Abstract
Introduction: Mepact® (mifamurtide) is the first drug approved for the treatment of high-grade resectable non-metastatic osteosarcoma in patients aged 2–30 in the last 20 years. It follows a randomized clinical trial showing a statistically-significant and clinically-relevant decrease in the risk of death without compromising safety. Aim: This study assessed the cost–effectiveness and budget impact of mifamurtide. Methods: The economic evaluation was done on a hypothetical cohort of young patients under the age of 30 with high-grade, non-metastatic, resectable osteosarcoma. Standard chemotherapy without mifamurtide was used as comparator. A Markov model was adapted using Spanish costs and the results of the INT-0133 Phase III study. The analysis has been carried out from the perspective of the Spanish National Health Service, with a time horizon of up to 60 years in the base analysis. Results: The observed greater efficacy of mifamurtide in the trial translates into a gain of 3.03 (undiscounted) and 1.33 (discounted) quality-adjusted life years at an additional cost of €102,000. The estimated budgetary impact of using mifamurtide in 10–100% of the potential population would cost €671,000 and €6.7 million respectively. Conclusion: The cost per quality-adjusted life years gained with mifamurtide in Spain is in the low band (<€100,000) of the iCERs obtained by other orphan drugs and would have a limited and affordable cost in Spain.
Mifamurtide (Mepact®) was designated as an ‘orphan’ drug in 2004 by the European Medicines Agency (EMA). In Europe, the rare diseases for which these drugs are intended are defined as those with a prevalence of <5/10,000 Citation[1].
A few years later (2009), mifamurtide obtained EMA approval for the treatment of high-grade, resectable, nonmetastatic osteosarcoma (rare tumor) in combination with postoperative chemotherapy after macroscopically complete surgical resection in children and young adults (aged 2–30).
A significant challenge regarding rare tumors (0.2% of all malignant tumors) is that both their financial cost and cost to society with regard to other spheres of life is often not appropriately estimated, despite being a significant public health problem Citation[2].
Osteosarcoma is a rare malignant pleomorphic bone tumor in which the proliferative fusiform cells produce osteoid or immature bone and can be localized or metastatic Citation[3]. It occurs in the early years of the patients’ lives, and so the loss of life years is considerable despite the relatively low incidence of the disease Citation[4].
Osteosarcoma is the most commonly diagnosed primary malignant bone tumor in children, adolescents and young adults, with an estimated incidence of three cases per million population per year in different countries, peaking in males aged 15–19 and in females aged 10–14 Citation[5,6]. In Spain, the National Registry of Childhood Tumors (RNTI-SEHOP) has recently shown that the incidence of osteosarcoma is 2.9 cases per million inhabitants between the ages of 0 and 14 Citation[7]. It is worth noting that the incidence rates have remained relatively constant over the last 30 years. Approximately 15–20% of patients have metastases at the time of diagnosis, which are located in the lungs in 85–90%.
Osteosarcoma has the fourth highest mortality rate of all the malignant disorders in adolescents and young adults aged 13–29 years Citation[8]. In Spain, the mortality rate for malignant neoplasms of the bone and joint cartilage is 3.3% in the 2- to 30-year age group Citation[9]. Despite efforts to improve the chemotherapy treatments, survival rates have remained below 60% in the last 20 years Citation[10]. The 5-year cumulative survival rates in Spain for bone tumors are 59% in patients aged 0–14 years and 51% for the 0- to 19-year age group Citation[11]. In patients with metastatic disease, this rate falls to 30% Citation[3]. Untreated, osteosarcoma claims the lives of over 80% of patients with the disease Citation[12]. Moreover, 35% of all newly diagnosed patients will relapse with the currently available treatment within 3–5 years. It is therefore necessary to find new treatment options for this disease.
Mifamurtide is the first drug approved for its indication in the last 20 years and follows a pivotal randomized clinical trial in over 600 patients, which showed a statistically significant and clinically relevant increase in overall survival without compromising safety Citation[3,13] in the arm containing mifamurtide. Based on these results, in the recently published clinical guidelines for the treatment of osteosarcoma, the Spanish Society of Medical Oncology states that it advisable to use this drug in patients with local osteosarcoma up to the age described in the trial Citation[14].
