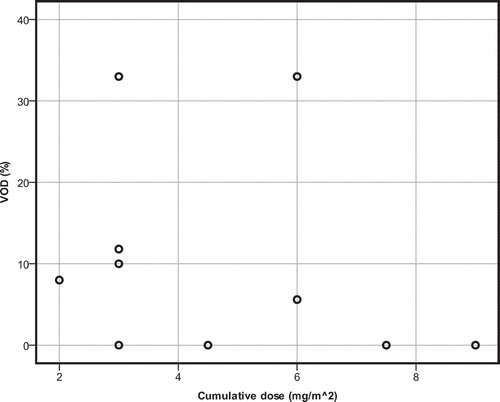
It should be noted that median total cumulative dose of GO combined with other chemotherapeutics is 6 mg/m2 (range 2–18 mg/m2) and when used as single agent is 18 mg/m2 (range 9–18 mg/m2). Both single dose and cumulative dose are respectively (p = 0.004 and 0.000) lower when used in combination chemotherapy than as a single agent.
Hematological toxicity
Hematological toxicity is one of the most common adverse events when GO is given. Four studies reported on the incidence of neutropenia [Citation4,Citation26,Citation30,Citation38]. Median incidence of neutropenia was 98% with a range from 40% to 100%. Median incidence of grade III/IV leucopenia was 71% (range 48–94%), and median incidence of grade III/IV thrombocytopenia was 37% (range 18–97%). Due to differences in chemotherapy and the interval between given chemotherapeutics, no relationship with the schedule of GO could be identified.
Efficacy
To estimate the efficacy of GO, CR and CRi were used as defined above. Remission rates varied between 17% and 100% among the different studies.
Single-agent therapy
The median remission rate for GO used as a single agent is 33% (range 17–100%). Median remission rate for a low cumulative dose of GO (arbitrarily defined as a dose ≤ 12 mg/m2) is 25% versus 35% (range 17–100%) for high cumulative dose (U = 1,000, p = 0.317). Since Roman et al.’s and Hasle et al.’s are post-consolidation studies, these studies were not taken into account in this section.
Combination therapy
When GO is combined with other chemotherapeutics, the median remission rate is 59% (range 40–100%). No study used ≤6 mg/m2 of GO as cumulative dose.
Discussion
In this systematic review of 14 studies including 640 patients on the use of GO in children with AML, we aimed to identify the relationship between the dose of GO and its toxicity and efficacy.
Since its approval in 2000 by the FDA, many studies on GO have been performed, and despite the withdrawal of GO in 2010 from the US market, studies on GO are still ongoing and very encouraging efficacy data have recently been published reporting both on adult and childhood AML [Citation17–Citation20]. In 2014, Gamis et al. published a study on the addition of GO to standard chemotherapy in children and adolescents and found an improved EFS through reduction of relapse risk in recipients of GO. This study could not be included since separate data in children were lacking. However, this study emphasizes the benefit of GO [Citation38].
Grade III/IV bilirubin elevation was frequently observed. However, a higher dose of GO was not associated with bilirubin elevation.
VOD is the most serious adverse event. Most studies used the Seattle criteria to define VOD. Since the Seattle criteria do not necessarily require hyperbilirubinemia to be present, we would advise to use the modified Seattle criteria to define VOD instead of the more stringent Baltimore criteria. We here show that there is a significantly increased incidence of VOD, when single dose and cumulative dose of GO are higher, when used as a single agent. In contrast, when the cumulative total dose of GO used as a part of combination therapy is higher, VOD incidence does not increase. This may be explained by a lower absolute dose of GO when it is used as combination therapy.
In this review, incidence of VOD ranged from 0% to 24% in individual studies with a total of 39 out of 640 patients (6.1%). In previous studies, it was found that not only GO can contribute to this side effect. Also, the combination of GO with thioguanine, busulfan or stem cell transplantation can account for the occurrence of VOD [Citation40].
In most of our selected studies, treatment with GO was followed by autologous or allogenic (matched unrelated donor) stem cell transplantation. Because indications for stem cell transplantation nor conditioning regimens were identical, no statistical analysis could be performed on these data. In previous studies, it was reported that stem cell transplantation within 30 days of gemtuzumab administration may result in a higher risk of developing VOD [Citation13,Citation33]. On the other hand, GO has been safely used as part of the conditioning regimen [Citation41]. It is possible that our data on toxicity and efficacy of GO have been influenced by stem cell transplantation.
