ABSTRACT
Parasitic diseases of the central nervous system are associated with high mortality and morbidity, especially in resource-limited settings. The burden of these diseases is amplified as survivors are often left with neurologic sequelae affecting mobility, sensory organs, and cognitive functions, as well as seizures/epilepsy. These diseases inflict suffering by causing lifelong disabilities, reducing economic productivity, and causing social stigma. The complexity of parasitic life cycles and geographic specificities, as well as overlapping clinical manifestations in the host reflecting the diverse pathogenesis of parasites, can present diagnostic challenges. We herein provide an overview of these parasitic diseases and summarize clinical aspects, diagnosis, therapeutic strategies and recent milestones, and aspects related to prevention and control.
Introduction
Parasitic diseases affecting the central nervous system (CNS) remain an important source of morbidity and mortality worldwide. The neurological, cognitive, and mental health problems caused by these parasitic infections affect millions of children and adults in low- and middle-income countries; however, sporadic cases also occur in nonendemic areas because of an increase in international travel and immunosuppression caused by post-transplantation therapy or HIV infection. Long-term immunosuppression caused by medications such as prednisone might also increase the risk for acquiring parasitic infections; however, information to support this hypothesis is very scarce [Citation1].
Parasites are a diverse group of organisms that can be broadly classified into single-celled organisms (i.e. protozoa) or multicellular helminths (i.e. metazoa) (). Protozoa have the ability to multiply in the immunosuppressed, which explains why most of the severe opportunistic infections in patients with HIV are caused by protozoan parasites. Helminths have the propensity to cause disease by physical disruption of tissue as they migrate, provoking an intense, often eosinophilic, inflammatory response [Citation2]. Some helminthic larvae can be very large, causing disease because of their expanding mass. A relatively large number of parasites infect humans, sometimes migrating through or lodging in tissues, including the CNS [Citation3]. Some parasites regularly cause symptomatic disease, while others cause few, if any, symptoms. Concomitant infections are possible in areas endemic for several parasites.
Table 1. Classification of parasitic infections of the central nervous system.
All parasites affecting humans might involve the CNS; however, the most common parasitic infection of the CNS is cysticercosis. Other less frequent infections are toxoplasmosis, echinococcosis and schistosomiasis. Rare parasitic diseases involving the CNS are paragonimiasis, malaria, toxocariasis, onchocerciasis, American trypanosomiasis [Chagas disease (CD)], human African trypanosomiasis (HAT) and angiostrongyliasis [Citation4]. These diseases have diverse causative organisms, vector or intermediate hosts, modes of transmission and endemic regions or geographic distributions ().
Table 2. Characteristics of main parasitic infections of the central nervous system.
Clinical manifestations of the main parasitic diseases of the CNS
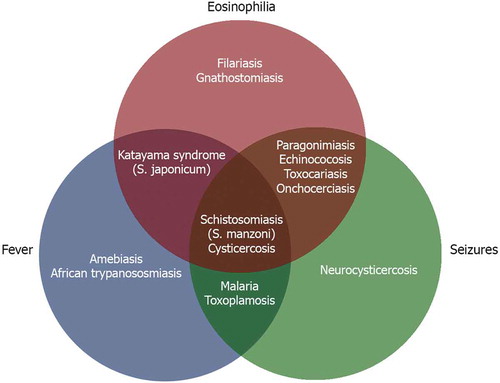
Almost all parasitoses involving the human brain can be associated with seizures and epilepsy, either by a diffuse encephalitis or encephalopathy, or by intracerebral location of the parasite [Citation5]. However, with the exception of neurocysticercosis (NCC) and malaria, which are likely responsible for most of the seizures attributed to parasitic disease worldwide, the frequency of seizures because of parasites is not well documented in the literature [Citation6,Citation7]. In a recent study of people with active epilepsy, exposure to multiple pathogens was common [Citation8]. The combined effect of Toxoplasma gondii and Onchocerca volvulus co-infection on the prevalence of epilepsy seemed more than additive. Some parasitic diseases cause eosinophilic meningoencephalitis, which is characterized by specific clinical manifestations and microscopic identification of eosinophils in cerebrospinal fluid (CSF).
Crossover between signs and symptoms, such as seizures, eosinophilia (in blood or CSF) and fever, occurs with many parasitic infections, thus making differential diagnosis a challenge (). For example, a patient with parenchymal NCC might only have seizures as a clinical manifestation; however, another patient with extraparenchymal NCC might also have eosinophilia and fever. Eosinophilia relates to the presence of >500 eosinophils/ml of blood or an eosinophil count of >7% of total leukocytes [Citation9], while eosinophilia in CSF has been arbitrarily defined by counts >10 eosinophils/ml or 10% of the total CSF leukocyte count [Citation2]. Helminthic infections are the most common cause of eosinophilic meningoencephalitis; nevertheless, other types of infections and neoplastic diseases may be other causes.
Neurocysticercosis
The clinical manifestations of NCC are heterogeneous and depend mainly on the localization of cysts and immune response by the host. Seizures, headache, focal deficits and cognitive abnormalities are the most frequent manifestations of cysts in the brain parenchyma [Citation10,Citation11]. NCC predominantly affects adults in their third and fourth decade of life, and is relatively uncommon in children and the elderly. Most pediatric patients have a single transitional cyst that resolves spontaneously over a few months [Citation12]. Contrary to cysts in the brain parenchyma, extraparenchymal cysts can be life threatening [Citation13,Citation14].
