Abstract
Using evidence from short-term randomized controlled trials, decision-analytic models project costs, risks and benefits of disease-modifying therapies (DMTs) for multiple sclerosis (MS). Such trial-informed models lack the breadth needed to generalize to clinical practice or policy due to limitations: lack of DMT switching/discontinuation, limited head-to-head DMT comparisons and efficacy, not effectiveness, designs. We present an illustrative example that incorporates treatment switching and discontinuation by estimating the cost–effectiveness (value) of first-line natalizumab versus second-line natalizumab treatment for relapsing-remitting MS patients negative for anti-JC virus antibodies. Treating JC virus-negative relapsing-remitting MS patients with natalizumab as first-line provided better value compared with second-line. Decision-makers should consider this evidence for treatment step-edit policies through modeling scenarios closer to clinical practice.
Multiple sclerosis (MS) is a chronic and debilitating inflammatory autoimmune disorder of the CNS with a global prevalence of approximately 2.3 million people Citation[1]. Because MS patients have increased healthcare utilization and typically have a long lifespan Citation[2], MS imposes a significant and long-term burden. Disease-modifying therapies (DMTs) have an ultimate treatment goal to delay or prevent the long-term disability of relapsing-remitting MS (RRMS) while minimizing DMT-related risks. The trial-based efficacy of DMTs as compared with placebo or standard of care are relatively well known over short-term 1- to 2-year time horizons in the RRMS population Citation[3].
Using evidence from short-term randomized controlled trials, decision-analytic models are commonly employed to project the risks and benefits of DMTs for RRMS over longer time horizons Citation[4–6]. Such trial-informed models, however, lack the breadth needed to generalize to clinical practice settings due to limitations such as: lack of real-world DMT treatment patterns such as switching between DMTs or discontinuing them, head-to-head comparisons among DMTs and clinical end points of efficacy not effectiveness. One example from clinical practice settings, treatment switching and discontinuation from DMTs has been associated with a significant return in disease activity and increased medical costs Citation[7,8]. Further, many US payers have restricted the access to more costly but highly effective DMTs by requiring step-edits (i.e., prior authorization) through lower cost but less effective DMTs.
To our knowledge, no decision-analytic models have included the impact of treatment switching, while few have included evidence such as adherence-adjusted treatment effects Citation[9] or discontinuation Citation[10,11]. Our objective was to incorporate treatment switching and discontinuation using real-world healthcare resource utilization inputs into a previously published and validated model Citation[12] to estimate the cost–effectiveness of first-line versus second-line natalizumab therapy for RRMS.
Treatment switching & discontinuation case example
In a previous trial-informed model, we evaluated the risks and benefits of first-line natalizumab (Tysabri, Biogen) compared with first-line glatiramer acetate (GA) for a specific MS subpopulation Citation[12]. A full explanation of our model assumptions is out of scope, but briefly, we simulated a cohort of patients who progressed through health states defined by the Expanded Disability Status Scale on clinical benefits, risks and quality-adjusted life years (QALYs). Inputs to the decision-analytic model included relapse and progression treatment effects, time-varying risk and disutility of progressive multifocal leukoencephalopathy (PML) including natalizumab-treated patients that seroconverted to positive anti-JC virus status, and utility inputs for estimating QALYs Citation[12]. Our results favored natalizumab over selected DMTs suggesting more aggressive early intervention with natalizumab in the negative anti-JC virus RRMS population. Our previous trial-informed modeling analysis did not include the impact of switching to or from natalizumab, or discontinuing Citation[12].
In this case example, we expand our previous modeling framework by including costs and treatment switching and discontinuation to estimate lifetime costs, QALYs and incremental cost–effectiveness ratios (ICERs) for first-line natalizumab (i.e., cohort initiates and continues on natalizumab) versus second-line natalizumab (i.e., cohort initiates GA and percent of cohort annually switches to natalizumab) treatment for the average RRMS patients negative for anti-JC virus antibodies . Natalizumab is a more effective but also more costly treatment option as compared with GA. Switching (e.g., lack of effectiveness) or discontinuing from natalizumab (e.g., risk of PML) has been associated with a return in disease activity Citation[13,14]. Additionally, studies have shown the use of natalizumab as a second-line agent (i.e., switching to natalizumab from IFNs or GA) has been effective in improving outcomes for MS patients Citation[15,16].
Figure 1. First-line and second-line natalizumab treatment pathway for JC-virus-negative RRMS patients.