Once the clinical benefits of mifamurtide were proven, its efficiency in the treatment of osteosarcoma was then evaluated with a cost–effectiveness analysis based on the clinical data from the INT-0133 study Citation[3,13] and on the use of resources and costs adapted to the Spanish context. The budgetary impact on the Spanish National Health Service of the treatment with mifamurtide was also estimated, since this would provide a comprehensive view of the potential financial consequences of the introduction of the drug into the health service. Thus, it has become a necessary element for decision-making Citation[15].
Methods
Study subjects
The economic evaluation was done on a hypothetical cohort of young patients under the age of 30 with high-grade, nonmetastatic, resectable osteosarcoma. The analysis of this population reflects the characteristics of the patients who took part in the pivotal clinical trial of the drug, leading to its approval.
For the budget impact analysis, the number of potential recipient patients in Spain was obtained from the RNTI-SEHOP run by the Spanish Society of Pediatric Hematology and Oncology Citation[7], which was projected over the total number of individuals aged between 0 and 29 years in the Spanish population. Of the total number of patients with osteosarcoma, the analysis included those in whom the treatment was indicated (80%) and excluded patients with metastasis and with localized osteosarcoma of the jaw.
Options for comparison
Treatment of osteosarcoma includes neoadjuvant chemotherapy followed by surgery aimed at removing the bone cancer, followed by adjuvant chemotherapy to reduce the chance of metastasis as much as possible. As mifamurtide is used together with adjuvant chemotherapy, the most relevant comparator treatment for analyzing the efficiency of mifamurtide is current clinical practice in its absence, i.e. standard chemotherapy without mifamurtide.
This analysis compared the use of mifamurtide added to the standard chemotherapy of cisplatin, doxorubicin and methotrexate with this same standard therapy without mifamurtide.
Description of the model
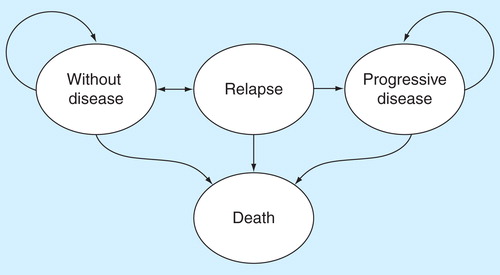
A Markov model was adapted using Spanish costs and the results of the INT-0133 Phase III study to replicate the natural history of the patients with osteosarcoma after having surgery, as a succession of disease-defining health states (see ). Patients in the disease-free health state at 12.25 years were assumed to have a mortality rate equivalent to the general population (using Spanish all-cause, sex and age-specific death rates) to model patients’ outcomes beyond the trial observation period. Patients in the post-recurrence disease-free state were assumed to have a mortality rate dependent on the time to recurrence, which was derived from a study by Ferrari et al. (2003) Citation[16]. Therefore, the study lasts for the patients’ lifetime.
The model calculates the incremental cost–effectiveness ratio (iCER) for the treatment with mifamurtide with respect to the standard therapy using the following formula:
where Costmifamurtide and CostST represent the costs associated with the treatment with and without mifamurtide respectively, while Effectivenessmifamurtide and EffectivenessST represent the clinical consequences in terms of quality-adjusted life years (QALY), in both groups. The iCER will express the additional cost of mifamurtide per QALY gained with respect to the standard therapy.
The net budgetary impact of mifamurtide was determined by multiplying the potential number of patients treated at the additional cost of the drug.
Model parameters
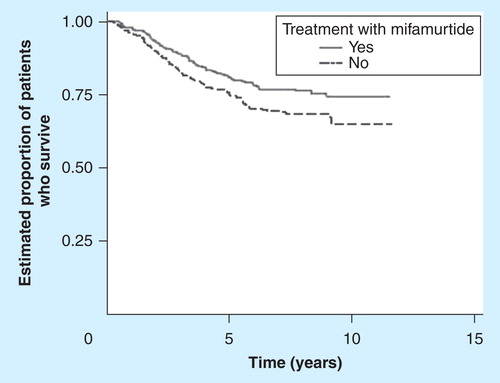
The effects on health were taken from the INT-0133 study, conducted on 662 patients with osteosarcoma, that found that when mifamurtide was added to the standard chemotherapy, survival was significantly improved with no increase in toxicity Citation[3,13]. At 6 years, mifamurtide increased the overall survival from 70 to 78% (p = 0.0313, hazard ratio: 0.72 [0.53–0.97]), with the survival curves reaching a plateau at around 60% and remaining divergent at 12 years, long past the time for risk of recurrence, indicating an increase in the cure rate and a 30% reduction in the risk of death Citation[3]. The tolerability profile for the treatment showed no differences between the arms for most of the adverse events reported Citation[13]. shows the clinical variables used in the model taken from the INT-0133 study (up to 12 years) and from the literature (long-term natural history of osteosarcoma patients).