Extraparenchymal disease occurs in about one-third of patients with NCC. Increased intracranial pressure also occurs in patients with the racemose form of NCC and in those with cysticercal encephalitis [Citation13]. Acute hydrocephalus related to intraventricular cysts, or chronic hydrocephalus because of arachnoiditis or ependymitis are the most frequent causes of this syndrome. Spinal cord cysticercosis is rare and patients may experience nonspecific clinical manifestations, such as nerve root pain or spinal cord compression syndromes, according to the level of the lesion. Massive infection of striated muscles can cause generalized weakness associated with muscle pseudohypertrophy.
Toxoplasmosis
After ingestion of the organism, Toxoplasma gondii cysts may develop in any tissue, but most commonly develop in the brain, retina, skeletal muscle and cardiac muscle. Rupture of the cysts releases the free tachyzoite, which causes acute illness [Citation4]. Fever, rash, lymphadenopathy and eye disturbances are typical in early stages. When lesions are in the CNS of immunocompromised patients, fever, headaches, confusion and seizures are common, as well as ocular disease (retinochoroiditis); however many infections are subclinical, and 20% of HIV-infected patients with toxoplasmosis develop encephalitis. The disease may also be transmitted transplacentally and have devastating effects on the fetal brain because maternal antibodies passed to the child will be limited by the blood–brain barrier. Seizures, microcephaly and chorioretinitis have been noted in most of these cases.
Chronic infection in immunocompetent people, usually considered asymptomatic, is suspected as a risk factor for various neurological disorders, including epilepsy [Citation5]. Few well-conducted studies are available; only six studies were identified in a systematic review [Citation15], which estimated an odds ratio of about 2. The odds of seizures are likely to be dependent on the number and location of cysts. Some studies have investigated whether Toxoplasma gondii infection may also be a risk factor for development of depression and other psychiatric disorders [Citation16]. A recent experimental study used Toxoplasma gondii to infect the brain of mice and showed that seizures arise because of a defect in signaling of GABA, which is the neurotransmitter primarily responsible for preventing the onset of seizures [Citation17].
Echinococcosis (hydatidosis)
The parasites Echinococcus granulosus and Echinococcus multilocularis causing cystic echinococcosis and alveolar echinococcosis, respectively, belong to the same genus; however, these pathologies are entirely different entities in terms of clinical manifestations, course of disease and prognosis [Citation18]. Alveolar echinococcosis is currently considered a neglected ‘malignant’ parasitic disease and a threat to public health in Europe [Citation19]. Recent reports have observed trends of Echinococcus multilocularis infection in the European fox and dog populations and expect an increase of annual case numbers of human alveolar echinococcosis in many areas of Europe within the next decades [Citation20,Citation21].
The infection may be primary or secondary to the spontaneous or traumatic rupture of a primary cerebral cyst or because of embolization of cardiac cysts [Citation22]. Cysts may remain asymptomatic until they are large enough to cause a mass effect. Cerebral lesions occur in 1–4% of individuals with cystic echinococcosis, with nonspecific clinical findings related to those of space occupying lesions, increased intracranial pressure and seizure activity.
Schistosomiasis
Schistosoma mansoni and Schistosoma haematobium are almost always associated with spinal infection and Schistosoma japonicum affects the brain. All three species that infect humans have CNS complications, usually as a result of the eggs causing infarction or granuloma formation. There are substantial differences in the pathogenesis, clinical presentation and outcome of the neurological disorder, depending on the phase and clinical form [Citation23].
Acute schistosomiasis, or Katayama syndrome, is an immune-mediated hypersensitivity reaction to maturation and migration of egg-laying schistosomes [Citation9]. Fever, urticarial rash, cough and pulmonary infiltrates are typical features, and are often accompanied by a moderate peripheral eosinophilia. Acute encephalitis, usually in the presence of a systemic illness with fever and eosinophilia, occurs in about 2–3% of cases of acute schistosomiasis [Citation23]. Schistosomiasis is regarded as an under-recognized cause of acute encephalopathy in the tropics [Citation24].
Patients with cerebral schistosomiasis are commonly asymptomatic and may present with signs of space occupying lesions, such as headache, seizures, papilledema, and visual and oral disturbances [Citation4]. Spinal schistosomiasis is often seen in young male patients. Its localization in the lower cord and conus region is explained by the free anastomosis between the pelvic veins and the vertebral venous plexus [Citation4]. The most frequent form is myeloradiculopathy. There may be multiple nodules in the spinal cord, cord compression from meningeal granulomata and cord necrosis. Blood and CSF analysis usually show eosinophilia and intrathecal specific antibodies. The CSF findings are variable from normal, a mild pleocytosis, to markedly raised protein. Identification of the Schistosoma is not common and occurs in only about one-quarter of the patients with myelopathy [Citation22].
Paragonimiasis
The CNS is affected by the anomalous migration of larvae through the bloodstream or through direct invasion of the neural foramina of the skull base and intervertebral foramina of the spine along the cranial and spinal vessels and nerves [Citation25]. Toxic substances produced by the parasite are responsible for aseptic inflammation or granulomatous reaction to the parasite or its eggs. The initial intracranial lesions include exudative aseptic inflammation, cerebral hemorrhage and infarction. The wandering adult paragonimus makes tunnels along the track of migration. The neurologic presentation is nonspecific and can include headache, seizures and focal neurologic deficits. Some patients can present intracranial vascular injury because of the granulomatous inflammatory reaction around tunnels along the migration track of the parasite, resulting in cerebral hemorrhage [Citation26].