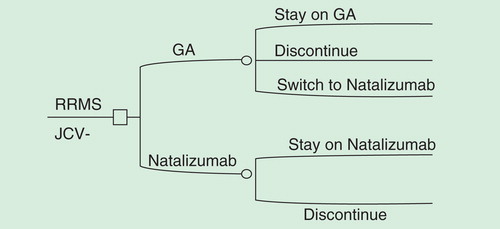
We evaluated real-world clinical practice patterns using the PharMetrics PlusTM claims database. PharMetrics Plus combines data from Blue Health Intelligence for a total of 150 million covered lives; approximately 87 million of those covered lives have both pharmacy and medical coverage across all years. We followed patients with at least one MS diagnosis (ICD-9 340.xx) for 12 months after their first initiation of a DMT (index date) based on a 6-month baseline period of no DMT prescriptions. The percent of subjects switching during the 12-month follow-up period was defined as at least two prescriptions for natalizumab or GA within the year and no 90-day or greater gap on the final DMT at the end of the year. The percentage of subjects who discontinued was based on those who were not persistent on their DMTs during the follow-up period (i.e., discontinue first DMT with no further use during year; discontinue and switch to another DMT but not persistent on second DMT).
Costs were derived using payer and subject paid claims for medical and prescription utilization. Relapse-related costs were defined as paid claims associated with a MS-related hospitalization or MS-related emergency or outpatient visit with intravenous or oral steroid burst claim (methylprednisolone, prednisolone, prednisone or adrenocorticotropic hormone) within 7 days Citation[17]. Previous research has suggested an increase in non-pharmacy-related costs associated with a switch or discontinue Citation[8]. Therefore, we estimated marginal total costs associated with switching or discontinuing during the 12-month follow-up period for the following cohorts: GA switch to natalizumab as compared with persistent GA use (no switch or discontinue); GA discontinue as compared with persistent GA use (no switch); natalizumab discontinue as compared with persistent on natalizumab (no switch). We used generalized linear modeling with log link and gamma error distribution adjusting for baseline costs, severity using the Charlson Comorbidity Index Citation[18], region, gender and insurance type. All costs were inflated to 2012 US dollars using an average of the medical inflation rate over the study period.
The switch and discontinuation rates derived from the claims database were used to create time-varying treatment transitions in the decision-analytic model. From our claims analysis, we found that approximately 7% of subjects were switching from GA to natalizumab, 14% were discontinuing GA and 13% were discontinuing natalizumab during the follow-up period. In the decision-analytic model, 100% of the cohort begins on either GA (natalizumab second-line treatment arm) or natalizumab (natalizumab first-line treatment arm). For the second-line natalizumab arm, we allowed 7% of the cohort to switch to natalizumab so that over the 20-year time horizon the cohort was split to create sub-cohorts of GA users, natalizumab users and best supportive care after discontinuation (i.e., symptom management only). We allowed discontinuation to occur during the first 2 years for both treatment arms of the model because the entire cohort would be exhausted into the best supportive care sub-cohort which is not reflective of clinical practice.
The time-varying transitions between therapies were used to create weighted average estimates of progression and relapse treatment effects, utilities, costs and risks. For example, the progression relative risk (RR) for the second-line natalizumab arm was a function of both GA and natalizumab treatment effects. Over time as more of the cohort switches to the natalizumab sub-cohort, the progression RR gets smaller toward the progression RR of natalizumab and away from the progression RR of GA. Therefore, the progression into worse Expanded Disability Status Scale states (i.e., greater disability) slows as the cohort moves to a majority of natalizumab users as compared with if the cohort was only treated with first-line GA. Irrespective of treatment history, we assumed trial-based treatment effects for each treatment arm.
Analyses yielded deterministic and probabilistic estimates of the cost–effectiveness of first-line versus second-line natalizumab. Compared with natalizumab as second-line treatment after switching from GA, first-line natalizumab treatment was associated with an increase in PML risk of 0.0049 (95% CI: 0.0045, 0.0068) per treated patient, 0.40 (95% CI: 0.008, 0.87) incremental QALYs gained, $36,779 (95% CI: $23,670, $50,786) more in 20-year costs for an ICER of $91,510 (95% CI: $25,923, $713,816) per QALY . Compared with first-line GA treatment without switching, first-line treatment with natalizumab was associated with an ICER of $95,764 (95% CI: $27,074, $858,829) per QALY (likelihood = 0.56 that first-line natalizumab treatment was cost-effective at a willingness-to-pay of $100,000/QALY). First-line natalizumab treatment dominated second-line natalizumab treatment through principles of extended dominance. As compared with GA first line, the ICER for natalizumab second line was greater (i.e. more costs per health unit gained) than the ICER for natalizumab first line.
Figure 2. Efficiency frontier showing results from the 1000 Monte Carlo draws and the base-case incremental cost–effectiveness ratios (ICERs) for each strategy. Natalizumab second-line is subject to extended dominance as the mean ICER lies to the left of the efficiency frontier defined by the line connecting the non-dominated options, natalizumab first-line and GA first-line. As compared with GA first line, the ICER for natalizumab second line was greater (i.e., more costs per health unit gained) than the ICER for natalizumab first line.