Table 1. Clinical and economic parameters of the model.
The costs of the treatment and the principal resources related to the management of the disease were adapted to the Spanish context . It should be pointed out that maximum vials administered of mifamurtide per patient are 48 Citation[1], but for the calculation of the total pharmacological cost, the number of doses used were those administered in the INT-0133 study, in which more than a third of the patients received less than 40 vials or courses.
Perspective, time horizon & discount rate
The analysis has been carried out from the perspective of the Spanish National Health Service, with a time horizon of up to 60 years in the base analysis. This period was identified because the treatment being evaluated is indicated in children, adolescents and young adults with a potential life expectancy that greatly exceeds the duration of the follow-up in the pivotal clinical trial Citation[3].
All the costs are expressed in euros for the year 2011 and, beyond the first year, both costs and effects (quality-adjusted survival) have been discounted with an annual rate of 3%, following local recommendations Citation[17]. Alternatively, effects have been discounted at 1.5% following National Institute for Health and Clinical Excellence (NICE) recommendations for interventions with long-term consequences.
Sensitivity analysis
In order to analyze the robustness of the analysis results, a probabilistic sensitivity analysis was carried out to assess the influence of the uncertainty of the variables introduced into the model. It was assumed that the survival curve parameters followed a normal distribution, the utilities a beta distribution and the costs a gamma distribution.
Results
Cost–effectiveness analysis
Basic results
The basic analysis showed that the long-term simulation (up to 60 years) of the treatment is associated with a gain of 3.03 QALYs (1.33 using a discount rate of 3%), with additional costs over the lifetime of the patients of approximately €100,000. The combination of those two results yielded to iCERs (cost per QALY gained with mifamurtide) of €33,684 and €76,620 (without and with discount for the benefits respectively) .
Table 2. Basic results of the cost–effectiveness analysis for mifamurtide.
It should be noted that given the long-term nature of the analysis, using a lower discount rate than that commonly recommended for the clinical benefits has been suggested by bodies such as NICE, which recommends 1.5% in analyses beyond 30 years when treatment effects are both substantial in restoring health and sustained over a very long period of time Citation[18], as is the case of mifamurtide. Using the rate of 1.5%, the results of the analysis would be €52,322 per QALY gained.
Sensitivity analysis results
The univariate sensitivity analysis showed that the time horizon and the discount rate used are the parameters that most influence the model results .
Table 3. Univariate sensitivity analysis results.
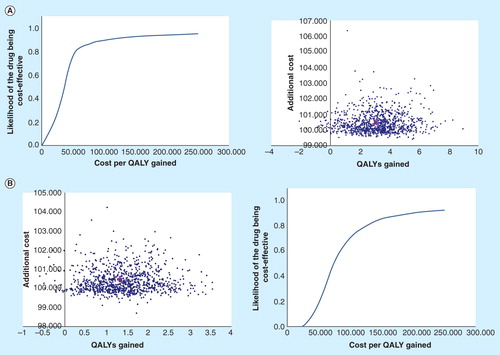
The probabilistic sensitivity analysis indicated that mifamurtide had a greater effectiveness and a higher cost. The flattened-out shape of the clouds of points denotes the little uncertainty in the costs of the treatment (the additional cost of the use of mifamurtide is known and predictable), and in virtually all the simulations, treatment with mifamurtide is more effective than the standard therapy .
Figure 3. Probabilistic sensitivity analyses. (A) Probabilistic sensitivity analysis (without discounting). (B) Probabilistic sensitivity analysis (3% discount). The individual points represent each of the 1000 patients simulated by the model. Approximately 99% of the cases are in the upper right quadrant of the cost–effectiveness graph, indicating greater effectiveness and a higher cost for mifamurtide.