Malaria
Malaria is the most common parasitic disease worldwide. It affects primarily African children and Asian adults, with the vast majority (>90%) of cases occurring in children 5 years old or younger. Cerebral malaria is caused by Plasmodium falciparum and may result in an acute encephalopathy (with febrile and acute seizures), which may be fatal or lead to polymorphic neurological sequelae.
The pathophysiology of brain involvement is multifactorial but is related to the degree of parasitemia, sequestration of schizonts in the brain venules, and the resulting vascular and perivascular damage [Citation22]. Suggested mechanisms of injury include anoxia, vascular leakage, parasite toxin, metabolic derangements, increased intracranial hypertension, including stroke and others [Citation5]. The first symptoms of malaria are nonspecific, and include headache, fatigue, abdominal discomfort, muscle aches and irregular fever. Nausea, vomiting and orthostatic hypotension also occur frequently. Generalized seizures might be followed by coma [Citation27]. For those who survive cerebral malaria, residual neurological abnormalities are common. Epilepsy, cognitive impairment, behavioral disorders and gross neurological deficits are frequent sequelae. There is a clear association with epilepsy, which has been reported by some epidemiological studies in endemic areas in Africa [Citation7].
Toxocariasis
There are three main syndromes associated with toxocariasis: visceral larva migrans, which encompasses diseases associated with major organs; covert toxocariasis, which is a milder version; and ocular larva migrans, in which the pathological effects on the host are restricted to the eye and the optic nerve. CNS infestation is rare, but these patients may present with seizures, eosinophilic meningitis, optic neuritis and meningomyelitis. A systematic review evaluating the strength of the association between epilepsy and Toxocara spp. suggested that seropositivity for Toxocara spp. is significantly higher among people with epilepsy [Citation28]. However, the inclusion of prevalent rather than incident cases does not allow distinguishing among the potential etiological factors that preceded the onset of epilepsy and thus, cause and effect become difficult, if not impossible, to establish.
Onchocerciasis
In onchocerciasis or river blindness, symptoms include severe itching, bumps under the skin and blindness. It has also been proposed to be a potential risk factor for epilepsy because of the high prevalence of onchocerciasis in areas with a high prevalence of epilepsy. Analytical work on this association has produced conflicting results, perhaps related to confounders (e.g. other CNS infections) or a lack of standardized methods [Citation5]. A recent meta-analysis showed a significant association with an odds ratio of around 3 [Citation29]. Onchocerciasis has been suggested to cause nodding syndrome, an epileptic encephalopathy characterized by nodding of the head, affecting mainly children in Africa [Citation30]. Some studies suggest an association with onchocerciasis, but this requires further investigation [Citation31].
American trypanosomiasis (Chagas disease)
CD is endemic to Latin America, but is increasingly found in other parts of the world, including countries previously free of disease [Citation32]. CNS involvement in CD may occur in a small percentage of patients in the acute phase. Reactivation of latent infection in chronic CD has been reported in immunosuppressed patients [Citation4]. Chagas meningoencephalitis may manifest as the first presentation of AIDS. The definitive diagnosis depends on characterizing the parasite in CSF tests or by histologic analysis of the cerebral parenchyma. CD is probably an under-recognized cause of ischemic stroke in South America, as some epidemiological studies have found an association between Trypanosoma cruzi infection and ischemic stroke [Citation33].
Human African trypanosomiasis (sleeping sickness)
HAT infects the brain parenchyma by early seeding in the choroid plexus and secondary passage into the CSF, or by direct passage into the cerebral capillaries. Once these vessels are involved, the extracellular spaces within the white matter allow the parasite to move into the brain tissue [Citation9]. The early stage (stage 1, early hemolymphatic stage) corresponds to the development of the parasite in blood and lymphatic tissue. Later (stage 2, late encephalitic stage) in the absence of treatment, the CNS becomes involved [Citation3]. The symptoms of HAT range from meningitis to meningoencephalitis with brain edema and arachnoiditis. Seizures may appear in the terminal phase of the disease and death occurs without treatment.
Angiostrongyliasis
Angiostrongylus cantonensis occurs widely in the tropics. Most infections occur in Southeast Asia, but small epidemics have been reported more recently from the Caribbean and elsewhere. Angiostrongylus cantonensis is a zoonotic parasite that affects rats as the primary hosts. Humans are infected by eating larvae in undercooked intermediate hosts (e.g. snails, slugs, crabs, or prawns). Angiostrongylus cantonensis is the most important etiological agent of eosinophilic meningitis. The larvae migrate to the CNS, where they can be identified in the meninges, blood vessels and perivascular spaces. Angiostrongyliasis is an acute disease that spontaneously resolves and rarely entails sequelae or fatality. It most often presents with headaches, nausea, vomiting and neck stiffness. Fever is uncommon, but other features of meningitis, encephalitis and radiculitis may occur. Lumbar puncture pressure is usually raised, with turbid CSF. The pleocytosis usually has eosinophils and occasionally the larvae are seen. There is no specific treatment and most illness resolves in 4–6 weeks.
Immunological and molecular diagnosis of the main parasitic infections of central nervous system
Parasitic infections of the CNS are often ‘silent’, with the classical neurological symptoms (e.g. headache, seizures, coma) appearing long after the initial invasion of the brain and, importantly, when considerable, sometimes irreversible, damage has occurred. Thus, early and reliable confirmatory diagnosis subsequent to clinical examination is an essential tool in the control and treatment of these debilitating infections, with detection of viable parasite as a key objective, rationally opening the door to appropriate and effective treatment. Direct visualization of the parasite, as for cerebral malaria, HAT and toxoplasmosis, is clearly definitive. In many parasite infections, however, this is not a feasible option, and so the development of specific and sensitive serodiagnostic and molecular biological [polymerase chain reaction (PCR)] assays for viable parasites is an urgent priority that will complement and confirm clinical examination.
