Prophylactic infectious disease vaccines represent one of the greatest successes of modern medicine. There are significant opportunities to harness the power of the immune system to cure cancer. However, the complexity of tumor biology, including the ability of transformed cells to react to stress through a multiplicity of signaling pathways and the diversity of immunotherapeutic interventions that are known to alter the etiopathogenesis of cancer, provide difficulties in identifying a precise molecular basis for tumor-specific immunity and rejection. The finding that cancer regression can be achieved by immune rejection of tumor antigens theoretically allows the long-term eradication of neoplastic cells without toxicity to normal tissues. While trials of cancer vaccines have failed to achieve overall survival (OS) or time-to-progression (TTP) benefits in prespecified intent-to-treat populations, subgroup analyses across studies suggest that they have their greatest benefits when the tumor antigen load is low. As a consequence, more weight should be given to subgroup analyses, particularly in the adjuvant setting where there is a strong mechanistic rationale for use of such therapies in addition to standard of care.
In 1908, Paul Ehrlich and Ilya Ilyich Mechnikov were awarded the Nobel prize for their hypothesis of the immune surveillance theory of cancer, in which they suggested that the immune system continually removes tumors that arise spontaneously (immune escape theory) Citation[1,2]. Their theories lay largely dormant until it was noticed, approximately 50 years later, that Kaposi’s sarcoma occurred in renal allograft recipients and these tumors regressed on cessation of immunosuppression Citation[3–7].
Interestingly, Ludwik Gross, George Klein and others had by then demonstrated the extraordinary phenomenon that one could immunize against tumors in the same manner, as was being performed with remarkable success against polio and smallpox Citation[8,9]. In contrast to viruses, the tumors were of self-origin but, like viruses, they were immunogenic. Inherent in this observation was the prediction that tumors express tumor-specific antigens. Burnett also suggested that transplantation antigens expressed on tumor cells could stimulate the immune system, leading to the generation of protective immunity Citation[10,11].
In 1983, the New York Times headline ‘Rare cancer seen in 41 homosexuals’ formed part of the events that heralded the onset of the HIV epidemic Citation[12]. The increased risk of cancers in patients with iatrogenic, primary or acquired immunosuppression has firmly established a role for the immune system in the control of cancer Citation[13,14].
Cancer vaccine studies
Advances in genomics and proteomics have enabled improved antigen selection in therapeutic cancer vaccine studies, which have been undertaken at all stages of disease . Conversely, vaccine development in oncology has been characterized by clinical failures, in particular, the failure to meet prespecified end points . Specifically, the most recent Phase III cancer vaccine trial failures were as follows:
| • | Therion’s (MA, USA) Panvac™-VF failed pancreatic Phase III trial, company put itself on the market; | ||||
| • | Aphton’s (PA, USA) Insegia™ (G17DT) failed pancreatic Phase III trial, no OS benefit, filed with noncompliance; | ||||
| • | CancerVax’s (CA, USA) Canvaxin™-melanoma Phase III, no OS benefit versus placebo, discontinued all further development and manufacturing, subset analysis showed benefit; | ||||
| • | Biomira’s (NJ, USA) Theratope® breast cancer Phase III, failed to meet end points of improving TTP and OS, partner Merck (NJ, USA) pulled out; | ||||
| • | Titan’s (CA, USA) CeaVac® and chemotherapy did not meet OS in Phase III trial for metastatic colorectal cancer; | ||||
| • | Genitope’s (CA, USA) MyVax® has not met primary end point of progression-free survival (PFS) in Phase III in non-Hodgkin’s lymphoma. | ||||
On the one hand, suboptimal product development (e.g., clinical trial design and patient selection) has led to poor clinical trial results. Conversely, it is agreed that earlier-stage patients are more likely to benefit but, paradoxically, the focus of most vaccine development has been in late-stage disease. Stage IV disease may complicate trial outcomes and reported efficacy owing to the unpredictable and rapid disease progression of that patient population, but results are often available sooner in trials of this population as end points occur faster. Vaccines will probably be beneficial in patients with minimal residual disease when there is less disease bulk, immune tolerance is less likely to have developed and immunosuppressive systemic therapies have yet to be administered. Indeed, it is difficult to provide an accurate assessment for a therapy designed to boost immunity when the immune system is already compromised Citation[15–19].