Budget impact analysis
Data from the RNTI-SEHOP Citation[7] were used to estimate the adjusted annual incidence by age of osteosarcoma in 2.9 cases per million inhabitants between the ages of 0 and 14 and assuming that from age 14 to 29 the incidence of the condition stabilizes. Each year in Spain, 67 new children or infant and young adult patients are diagnosed with osteosarcoma. Of these, patients not eligible for the treatment (20%) were excluded from the analysis Citation[19], resulting in a maximum number of 54 potential patients candidates for treatment with mifamurtide each year.
Multiplying the cost per vial of mifamurtide (ex-factory price = €2708; €2600 after applying the 4% discount for orphan drugs as established by Royal Decree 8/2010 of 20th May Citation[20], by which special measures are adopted to reduce the public deficit) by the 39 or 48 vials per patient gives a cost per treatment of between €101,388 and €124,785.
It has thus been estimated that the gradual introduction of mifamurtide in the potential recipient population would have a minimum yearly cost of €671,000 (treatment of 10% of potential patients) and a maximum cost of €6.7 million (treatment of 100% of potential patients), assuming the cost of using the number of vials foreseen in the summary of product characteristics. A more realistic analysis, taking into account the average number of vials used in the clinical trial, results in an impact of between €0.5 and €5.0 million a year in Spain.
Discussion
Osteosarcoma in Spain is not the most common malignant bone tumor occurring in children and adolescents, but the second after Ewing sarcoma Citation[7]. The treatment of osteosarcoma requires a multidisciplinary team and involves careful coordination of complete tumor surgical removal of the primary tumor combined with 6–9 months of systemic chemotherapy. Currently, there are four chemotherapeutic agents that are considered active standard agents for the treatment of osteosarcoma, i.e., doxorubicin, cisplatin, high-dose methotrexate with leucovorin rescue and ifosfamide. All studies evaluating treatment regimens from the 1980s and 1990s using the standard cytotoxic chemotherapeutic agents have shown that the overall survival rates for patients with nonmetastatic osteosarcoma was 60–70%. Since the 1990s until today, 5-year overall survival for all childhood/adolescent cancers has improved from 58 to 82% Citation[7]. In contrast, 10-year overall survival for osteosarcoma has remained static over three decades at 60%. Thus, the consensus is that a plateau of efficacy has been reached with traditional cytotoxic chemotherapy regimens and that new agents or therapies are required to improve the survival outcome for patients with osteosarcoma. For two decades now, all intents of targeted therapy against the multiple genetic abnormalities harboring osteosarcoma cells have failed.
A significant interest in the role of enhancing the innate immune system for bone tumor therapy stems from the original observations by William B Coley in the late 1900s of complete responses after bacterial infections Citation[21]. Both the innate and adaptive immune systems have mechanisms capable of recognizing and killing tumor cells. The innate immune response, including NK and NKT cells, provides first-line, non-specific, defense mechanisms. In addition to providing direct protection against tumor, these cells are also important in priming the adaptive immune response by releasing cytokines that help to activate CD8 T cells. A study by Jeys LM et al. found that among 412 patients with osteosarcoma, those who experienced a postoperative infection within 1 year of their limb salvage surgery had a significantly improved 10-year overall survival outcome compared with those patients without a history of infection Citation[22]. This finding was rather surprising considering that infections were local but had an impact against distant lung metastases. Other studies have investigated the role of immunostimulatory agents, such as interferon, interleukins and GM-CSF for patients with osteosarcoma, but the results have been confusing.