In the hospital situation, where the neurologist routinely takes CSF samples from patients exhibiting neurological symptoms, detection of secretions from viable parasites or detection of parasite DNA in CSF would be the preferred option, although the location of the parasite in the brain may be an important factor. For example, in the diagnosis of NCC, through detection of the secreted metacestode glycoprotein using the HP10 antigen assay, the location of the cyst is an important factor functioning well for extraparenchymal NCC, but not so sensitive for parenchymal NCC [Citation14]. On the other hand, for investigation in a rural endemic setting, serum is clearly the only practical possibility for HP10 assay, and one must accept the fact that while there may be false positives and false negatives, an endemic focus has been identified for further studies and for referral to appropriated hospital facilities.
The detection of parasite DNA through the PCR is an experimentally simple approach and is currently receiving much more attention than serological detection of secreted products of viable parasites. Unfortunately, parasite DNA can originate from both the live and dead organism and thus, a positive PCR is not necessarily definitive proof of a viable parasite infection. The strength of the PCR, however, is its sensitivity and exquisite specificity, which are characteristics that provide a powerful tool for the differential diagnosis of parasite subtypes and polymorphisms and for molecular epidemiological investigations.
Anti-parasite antibodies are synthesized soon after host invasion and so their detection is still the most frequently employed diagnostic tool. Once again, however, their detection is not definitive proof of a current, viable infection as antibodies can persist for months, even years, after elimination of the parasite, for example by drug treatment.
For antibody diagnosis of cerebral parasitic infections, both serum and CSF samples are commonly employed. The presence of antibodies in CSF is a clear indication of cerebral involvement and damage, but does not necessarily exclude the presence of the parasite in other body locations. Similarly, the presence of serum antibodies may, or may not, indicate a cerebral infection with consequent impact on the blood–brain barrier. Importantly, the demonstration of anti-parasite antibodies, being clear evidence of exposure to the parasite, is an extremely useful tool for the evaluation of parasite endemicity at the level of a population before, during and after control programs.
Finally, in spite of their recognized lack of specificity, soluble parasite extracts are still commonly used as ‘antigens’ in anti-parasite antibody assays. Today, with the ready availability and reproducibility of recombinant and monoclonal antibody technology, this practice should cease. With these preliminary considerations in mind, current diagnostic procedures for the detection of main parasitic infections of CNS are herein summarized.
There has been considerable investigation into the development of serological and molecular biological procedures for the diagnosis of NCC [Citation34]. Not surprisingly, therefore, a variety of recombinant metacestode antigens and synthetic peptides have been tested as target for antibody detection. Amongst these, particularly useful examples are the cathepsin L-like protease and the T24H and Ts8B2 recombinant proteins [Citation35,Citation36]. Simple, sensitive, specific and economic enzyme-linked immunosorbent assay (ELISA) assays detecting antibodies to reproducible, recombinant antigens are clearly a preferred alternative to the enzyme-linked immunotransfer blot (EITB), which is expensive, technically more complicated and requires parasite material.
As an alternative to the antibody assays, the detection of molecules secreted by viable metacestodes has been met with success [Citation37,Citation38], and their utility in the diagnosis and treatment of NCC has been demonstrated [Citation14,Citation39–Citation41]. A particularly useful application of the viable metacestode secreted antigen detection assay is the long-term follow up of patients with NCC before, during and after drug treatment. Thus, effective drug treatment of patients with NCC is clearly established by the decreased antigens levels in CSF, whereas ineffective treatment is revealed by the continued presence of the secreted parasite product in the CSF. Finally, some success in the diagnosis of NCC in CSF has been realized through PCR [Citation42].
Like malaria, the ‘gold standard’ for diagnosis of toxoplasmosis is detection of the parasite by microscopy. Complementary techniques include detection of circulating Toxoplasma gondii antigens, anti-toxoplasma antibodies, and a variety of PCR protocols [Citation43]. Diagnosis at the level of clonal types is now possible through DNA sequencing and the serological identification of antibodies to polymorphic toxoplasma peptide sequences, and these are useful tools for both clinical and epidemiological studies. Confirmation of neonatal toxoplasmosis is highly important and definitive by the detection of IgM antibody in CSF, typically using either the indirect fluorescein antibody test or the Sabin–Feldman dye test. Diagnosis of toxoplasmosis in immunocompromised patients is complicated by immunosuppression and frequently low antibody titers. A serious practical problem is to distinguish infection with Toxoplasma gondii from cerebral infection with Trypanosoma cruzi; in these patients, diagnosis demands a combination of serology, PCR and examination of CSF.
Diagnosis of cerebral echinococcosis follows the common pattern of serological confirmation subsequent to the observation of neurological symptoms. Diagnostic testing for anti-parasite antibodies using parasite extracts or a western blotting protocol has been described [Citation34].
Invasion of the brain has been described for all of the Schistosoma species infecting humans, and the full importance of this clinical entity and its consequence for the proposed massive population dosing of the drug praziquantel is yet to be fully appreciated. Diagnosis following suspicious clinical symptoms is usually through the demonstration of Schistosoma eggs in feces or urine and through the detection of antibodies to the soluble egg antigen [Citation2]. There is a need for more work here.