Thrombocytopenia and neutropenia have been frequently observed after GO-based regimens, and these adverse events may lead to alterations in the therapeutic schedule [Citation3]. The incidence of neutropenia ranged from 40% to 100% (median incidence of 98%) in the 14 studies reviewed here. Similarly, the incidence of thrombopenia ranged from 18% to 97% (median incidence 37%). It appeared impossible to investigate the relationship, if any, between the dose of GO and hematological toxicity.
Since GO includes an antibody against the CD33 surface antigen, the correlation between the expression of CD33 in individual patients and their response to GO would have clinical implications. Pollard et al. investigated the relationship between expression of CD33 and the overall survival. They reported that high expression of CD33 is associated with an inferior outcome. Nowadays, the correlation between CD33 expression and GO response is being studied. Also, CD33 saturation is an important factor when considering the pharmacokinetics of GO and required doses [Citation42]. In this review, not all included studies mentioned the mean expression of CD33, so no conclusions could be drawn regarding this relationship. Buckwalter et al. found that the pharmacokinetics of GO in pediatric patients are similar to the profile and variability of adult patients [Citation43]. Gemtuzumab concentrations resulting in near-complete CD33 saturation levels, defined as ≤90%, are required for efficient cell kill. Data from van der Velden et al. indicate that a high peripheral CD33-antigen load utilizes a large part of the gemtuzumab dose, which results in reduced gemtuzumab penetration in bone marrow and subsequently in a less efficient cell kill. The authors suggest that better CD33 saturation can be realized by increasing the dose to 9 mg/m2 or by giving repeated fractionated doses at subsequent days [Citation44,Citation45]. However, 9 mg/m2 gives more severe side effects as shown in this review. Fractioned therapy has been given in some studies, but no conclusion can yet been drawn about whether side effects occur less often when gemtuzumab is given as such.
In our study, median remission rate of gemtuzumab combined with other chemotherapeutics was 59%, when single-agent therapy shows a median remission rate of 33%. This difference can be explained by the addition of other antileukemic agents that influence the remission rate. Since incidence of VOD does not increase with higher doses of GO combined with other chemotherapeutics, we would advise to use gemtuzumab in combination with other chemotherapeutics. Furthermore, our study shows that even a low dose of 3 mg/m2 can have an antileukemic effect and does not cause excessive incidence of VOD.
In conclusion, further research is warranted to investigate the role of GO in the treatment of pediatric AML. Future studies should focus on the most effective and least toxic dose and schedule of GO and fractioning of GO.
Expert commentary
The treatment and outcome of AML has made tremendous progress in recent years. Survival rates have increased, and there is now more interest in the side effects of currently used drugs. Treatment of AML still needs further improvement as survival rates are approximately 70%, mainly due to relapse. Treatment of AML continues to improve since new therapies are being designed for both initial disease and relapsed AML and international collaboration gives the opportunity to perform randomized clinical trials. Fortunately, more interest is shown in the side effects and late effects of therapy, which can be life-threatening.
Five-year view
In recent years, antigen-targeted therapies such as GO have been developed and today’s research focuses on the development of new antigen-targeted therapies. GO has been on the market from 2000 until its withdrawal in 2010. Currently, some hospitals still use GO as part of their treatment for AML. Currently, there are four pediatric trials enrolling patients in studies where GO is being used as part of chemotherapy treatment. We believe that in 5 years time gemtuzumab will have recaptured a position in the chemotherapy treatment of AML in children. We expect that GO will have proven its beneficial role in the treatment of AML in children and hopefully also in adults. We hope future trials on GO will focus on the frequency and dose of GO as well.
Key issues
Gemtuzumab ozogamicin has been on the market from 2000 until its withdrawal in 2010 for acute myeloid leukemia (AML) in adults.
Veno-occlusive disease (VOD) is the most feared side effect of gemtuzumab ozogamicin.
No study has yet reviewed the dose-related toxicity and efficacy of gemtuzumab ozogamicin in children.
Fourteen pediatric studies have been included in this review to investigate the dose-limited toxicity and efficacy of gemtuzumab ozogamicin as single agent and in combination therapy.
A nonsignificant increase in bilirubin and VOD was seen with higher doses of gemtuzumab ozogamicin.
Even a low dose of 3 mg/m2 can have an antileukemic effect.
Only 6.1% patients developed VOD.
Further research is warranted to investigate the role of gemtuzumab ozogamicin in pediatric AML.
Studies should focus on the most effective and least toxic dose of gemtuzumab ozogamicin.
Financial & competing interests disclosure
CM Zwaan has served as a consultant for Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Kaspers GJ. Pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2012;12(3):405–413.
- Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27(24):4007–4013.