Muramyl tripeptide phosphatidylethanolamine is a synthetic derivative of muramyl dipeptide, which is a peptidoglycan component found in bacterial cell walls. Mifamurtide is a formulation of muramyl tripeptide phosphatidylethanolamine encapsulated into multilamellar liposomes and functions as a potent activator of macrophages and monocytes. Much work has been done to demonstrate that mifamurtide potentiates the tumoricidal ability of macrophages and monocytes. Given that the lungs harbor a significant population of macrophages and are the most common site of metastatic involvement and recurrence of osteosarcoma, there was plenty of rationale for developing mifamurtide as a therapeutic agent for osteosarcoma. The first clinical evidence of antitumor activity in patients was in dogs. In 1989, veterinarians MacEwen EG et al. reported the first randomized trial of spontaneous osteosarcoma in dogs where after amputation, dogs would be treated with mifamurtide twice weekly for 8 weeks versus placebo liposomes Citation[23]. The overall survival advantage was highly statistically significant. Since then, more than 10 early clinical trials were performed in humans. The most significant clinical trial showing the benefit of mifamurtide for osteosarcoma was the Phase III, randomized, prospective Intergroup 0133 study for patients with newly diagnosed osteosarcoma including more than 600 patients Citation[3]. The addition of mifamurtide resulted in a significant improvement in 6-year overall survival (78 vs 70%; p = 0.03). The outcome for patients with metastatic disease showed a trend toward improvement in 5-year overall survival (53 vs 40%; p = 0.19). A compassionate access trial for patients with high-risk osteosarcoma has recently completed enrollment in the USA including 200 patients, and the data from this trial have also shown clinical benefit in this group of patients.
With all this background, surprisingly in the US, mifamurtide has not received approval from the US FDA, while it was approved by the EMA.
Immunotherapy has been the only therapeutic advance in osteosarcoma for the last three decades. In the next 5 years, strategies to improve immunotherapy will provide better chances for long-term disease control of osteosarcoma.
While the combination of mifamurtide with cytotoxic chemotherapy has been studied, the combination of mifamurtide with other therapeutic agents, such as Samarium-153 ethylene-diamine-tetramethylene phosphonic acid (153Sm-EDTMP) or radium-223, bone-seeking radiopharmaceutical agents that are in use for patients with bone metastases, could represent a novel avenue to explore. Immunomodulatory agents, such as inhaled GM-CSF recently evaluated in a clinical trial by the Children’s Oncology Group, could be another option for future studies.
Mifamurtide is a non-specific immunostimulant of the innate branch of the immune system. Potential developments should be expected in the field with immunomodulatory approaches of the adaptive immune system using osteosarcoma antigen targets. For instance, the GD2 ganglioside is overexpressed in osteosarcoma Citation[24,25] and recent definitive studies demonstrated that anti-GD2 antibody therapy improves survival in high-risk neuroblastoma Citation[26]. GD2 has also been targeted in clinical trials using first-generation CARs with some evidence for clinical activity Citation[27,28]. In the next coming years, we will see anti-GD2 and other targeted immune strategies applied to patients with osteosarcoma.
In this study, the development of a model using the results of a randomized clinical trial that showed that mifamurtide was associated with a statistically significant increase in overall survival has made it possible to estimate the long-term effectiveness of the use of this treatment in Spain for the management of osteosarcoma.
The long-term benefits of mifamurtide exceed 1.3 QALYs (3.03 QALYs when the clinical results are not discounted), leading to a cost per QALY gained of €76,620 and €33,623 respectively. These iCER are relatively low, especially considering that osteosarcoma is an ultra-rare disease and there is consensus on the specificity of the consideration of efficiency thresholds in these diseases.
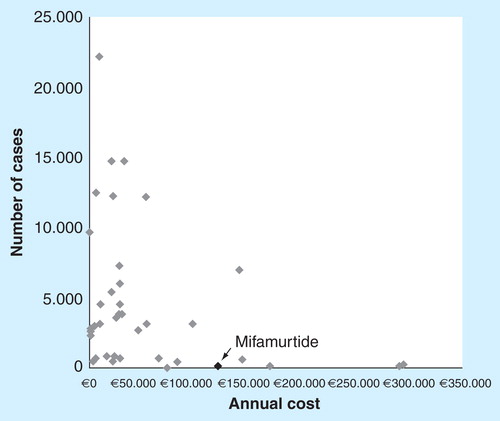
Even in the worst of the scenarios analyzed (time horizon of 12.5 years and 3% discount for the future benefits), the iCER for mifamurtide is within the range of efficiency defined by the National Institute for Health and Clinical Excellence for interventions forming part of the management of ultra-orphan diseases Citation[29] such as osteosarcoma. If we consider the threshold of £200,000–300,000 (€240,000–€360,000) proposed by NICE for the ultra-orphan drugs, mifamurtide can be considered to be a cost–effective ultra-orphan drug. It is not uncommon (see ) for the cost–effectiveness ratios of orphan drugs to be above the thresholds commonly used to define efficient interventions (between €30,000 and €50,000 in a number of countries in Europe). It is known that the standard procedures for evaluating healthcare technologies do not adapt particularly well to orphan drugs. In this context, it is important to take into consideration that both the European and Spanish healthcare authorities promote the research and development of orphan drugs, knowing that the low incidence of the diseases they are aimed at is a disincentive to pharmaceutical companies to market them and that these companies are going to set relatively high prices in order to make some return on their investment. The Spanish Ministry of Health and Social Policy [Ministerio de Sanidad y Política Social (MSPS)] in fact recognizes that: ‘These are medications which, for reasons of economics, pharmaceutical companies are unlikely to research without incentives’ Citation[30].