Paragonimiasis is confirmed by detection of the parasite and the demonstration of anti-parasite antibodies following the observation of neurological symptoms. The preferred serological diagnostic test uses a western blotting procedure to detect antibodies to an 8 kD protein of the adult [Citation44].
Like toxoplasmosis, the standard for diagnosis of cerebral falciparum malaria is direct visualization of the parasite in blood smears. There are PCR protocols and immunodiagnostic tests detecting antibodies to parasite proteins, such as the histidine-rich protein and lactate dehydrogenase [Citation45]. Apart from their expense, the latter tests are criticized for not providing a reliable estimate of parasite load.
Although rare, cerebral toxocariasis does occur and, following neurological symptoms, the preferred confirmation is through detection of antibodies to eggs antigens or secretions of infective larvae, preferably in CSF [Citation46].
Cerebral complications associated with HAT are usually first detected through antibody-mediated agglutination of fixed trypanosomes, a technique known as the ‘Card Agglutination Test for Trypanosomes’ (CATT), and then confirming the diagnosis by direct visualization of the parasite in CSF. Both false positive and false negative have been reported using the CATT [Citation34].
As for toxoplasmosis, the main challenges for CD are: the low antibody titer in young children and immunosuppressed individuals, and the difficulty of distinguishing Trypanosoma cruzi from Toxoplasma gondii. Once again, a combination of serology, PCR and examination of CSF for parasite is required. In addition to various antibody detection tests, a technique to detect antigens present in a crude excretion/secretion product has been described [Citation47].
Neuroimaging diagnosis of parasitic infections of the CNS
Neuroimaging studies (CT scan and MRI) play an important role in early diagnosis; however, there is a wide range of neuroimaging findings in parasitic infections of the CNS, often with considerable overlap, which makes determination of a specific diagnosis difficult. Therefore, correlation with laboratory tests, especially CSF analysis, is considered to be fundamental in establishing a definitive diagnosis. In addition to conventional CT and MRI, advanced neuroimaging techniques, such as fluid attenuation inversion recovery (FLAIR), diffusion MR, perfusion MR, and MR spectroscopy have been employed, as well as the 3D MRI sequences [Fast Imaging Employing Steady-state Acquisition (FIESTA) and Spoiled Gradient Recalled Echo (SPGR)] which provide more clues for differentiation of CNS parasitic diseases, especially for NCC [Citation11].
Parasitic disease in the CNS develops inflammation revealed by hypercellularity and as a consequence of edema. The cellular malfunctions develop breakdown of the blood–brain barrier, which is evident by imaging studies. These abnormalities are overlapping for several other inflammatory, infectious, and space-occupying lesions, such as metastatic disease, gliomas, demyelinating disease, resolving cerebral hemorrhages, subacute infarctions, tuberculomas, and cerebral abscesses [Citation48]. Infectious and noninfectious inflammatory lesions characteristically show low-density change (CT) or high signal change (T2 MR imaging) consistent with edema and associated swelling, mass effect, and shift may be present. Therefore, acuity on neuroimaging is variable; it may wax and wane. It may be a one-time manifestation; it may vary with treatment; or may be chronic [Citation49]. At times, imaging shows more specific disease features that may allow a more precise diagnosis, as in cases of NCC and echinococcosis.
Neuroimaging is useful in diagnosis of NCC, as it permits visualization of the parasite evolutionary stage, as well as number and localization of lesions [Citation11]. Specifically, imaging procedures allow visualization of vesicular, colloidal, granular-nodular and calcified phases of the parasite in the CNS (). MRI is more sensitive than CT for the detection of the scolex and for the diagnosis of extraparenchymal NCC [Citation4,Citation49].
Figure 2. Imaging findings in patients with neurocysticercosis. A) MRI of parenchymal vesicular cyst with scolex. B) MRI of colloidal cysticerci with perilesional edema. C) CT scan of many calcified parasites. D) MRI of intraventricular cysts.
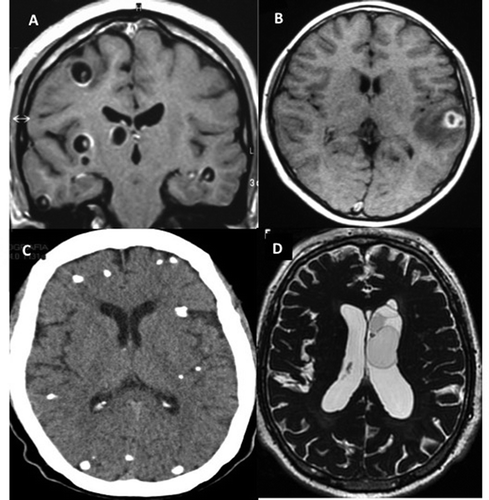
In the vesicular phase, the CT scan depicts circumscribed, round, hypodense areas, varying in size and number, without enhancement by contrast media. In the MRI, the vesicular larva appears with a CSF-like intensity signal on all sequences, with no surrounding high signal on T2-weighted images. Both MRI and CT may show a high intensity or hyperdense, 2–3 mm mural nodule depicting the scolex, within some vesicular cysts. As the cyst degenerates, the contrast-enhanced CT scan shows an annular (colloidal phase) or nodular (nodular phase) enhancement surrounded by irregular perilesional edema. In this phase, the fluid content gives a slightly higher signal than CSF and is occasionally isodense with the parenchyma on MRI-T1 and/or proton density-weighted, and high signal on T2 images. The capsule shows a higher signal than the adjacent brain, with thick ring enhancement on T1 images, while on T2 images there is a low ring signal surrounded by high signal lesion, due mostly to edema. When the cyst dies it may disappear or become an inactive calcified nodule with homogeneous high density on CT or low intensity on proton-weighted MRI.