As we know that cancer vaccines do not usually induce dramatic tumor responses, patients most likely to benefit are those with totally resected disease with a high risk of relapse. These include, for example, patients with stage III melanoma or renal cancer without vascular invasion, and with minimal tumor volumes Citation[20]. Although institutional variability in patient staging and grading leads to heterogenous populations (and this can be improved upon), in general, the lower the tumor burden, the more likely a vaccine is to be successful.
Future directions
Therapeutic cancer vaccine studies should occur in the adjuvant setting. Since relapse rates are often low here, timelines are long and there is high commercial risk inherent in this approach. By using later-stage patients in clinical trials, results may be achieved in a shorter time period, but efficacy has not been convincingly demonstrated here in the major subsets in any of the studies Citation[18,21]. There remains a lack of consensus of defined surrogate markers, but accurate biomarkers could reduce the time required to address a vaccine’s clinical activity at the Phase II stage, before progressing to Phase III trials. A good example here would be the use of real-time or multimarker reverse transcriptase polymerase chain reaction technology to detect minimal residual disease Citation[22,23], or confirm molecular elimination.
There are increasing data showing that immune monitoring may constitute a viable clinical trial end point in cancer vaccine development and several vaccines have demonstrated some benefits in certain subsets, as follows:
| • | GV1001 demonstrated a large survival advantage in pancreatic cancer in small Phase I/II trials over Gemzar® (results need to be replicated in Phase III setting) | ||||
| • | Biomira’s (Merck) L-BLP25 (Stimuvax®) improvesd survival versus best supportive care in Phase IIb trials in Stage IIIb or IV nonsmall cell lung cancer (NSCLC) after first-line therapy (received single dose of cyclophosphamide); patient groups were too small to be significant. New Phase III trial focuses on Stage IIIa patient group (in combination with chemotherapy and radiotherapy) | ||||
| • | Oncophage demonstrated benefit in Phase III Melanoma trial in Stage M1a–1b subsets; | ||||
| • | Oncophage demonstrated benefit in relapse-free survival (RFS) in Phase III trial of renal cell carcinoma in early stage patients; | ||||
| • | Provenge® (Dendreon) demonstrated improved survival in prostate cancer, including an increased median survival in patients with Gleason Score of less than 7 in a Phase III trial; | ||||
| • | Therion’s ProstVac®-VF demonstrated a trend in survival advantage in hormone-refractory prostate cancer (HRPC) patients not taking bisphosphonates at baseline (early stage patients). Study did not meet its end point; | ||||
| • | Liponova’s Renial® demonstrates benefit in T3 patients (intermediate risk) renal cell cancer ; | ||||
| • | Intracel’s Oncovax® demonstrates improved survival in Phase III trial in stage II colon cancer patients. Reached agreement with the US FDA; | ||||
| • | CancerVax’s Canvaxin demonstrated a 5-year survival rate of 70% and median survival of more than 76 months compared with 8% and 19 months in patients showing an immunoglobulin M antibody response to antigen TA90. | ||||
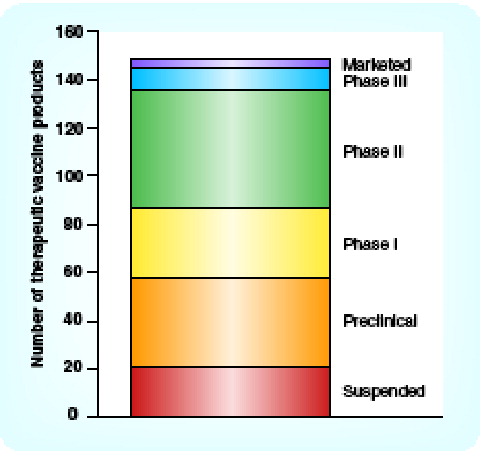
The therapeutic vaccine pipeline shows strong progression up to Phase II but a low success rate from Phase II onwards. As for the recent FDA workshops, most recently on ovarian cancer trial end points, consensus is required for end points in Phase III vaccine studies that should, in some way, give companies an incentive to undertake such trials, due to the time required to yield a result. It is unlikely that companies will perform 5-year adjuvant studies to identify patients in a subgroup likely to benefit and then spend a further 5 years demonstrating efficacy in that subgroup. The lack of regulatory guidance in trial designs leads to variability and the vague direction of comparator arms can lead to inability to compare with control groups.