Table 4. Incremental cost–effectiveness ratios for different orphan drugs.
Nonetheless, the pharmacological cost of mifamurtide is comparable to, and in fact less than, the cost of a number of the different orphan drugs available in Spain, and the maximum expenditure potentially generated by mifamurtide is limited, since the combination of the number of potential patients and the cost of the treatment (a limited duration of less than 1 year) results in the maximum theoretical cost being relatively low (compared to the majority of other orphan drugs) . Since osteosarcoma is an ultra-orphan disease, the maximum number of potential recipients of mifamurtide is extremely low (less than 60 patients per year in Spain) and the potential budgetary impact is therefore very limited too. The use of mifamurtide in half the potential patients in Spain would result in pharmaceutical expenditure of between 2.5 and 3.4 million euros, with a low margin of uncertainty in terms of cost per patient in view of the fact that mifamurtide is administered only once in the entire process. This means lower costs compared to the long-term administration of other oncology orphan therapies that involve higher annual costs for treatment.
It is important to point out that the potential budgetary impact of extending the use of mifamurtide in Spain would, to a large extent, occur as a result of the lack of innovative treatments for a disease like osteosarcoma, for which long-term survival rates have not improved since the introduction of combined chemotherapy 20 years ago Citation[10].
We also have to take into account the fact that the need for new, more specific treatments that improve survival and patient quality of life and do not always replace the standard treatments for cancer (in this case standard chemotherapy), and the rapid increase in cancer cases, in general, mean that pharmaceutical expenditure is increasing. Hospital pharmaceutical expenditure on anticancer treatments (ATC L1) in 2010 represented 20% (€1128 million) of the total hospital pharmaceutical market (€5639 million; IMS data). Considering these figures, the cost involved in treating up to half the potential patients with mifamurtide in the overall hospital pharmaceutical expenditure would under no circumstances exceed 0.3%, representing a maximum of 0.6% of the hospital pharmaceutical expenditure associated with oncological diseases.
In the context of pharmaceutical expenditure on orphan drugs, it must be remembered that, although osteosarcoma is the most common bone cancer in children, adolescents and young adults, it is still considered a rare ultra-orphan disease. According to EMA definition, orphan diseases are life-threatening or chronically debilitating diseases that have a prevalence of less than five cases per 10,000 population Citation[31]. Although it is difficult to specify the number of rare diseases, it is estimated that their numbers could be somewhere between 5000 and 8000. Therefore, despite the fact that they are uncommon or very uncommon diseases with only isolated cases, they become significant when considered together since they affect between 6 and 8% of the population in developed countries; they affect over 3 million people in Spain Citation[30,31]. Orphan drugs are increasingly becoming more accessible for patients in Spain and in the last 5 years (2004–2008), 38 orphan drugs have been authorized in Spain, 87% of those positively evaluated by the EMA Citation[30]. Of the orphan drugs marketed to date, the majority are for use in oncology and endocrinology or metabolism Citation[30].
According to the study by Orofino et al. (2010) Citation[32], in 2007, expenditure on orphan drugs accounted for 2% of total pharmaceutical expenditure in Spain (12,800 million euros), that is, about 256 million euros. In this context, the use of mifamurtide in half of the potential patients would represent less than 1.3% of pharmaceutical spending on orphan drugs and less than 0.03% of total pharmaceutical expenditure.
Moreover, pharmaceutical expenditure generated by orphan drugs obviously does not depend solely on their cost, but on the combination of cost and the number of patients effectively treated . Although the annual cost multiplied by the number of potential cases provides a purely theoretical estimation (and, moreover, maximum) of the pharmaceutical costs that may be generated by the orphan drugs, calculating this figure allows their potential financial impact to be seen in perspective.