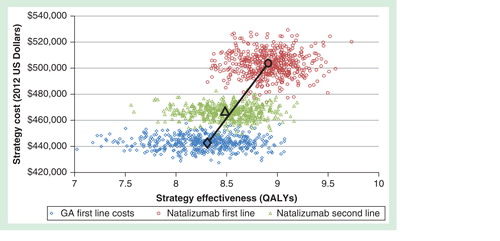
Findings suggest treating JC virus negative RRMS patients with natalizumab as a first-line treatment provided better value for money compared with natalizumab as a second-line treatment. In other words, if a decision-maker is willing to pay $100,000 per QALY, natalizumab provides more health gained per dollar spent with limited increased risk over 20 years when compared with initiating a traditional first-line therapy such as GA and later switching to natalizumab.
Future modeling advancements to consider
The model framework using switching and discontinuation is only one possible expansion on our trial-informed model. We did not incorporate many assumptions that are needed for future effectiveness and cost–effectiveness analyses: regression of disease from the use of highly effective DMTs, observational treatment effects or indirect treatment comparisons from clinical practice and the inclusion of other RRMS subpopulations. While many additional expansions on our assumptions are possible and necessary for future analyses, the model expansion presented here addresses closer to real-world clinical practice questions than our previous trial-informed model.
Past original research and review articles demonstrate the importance of including real-world scenarios or inputs into decision-analytic models Citation[9,19,20]. In RRMS, the rapidly changing treatment landscape serves as an additional motivation to develop and present modeling analyses that are relevant to clinicians and decision-makers. Omission of important clinical practice trends and scenarios in future modeling analyses in RRMS may at best have little relevance for payers and practitioners, and at worse lead to incorrect decisions on the adoption or rejection of a particular DMT.
Acknowledgements
The authors would like to acknowledge RR Allen, Peak Statistical Service, for statistical programming.
Financial & competing interests disclosure
RB McQueen was awarded a PhRMA Foundation Post-Doctoral Fellowship that in part funded this work. TL Vollmer has received consulting fees from separate unrelated work from Consortium of MS Centers, Teva, Novartis, Genentech, Acorda and Biogen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- National Multiple Sclerosis Society. Who Gets MS? (Epidemiology). 2015. Available from: www.nationalmssociety.org/What-is-MS/Who-Gets-MS#section-2
- Prescott JD, Factor S, Pill M, Levi GW. Descriptive analysis of the direct medical costs of multiple sclerosis in 2004 using administrative claims in a large nationwide database. J Manag Care Pharm 2007;13(1):44-52
- Goodin DS, Cohen BA, O’Connor P, et al. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology 2008;71(10):766-73
- Earnshaw SR, Graham J, Oleen-Burkey M, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy 2009;7(2):91-108
- Goldberg LD, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm 2009;15(7):543-55
- Thompson JP, Edwards NC, Fincher C, et al. Quantitative risk-benefit analysis of natalizumab. Neurology 2008;71(5):357-64
- Fox RJ, Salter AR, Tyry T, et al. Treatment discontinuation and disease progression with injectable disease-modifying therapies: findings from the north American Research Committee on multiple sclerosis database. Int J MS Care 2013;15(4):194-201
- Reynolds MW, Stephen R, Seaman C, et al. Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ 2010;13(1):90-8
- Brandes DW, Raimundo K, Agashivala N, et al. Implications of real-world adherence on cost-effectiveness analysis in multiple sclerosis. J Med Econ 2013;16(4):547-51
- Lee S, Baxter DC, Limone B, et al. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ 2012;15(6):1088-96
- Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health 2009;12(5):657-65
- Campbell JD, et al. Comparative effectiveness of early natalizumab treatment in JC virus-negative relapsing-remitting multiple sclerosis. Am J Manag Care 2013;19(4):278-85
- O’Connor PW, McQueen RB, Miravalle A, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011;76(22):1858-65
- Havla J, Gerdes LA, Meinl I, et al. De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 2011;258(9):1665-9
- Castillo-Trivino T, Mowry EM, Gajofatto A, et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One 2011;6(2):e16664
- Lanzillo R, Bonavita S, Quarantelli M, et al. Natalizumab is effective in multiple sclerosis patients switching from other disease modifying therapies in clinical practice. Neurol Sci 2013;34(4):521-8
- Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ 2010;13(4):618-25
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130-9
- Campbell JD, McQueen RB, Briggs A. The “e” in cost-effectiveness analyses. A case study of omalizumab efficacy and effectiveness for cost-effectiveness analysis evidence. Ann Am Thorac Soc 2014;11(Suppl 2):S105-11
- Thompson JP, Abdolahi A, Noyes K. Modelling the cost effectiveness of disease-modifying treatments for multiple sclerosis: issues to consider. Pharmacoeconomics 2013;31(6):455-69