Extraparenchymal NCC is more difficult to detect by imaging because the attenuation and signal intensity of the cyst’s content is similar to that of CSF, the cystic wall is usually not detected, there is not enhancement after intravenous contrast administration, and the cysts frequently lack a scolex. MRI techniques such as FLAIR and FIESTA sequences permit better detection of the parasites (). In case of meningeal inflammatory process, gadolinium enhancement of MRI or contrast-enhanced CT may depict leptomeningeal thickening. Occasionally, a grape-like cluster of cysts (racemose form) without scolices may arise in the cisterns and sylvian fissure and does not show associated enhancement or progression through the stages described above [Citation50].
Neuroimaging studies in patients with toxoplasmosis commonly show multifocal abscesses with a predilection for the basal ganglia. However, solitary lesions have been noted in about one-third of patients. Most lesions show enhancement, often in a ring-like pattern. The eccentric target sign, which is characterized by a small nodule, along the wall of the enhancing ring, has been reported to be highly suggestive of a diagnosis of toxoplasmosis [Citation51].
The imaging appearance of toxoplasmosis may overlap that of CNS lymphoma and other neoplasms. Subcortical location, eccentric target sign, absence of corpus callosal or leptomeningeal involvement, and marked edema are imaging findings that favor toxoplasmosis, whereas hyperattenuation, hypointensity on T2-weighted images, restricted diffusion, and periventricular location favor CNS lymphoma. MR spectroscopy can be helpful in the differential diagnosis [Citation52].
Hydatid cysts in imaging studies appear as large, well-defined, smooth, thin-walled, cystic lesions that are spherical or oval in shape, usually with no surrounding edema. The cyst contents typically have CSF-like density on CT and CSF-like signal characteristics on all MR sequences. On post contrast images, a thin rim of enhancement may be seen. The presence of a daughter cyst within a cystic lesion is considered pathognomonic of an echinococcus cyst [Citation53].
Neuroimaging findings in schistosomiasis are variable and nonspecific. On CT, single or multiple variably enhancing hyperattenuated lesions with surrounding hypoattenuated edema may be present and reflect a focal granulomatous reaction [Citation22]. MRI shows foci of low signal on T1 and high signal on T2, with contrast enhancement. A characteristic MR imaging pattern of lesions is a large mass comprising multiple intensely enhancing nodules, sometimes with areas of linear enhancement. Central linear enhancement surrounds multiple enhancing punctate nodules, forming an ‘arborized’ appearance [Citation54]. Although this pattern is highly suggestive, it is a rare presentation.
On MRI, conglomerates of multiple ring shaped shadows or enhancement of so-called ‘soap bubble’ forms in one hemisphere is a suggestive image of paragonimiasis. On CT, multiple calcifications with round or oval shapes surrounded by low-density areas, cortical atrophy and ventricular dilation are seen [Citation4]. Chagas meningoencephalitis usually manifests as multiple expanding hyperintense lesions on T2-weighted images with nodular or annular enhancement in imaging studies. Additionally, it may be seen in the spinal cord. These findings are quite similar to those displayed in toxoplasmosis and CNS lymphoma. On MRI, HAT shows meningeal thickening, with multiple white-matter T2 hyperintense lesions or meningeal enhancement and diffuse hypoattenuated areas affecting the white matter in both hemispheres on CT. MRI toxocariasis images show unspecific circumscribed, multifocal lesions in the brain white matter, with contrast enhancement. Imaging findings in cerebral malaria include cerebral edema, cortical and subcortical ischemic lesions, and multiple petechial hemorrhages [Citation53].
On MRI, angiostrongyliasis often reveals multiple micronodular enhancements in brain tissues and linear enhancement in the pia mater. Complete resolution of abnormal MRI findings typically occurs after 4–8 weeks. Micronodules have also been detected in MR imaging of lungs, which may reflect the presence of worms [Citation2].
Overview of medical treatment of parasitic infections of the CNS
Analogous to their heterogeneity in clinical presentation, treatment of parasitic infections of the CNS is diverse and spans both medical and surgical treatment. Medical treatment often involves pharmacotherapy to kill the parasite and adjunctive care to prevent or treat complications related to infiltration of the parasite (e.g. anticonvulsants for patients presenting with seizures). Similarly, surgical treatment might involve physical removal of the parasite (e.g. cysts in the CNS) or treating complications of the parasite (e.g. hydrocephalus).
Antiparasitic drugs are indicated for most parasitic infections of the CNS () [Citation22,Citation55]. However, there are some exceptions when the risks of treatment outweigh its potential benefits, particularly when antiparasitic treatment may result in host inflammatory responses that are uncontrollable, dangerous, or destructive. In other instances, antiparasitic treatment may simply be ineffective because the parasite is already dead, but still causing symptoms (e.g. calcified NCC). Antiparasitic treatment may also be adjunctive to surgery, such as in echinococcosis, where perioperative antihelminthic drugs can facilitate cyst removal and prevent cyst regeneration.
Table 3. Currently available antiparasitic drug regimens for parasitic infections of the CNS.