To conclude, regardless of whether or not the usual cost–effectiveness criteria (general efficiency thresholds) apply in the evaluation of orphan drugs, the cost per QALY gained with mifamurtide in Spain is in the low band (below €100,000 per QALY) of the iCER obtained by other orphan drugs used in this country.
Osteosarcoma is the second most commonly diagnosed primary malignant bone tumor in children, adolescents and young adults in Spain, with an estimated incidence of three cases per million population.
Osteosarcoma has the fourth highest mortality rate of all the malignant disorders in adolescents and young adults aged 13–29 years. Despite efforts to improve the chemotherapy treatments, survival rates have remained below 60% in the last 20 years.
Mifamurtide is a drug indicated in the treatment of high-grade, resectable, nonmetastatic osteosarcoma in combination with postoperative chemotherapy after macroscopically complete surgical resection of the tumor in patients aged 2–30. This is the first drug approved for such indication in the last 20 years and follows a pivotal randomized clinical trial in over 600 patients that demonstrated a statistically significant and clinically-relevant increase in overall survival without compromising safety.
Our study shows that the long-term benefits of mifamurtide exceed 1.3 QALYs (3.03 QALYs when the clinical results are not discounted), leading to cost per QALY gained of €76,620 and €33,623, respectively. These iCER are relatively low, especially considering that osteosarcoma is an ultra-rare disease and there is consensus on the specificity of the consideration of efficiency thresholds in these diseases.
The cost per QALY gained with mifamurtide in Spain is in the low band (below €100,000 per QALY) of the iCER obtained by other orphan drugs used in this country.
Acknowledgements
We thank Ana Fernández-Teijeiro (Jefe de Sección, Unidad de Oncología Pediátrica, Hospital Virgen Macarena y Hospital Infantil Virgen del Rocío, Sevilla) for her kindly review of the paper. Also Lucía Catania from Nova Language Services for medical writing assistance.
Financial & competing interests disclosure
This article was sponsored by Takeda Farmacéutica España. M Brosa and A Villacampa received an unrestricted grant/consultancy fees from Takeda Farmacéutica España in relation to their participation in this study. C Montoto, T Pozo-Rubio & L Cubells are employees of Takeda Farmacéutica España. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- European Medicines Agency (EMA). Human medicines - Mepact (mifamurtide). [Updated 6 September 2012]. Available from: www.emea.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000802/human_med_000899.jsp&mid=WC0b01ac058001d12
- Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, et al. Cancer in children and adolescents in Europe: developments over 20 years and future challenges. Eur J Cancer 2006;42(13):2183-90
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. J Clin Oncol 2008;26(4):633-8
- National Cancer Institute (NCI). Osteosarcoma and malignant fibrous histiocytoma of bone treatment. Available from: www.cancer.gov/cancertopics/pdq/treatment/osteosarcoma [Last accessed 2009]
- Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis 2007;2:6
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009;125(1):229-34
- Peris Bonet R, et al. Cáncer infantile en España. Estadísticas 1980-2011. In: Registro Nacional de Tumores Infantiles (RNTI-SEHOP). Universitat de València; Ed Valencia: 2012
- Geraci M, Birch JM, Alston RD, et al. Cancer mortality in 13 to 29-year-olds in England and Wales, 1981-2005. Br J Cancer 2007;97(11):1588-94
- World Health Organisation (WHO). Global Health Observatory/WHOSIS. Available from: www.who.int/whosis/mort/download/en/index.html [Last accessed 2009]
- Longhi A, Errani C, De PM, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006;32(6):423-36
- Automated Childhood Cancer Information System. Osteosarcoma incidence and survival by registry and tumour. Available from: www-dep.iarc.fr/accis/data.htm [Last accessed 2009]
- Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol 2003;10(5):498-507
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23(9):2004-11
- Redondo A, Cruz J, Lopez-Pousa A, Barón F. SEOM clinical guidelines for the treatment of osteosarcoma in adults-2013. Clin Transl Oncol 2013;15(12):1037-43