- Sievers EL. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukaemia in first relapse. Expert Opin Biol Ther. 2001;1(5):893–901.
- Aplenc R, Alonzo TA, Gerbing RB, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(14):2390–3295.
- Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–552.
- Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29(3):310–315.
- Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155(3):366–376.
- Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607.
- Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011;17(20):6417–6427.
- Legrand O, Perrot JY, Baudard M, et al. The immunophenotype of 177 adults with acute myeloid leukemia: proposal of a prognostic score. Blood. 2000;96(3):870–877.
- Sievers EL, Appelbaum FR, Spielberger RT, et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93(11):3678–3684.
- Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23(1):11–25.
- Wadleigh M, Richardson PG, Zahrieh D, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102(5):1578–1582.
- Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104(7):1442–1452.
- McKoy JM, Angelotta C, Bennett CL, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31(5):599–604.
- Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860.
- Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377.
- Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–3931.
- Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516.
- Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–996.
- Zwaan CM, Reinhardt D, Corbacioglu S, et al. Gemtuzumab ozogamicin: first clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate-use basis. Blood. 2003;101(10):3868–3871.
- Hasle H, Abrahamsson J, Forestier E, et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. 2012;120(5):978–984.
- Yoshida N, Sakaguchi H, Matsumoto K, et al. Successful treatment with low-dose gemtuzumab ozogamicin in combination chemotherapy followed by stem cell transplantation for children with refractory acute myeloid leukaemia. Br J Haematol. 2012;158(5):666–668.
- Satwani P, Bhatia M, Garvin JH, et al. A phase I Study of Gemtuzumab Ozogamicin (GO) in combination with Busulfan and Cyclophosphamide (Bu/Cy) and allogeneic stem cell transplantation in children with poor-risk CD33+ AML: a new targeted immunochemotherapy myeloablative conditioning (MAC) regimen. Biol Blood Marrow Transplant. 2012;18(2):324–329.
- Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118(3):761–769.
- Reinhardt D, Zwaan CM, Sander A, et al. Gemtuzumab ozogamicin in refractory childhood acute myeloid leukemia. Blood. 2010;116(21):1075.
- Sayar D, Burstein Y, Bielorai B, et al. Upfront use of gemtuzumab ozogamicin in young children with CD33-positive AML. Pediatr Blood Cancer. 2010;55(1):183–185.
- Zwaan CM, Reinhardt D, Zimmerman M, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a phase II study. Br J Haematol. 2010;148(5):768–776.
- Sibson K, Steward C, Moppett J, et al. Dismal long-term prognosis for children with refractory acute myeloid leukaemia treated with gemtuzumab ozogamicin and stem cell transplantation: where now? Br J Haematol. 2009;146(3):342–344.
- Brethon B, Yakouben K, Oudot C, et al. Efficacy of fractionated gemtuzumab ozogamicin combined with cytarabine in advanced childhood myeloid leukaemia. Br J Haematol. 2008;143(4):541–547.
- Brethon B, Auvrignon A, Galambrun C, et al. Efficacy and tolerability of gemtuzumab ozogamicin (anti-CD33 monoclonal antibody, CMA-676, Mylotarg) in children with relapsed/refractory myeloid leukemia. BMC Cancer. 2006;6:172.
- Roman E, Cooney E, Harrison L, et al. Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin Cancer Res. 2005;11(19 Pt 2):7164s–70s.
- Arceci RJ, Sande J, Lange B, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106(4):1183–1188.
- IBM Corp. IBM SPSS statistics for Windows. Version 20.0. Armonk, NY: IBM Corp.; 2011.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649.
- McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267.
- Bearman SI. Veno-occlusive disease of the liver. Curr Opin Oncol. 2000;12(2):103–109.
- Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–3032.
- De Vries JF, Zwaan CM, De BM, et al. The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia. 2012;26(2):255–264.
- Pai RK, van BK HJ, Artz AS, et al. Clinicopathologic features of late-onset veno-occlusive disease/sinusoidal obstruction syndrome after high dose intravenous busulfan and hematopoietic cell transplant. Leuk Lymphoma. 2012;53(8):1552–1557.
- Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008;36(7):755–768.
- Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119(16):3705–3711.
- Buckwalter M, Dowell JA, Korth-Bradley J, et al. Pharmacokinetics of gemtuzumab ozogamicin as a single-agent treatment of pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Pharmacol. 2004;44(8):873–880.
- van der Velden VH, Boeckx N, Jedema I, et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia. 2004;18(5):983–988.
- van der Velden VH, Te Marvelde JG, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97(10):3197–3204.