The efficacy of antiparasitic drugs varies widely. In cerebral malaria, for example, the drugs are effective, but mortality remains high, so much research has focused on optimizing adjunctive care to reduce mortality. In other infections, antiparasitic drugs may have suboptimal efficacy or be highly toxic, demonstrating an unmet need for new antiparasitic drugs, some of which are in the clinical pipeline, such as fexinidazole for CD and HAT. Despite these challenges, high quality research has been able to establish a more reliable evidence base for treating and managing some of these ‘neglected’ infections.
Recent progress in the treatment and management of parasitic infections of the CNS
For NCC, substantial progress has been made in treatment of patients with active cysts in the brain parenchyma. A recent randomized controlled trial enrolling patients with at least one active cyst and a history of seizures found that the combination of albendazole and praziquantel was significantly more effective than albendazole monotherapy in resulting in complete cyst disappearance in patients with three or more active cysts [Citation56], and not significantly more effective in patients with one or two cysts, confirming results of a previous study in children [Citation57]. Although this treatment was more effective in eradicating the parasite in patients with multiple active cysts, it was not associated with a greater proportion of patients with complete seizure remission. Despite this progress for parenchymal NCC, there is little evidence for treating extraparenchymal disease. As this form of the disease has a much worse prognosis and is associated with higher fatality, many questions remain about which antihelminthic agent (or potentially combination of agents), dose, and treatment duration is necessary.
There are no specific trials providing evidence for managing or treating CNS involvement in acute or chronic CD. In general, the role of antiparasitic treatment has been unclear in the chronic stage of disease, but a recent large randomized controlled trial of a course of benznidazole versus placebo in patients with chronic CD cardiomyopathy has provided important evidence that may be relevant for patients with different manifestations of the disease [Citation58]. This trial found that benznidazole treatment was associated with a significant reduction in parasitemia, but unfortunately, there were no significant effects of the drug seen in clinical efficacy end points. However, a reassuring aspect of this study is that the safety and tolerability of benznidazole were much better than observed in previous observational studies.
The arsenal for treatment of the second, meningoencephalitic stage of Trypanosoma brucei gambiense HAT has been long characterized by highly toxic compounds such as melarsoprol, which is associated with a substantial rate of reactive meningoencephalopathy and on-treatment death. A milestone for the treatment of second-stage Trypanosoma brucei gambiense HAT has been nifurtimox–eflornithine combination therapy (NECT), which consists of IV nifurtimox for 10 days and intravenous eflornithine for 7 days, and is now on the World Health Organization (WHO) Essential Medicines List. A recent retrospective cohort study of patients treated at Médicins Sans Frontièrs-supported hospitals in northeastern Democratic Republic of the Congo provided information about NECT in the ‘real world’ setting outside of clinical trials [Citation59]. This study provided strong confirmatory evidence of the efficacy and safety observed in clinical trials, with high cure rates, very low rates of in-hospital mortality and low rates of major adverse events. Of particular note, children appeared to tolerate NECT particularly well compared with adults, who had about four times greater odds of experiencing an adverse event.
For Trypanosoma brucei rhodesiense, NECT is unlikely to be effective against second-stage HAT, leaving melarsoprol as the only effect treatment option. Despite the safety concerns with this agent, some progress has been made to improve its use in clinical practice. Treatment for second stage Trypanosoma brucei rhodesiense HAT is country protocol-specific and has typically included suramin pre-treatment, followed by melarsoprol infusions, which are separated by 5–7 day resting periods, with an overall hospitalization of approximately 1 month. A dual-publication of a proof-of-concept trial and utilization study explored a 10-day melarsoprol regimen for Trypanosoma brucei rhodesiense [Citation60]. In this study, the 10-day melarsoprol regimen had a similar incidence of encephalopathic syndrome and case fatality relative to historic data. Parasitic and clinical cure rates were high, with mean hospitalization time reduced from 29 to 13 days. Furthermore, there was no observed benefit of suramin pre-treatment, which has not been an evidence-based practice. This regimen should now be considered the preferred regimen for treating second-stage Trypanosoma brucei rhodesiense HAT.
Ivermectin has been the gold-standard treatment for onchocerciasis, but there is concern about the reliance on one solitary drug, and uncertainty about how to handle apparent treatment failures and situations where ivermectin cannot be used. A recent randomized controlled trial conducted in Ghana provided formal data on the efficacy and safety of doxycycline [Citation61]. This trial compared a 6-week course of daily doxycycline versus placebo among patients recruited in areas with suboptimal response to ivermectin (i.e. significant microfilaridermia despite a history of multiple rounds of ivermectin treatment). At 20 months’ follow-up, doxycycline treatment was associated with a much lower proportion of Wolbachia positive living female worms, much greater freedom from microfilaridermia, and greater number of dead worms compared with placebo. These data on doxycycline treatment are particularly relevant when treating patients co-infected with Loa loa, who are at increased risk for life-threatening encephalopathy when given ivermectin.
Aside from antiparasitic treatment, adjunctive treatment is a cornerstone of managing parasitic infections of the CNS, particularly in cerebral malaria, where patients are managed in intensive care units and mortality is high. Two issues related to adjunctive care in cerebral malaria are managing cerebral edema and starting enteral feeding.
Mild to moderate edema is a common finding on CT in patients with cerebral malaria, but its role in the pathophysiology of cerebral malaria has been unclear. Nevertheless, osmotic diuretics, such a mannitol, have often been routinely used to treat this cerebral edema. A randomized controlled trial conducted in India sought to determine if this treatment was in fact beneficial [Citation62]. In this open-label trial, adult patients with cerebral malaria and cerebral edema were randomized to receive either no treatment or a regimen of mannitol infusions. Not only was degree of cerebral edema not associated with coma depth or mortality, but patients who received mannitol had significantly longer coma recovery time and nonsignificantly higher mortality.