Conclusions
Strategic planning is required, including manufacturing considerations, in order to realize a standardized and reproducible production process, especially for autologous formulations. Such standardization should help ease regulatory concerns and facilitate commercialization. A single cancer vaccine is likely to constitute several different components that are developed from several different technologies and, as it is highly unlikely that a single company owns the various relevant components and technologies, collaboration between companies to prevent relapse of cancer will be critical to facilitate development and reduce costs. The lack of precedent within the cancer market indicates that a consistent assessment protocol needs to be established to facilitate approval. Coordinated efforts between the major regulatory bodies and cancer vaccine researchers should occur to aid realization of this goal and this should ideally include prespecification of the more obvious subgroup analyses. When this has not occurred, larger studies with low event rates should be carefully examined to determine whether or not there are true benefits in certain individuals.
On a futuristic note, advances in genomic sequencing should allow the identification of relevant mutated unique personalized antigenic epitopes, which may be synthesized and then used to immunize that particular patient.
Table 1. Randomized Phase II and III immunotherapies.
References
- Sulek K. Prize in 1908 awarded to P Ehrlich and E Metchnikoff for their work in immunology. Wiad. Lek.20, 1117–1118 (1967).
- Ehrlich P. The partial function of cells: Nobel prize address given on 11 December 1908 at Stockholm. Int. Arch. Allergy Appl. Immunol.5, 67–86 (1954).
- Siegel JH, Janis R, Alper JC, Schutte H, Robbins L, Blaufox MD. Disseminated visceral Kaposi’s sarcoma. Appearance after human renal homograft operation. JAMA207, 1493–1496 (1969).
- Myers BD, Kessler E, Levi J, Pick A, Rosenfeld JB, Tikvah P. Kaposi sarcoma in kidney transplant recipients. Arch. Intern. Med.133, 307–311 (1974).
- Penn I. Kaposi’s sarcoma in organ transplant recipients: report of 20 cases. Transplantation27, 8–11 (1979).
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N. Engl. J. Med.348, 1681–1691 (2003).
- Stebbing J, Bower M. What can oncologists learn from HIV? Lancet Oncol.4, 438–445 (2003).
- Klein G, Sjorgen HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res.20, 1561–1562 (1960).
- Klein G. Tumor antigens. Annu. Rev. Microbiol.20, 223–252 (1966).
- Burnet FM. Immunological aspects of malignant disease. Lancet1, 1171–1174 (1967).
- Burnet FM. Immunological surveillance in neoplasia. Transplant Rev.7, 3–25 (1971).
- Bayer R, Oppenheimer GM. AIDS Doctors, Voices from the Epidemic: an Oral History. Oxford University Press, Oxford, UK (2002).
- Stebbing J, Portsmouth S, Bower M. Insights into the molecular biology and sero-epidemiology of Kaposi’s sarcoma. Curr. Opin. Infect. Dis.16, 25–31 (2003).
- Stebbing J, Bower M, Srivastava P. Kaposi’s sarcoma as a model for cancer immunotherapy. Trends Mol. Med.10, 187–193 (2004).
- Whelan M, Whelan J, Russell N, Dalgleish A. Cancer immunotherapy: an embarrassment of riches? Drug Discov. Today8, 253–258 (2003).
- Ward S, Casey D, Labarthe MC et al. Immunotherapeutic potential of whole tumour cells. Cancer Immunol. Immunother.51, 351–357 (2002).
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol.3, 630–641 (2003).
- Dalgleish AG, Whelan MA. Cancer vaccines as a therapeutic modality: the long trek. Cancer. Immunol. Immunother.55, 1025–1032 (2006).
- Slingluff CL Jr, Engelhard VH, Ferrone S. Peptide and dendritic cell vaccines. Clin. Cancer Res.12, S2342–S2345 (2006).
- Sondak VK, Sabel MS, Mule JJ. Allogeneic and autologous melanoma vaccines: where have we been and where are we going? Clin. Cancer Res.12, S2337–S2341 (2006).
- Labarthe MC, Halanek N, Birchall L et al. The biological effects of syngeneic and allogeneic cytokine-expressing prophylactic whole cell vaccines and the influence of irradiation in a murine melanoma model. Cancer. Immunol. Immunother.55, 277–288 (2006).
- Schnittger S, Schoch C. Quantitative PCR based minimal residual disease detection in core binding factor leukemias: prognostication and guiding of therapy. Leuk. Res.30, 657–658 (2006).
- Ireland R. Multimarker RT-PCR for detection of minimal residual disease in breast cancer patients. Nat. Clin. Pract. Oncol.3, 293 (2006).