- Brosa M, Gisbert R, Rodríguez JM y Soto J. Principios, métodos y aplicaciones del análisis del impacto presupuestario en el sector sanitario. Pharmacoeconomics Spanish Res Articles 2005;2(2):64-78
- Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol 2003;21(4):710-15
- López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010;11(5):513-20
- National Institute for Health and Clinical Excellence. Discounting of health benefits in special circumstances. Available from: www.nice.org.uk/media/955/4F/Clarification_to_section_5.6_of_the_Guide_to_Methods_of_Technology_Appraisals.pdf
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009;152:3-13
- Real Decreto Ley 8/2010 de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público In: Boletín oficial del estado. 126:45070-128.Spain: 2010
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154-8
- Jeys LM, Grimer RJ, Carter SR, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol 2007;14(10):2887-95
- MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome encapsulated muramyl tripeptide. J Natl Can Inst 1989;81:935-8
- Schulz G, Cheresh DA, Varki NM, et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res 1984;44(12 Pt 1):5914-20
- Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets 2010;10(2):200-9
- Yu AL, Gilman AL, Ozkaynak MF, et al. Children’s Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324-34
- Park JR, Bagatell R, London WB, COG Neuroblastoma Committee. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013;60(6):985-93
- Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14(11):1264-70
- National Institute for Health and Clinical Excellence. Appraising Orphan Drugs. Available from: www.nice.org.uk/niceMedia/pdf/smt/120705item4.pdf
- Ministerio de Sanidad y Política Social (MSPS). El Ministerio de Sanidad y Consumo incluye en la financiación pública dos nuevos medicamentos para enfermedades raras. Available from: www.msps.es/gabinetePrensa/notaPrensa/desarrolloNotaPrensa.jsp?id=1421
- European Medicines Agency (EMA). Medicines for rare diseases, 2011. Available at www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000034.jsp&mid=WC0b01ac058002d4eb
- Orofino J, Soto J, Casado MA, Oyagüez I. Global spending on orphan drugs in France, Germany, the UK, Italy and Spain during 2007. Appl Health Econ Health Policy 2010;8(5):301-15
- Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99(2):112-28
- Instituto Nacional de Estadística - INE. Tablas de mortalidad de la población de España 1991-2008. Available from: www.ine.es/jaxi/tabla.do?path=/t20/p319a/serie/l0/&file=01001.px&type=pcaxis&L=0 [Last accessed 17 June 2010]
- CGCOF (Consejo General del Colegio Oficial de Farmacéuticos) 2011. Available from: www.portalfarma.com [Last accessed December 2011]
- E-Salud. Base de datos de costes sanitarios españoles (2011). Oblikue Consulting. Available from: www.oblikue.com/bddcostes/ [Last accessed December 2011]
- Ministerio de sanidad, política social e igualdad. Portal estadístico. Available from: www.msps.es/estadEstudios/estadisticas/sisInfSanSNS/home.htm [Last accessed December 2011]
- Burls A, Austin D, Moore D. Commissioning for rare diseases: view from the frontline. BMJ 2005;331(7523):1019-21
- Gildea TR, Shermock KM, Singer ME, Stoller JK. Cost-effectiveness analysis of augmentation ther-apy for severe alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2003;167(10):1387-92
- García Quetglas E, Azanza Perea JR, Lecumberri Villame-diana R. New therapeutic strategies for Mieloma múltiple. Efficacy and cost-effectiveness analyses. Med Clin (Barc) 2008;130(16):626-35
- Cannock M, Wang D, Fry-Smith A, Moore D. Prevalence and prognosis of paroxysmal nocturnal haemoglobinurea and the clinical and cost-effectiveness of eculizumab [Report Number 69]. Birmingham (UK): University of Birmingham; 2008. Available from: www.rep.bham.ac.uk/2008/PNH.pdf
- Hoyle M, Green C, Thompson-Coon J, et al. Cost-effectiveness of temsiro-limus for first line treatment of advanced renal cell carcinoma. Value Health 2010;13(1):61-8
- Paz-Ares L, García del Muro X, Grande E, et al. Cost-effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumors (GIST) after progression or intolerance with imatinib. Clin Transl Oncol 2008;10(12):831-9
- Maroto P, Villavicencio H, Piñol C, et al. Análisis coste-efectividad de sorafenib oral en el tratamiento del carcinoma de células renales avanzado. Rev Esp Econ Salud 2008;7(4):173-80