In general, enteral feeding is often started soon after admission and intubation in the intensive care unit as it can improve outcomes; however, endotracheal intubation is not always realistic, particularly in resource-poor settings. To explore the clinical utility and safety of early enteral feeding in non-intubated patients with cerebral malaria, a trial in Bangladesh randomized patients to receive enteral feeding through nasogastric tube either early (upon admission) or late (up to 60 h in adults or 36 h in children) after hospitalization [Citation63]. The trial was stopped early because of much higher incidence of aspiration pneumonia in the early feeding group relative to the late feeding group. Aside from the applicability of these results to practice, this trial highlights the importance considering the practicalities of resource-poor settings when using an evidence-based approach to manage and treat parasitic infections of the CNS.
Burden, control and elimination of parasitic diseases of the CNS
There are approximately one billion people who are affected by one or more neglected tropical disease (NTD). These diseases are considered ‘neglected’ because they occur mostly in the poorest of populations [Citation19]. Among these NTDs are several parasitic infections that affect the CNS. details the approximate prevalence and burden of some parasitic diseases that can affect the CNS [Citation64–Citation69].
Table 4. Approximate prevalence and disability-adjusted life years (DALYs) of common parasitic infections.
While disability-adjusted life years (DALYs) are a good objective measure of the burden of disease, because of methodological challenges, these figures generally underestimate the disability associated with parasitic infections. In addition, they do not take into account the economic or social losses because of impact on productivity or stigma [Citation68].
Parasitic infections of CNS are largely preventable and highly burdensome. For example, NCC is known to cause epilepsy and other neurological sequelae, and is the most frequent preventable cause of epilepsy in developing countries [Citation70]. A recent meta-analysis revealed that brain lesions attributed to NCC are present in approximately 29.0% (95% CI: 22.9–35.5%) of people with epilepsy in populations living in Taenia solium endemic areas in settings with poor sanitation and pig management practices and where pork is consumed [Citation71]. In these areas, the burden of epilepsy is almost 6.8 million DALYs per year [Citation72] and mortality because of NCC is about 3–6 times higher than in the general population [Citation70]. However, these rates are likely underestimations, because of inadequate diagnosis and incomplete reporting in low resource settings and because other important associated symptoms, such as chronic headache, hydrocephalus, stroke, and depressive disorders have not yet been taken into account when calculating burden estimations [Citation10]. illustrates countries and areas at risk for cysticercosis [Citation73].
Figure 3. Countries and areas at risk for cysticercosis.
Reprinted from: World Health Organization. Assembling a Framework for Intensified Control of Taeniasis and Neurocysicercosis caused by Taenia solium, 2013. Available at: http://apps.who.int/iris/bitstream/10665/153237/1/9789241508452_eng.pdf?ua=1 [Last accessed 29 December 2015].
![Figure 3. Countries and areas at risk for cysticercosis.Reprinted from: World Health Organization. Assembling a Framework for Intensified Control of Taeniasis and Neurocysicercosis caused by Taenia solium, 2013. Available at: http://apps.who.int/iris/bitstream/10665/153237/1/9789241508452_eng.pdf?ua=1 [Last accessed 29 December 2015].](/cms/asset/7f35c2a3-8c23-4282-a7e0-30ddb3f9a83c/iern_a_1155454_f0003_oc.jpg)
In the year 2015, approximately 3.2 billion people – nearly half of the world’s population – were at risk for malaria. Malaria accounts for 3.3% of all DALYs and is ranked seventh among the top leading causes of DALYs globally [Citation69]. Sub-Saharan Africa continues to carry a disproportionately high share of the global malaria burden, being home to 89% of malaria cases and 91% of malaria deaths. In 2015, an estimated 438,000 deaths were related to malaria. Young children, pregnant women and non-immune travelers from malaria-free areas are particularly vulnerable to the disease when they become infected [Citation66].
Although data on the economic burden of parasitic diseases are confined to small studies in limited geographical areas, the costs for individuals, healthcare systems and economies are grossly high. For example, the monetary cost of cysticercosis is estimated at US$15.27 million in India, US$28.3 million in Honduras and US$16.6 million in the Eastern Cape province (South Africa). In Latin America, CD is estimated to account for 752,000 lost working days per year because of premature deaths, at a cost of over US$1.2 billion per year [Citation74]. There is also an unquantifiable dimension to the burden of parasitic diseases that reduces the productivity of millions of women who care for families and maintain households in countries where such diseases are endemic. Additionally, in low- and middle-income countries, children are an economic resource and by improving their health, these economies would be much better off.
Prevention and control
In the year 2011, WHO adopted a roadmap for the control and elimination of NTDs, including many parasites that affect the CNS [Citation19]. Principles for action include:
A focus on populations and interventions rather than specific diseases;
The introduction of innovative tools for parasite detection and control;
A multi-disease, intersectorial and interprogrammatic approach.
This framework endorses the combination of several strategic approaches, including (i) community sensitization and mobilization campaigns; (ii) chemoprevention; (iii) intensified case-management; (iv) symptomatic management; (v) vector control; (vi) provision of safe food and water, sanitation and hygiene; and (vii) veterinary public health to prevent animal to human transmission. Furthermore, strategies should be cost-effective, ethical, environmentally sustainable, and applicable within local health structures and agricultural systems [Citation19].
Many large-scale programs for control, elimination or eradication are being implemented by WHO and other partners and stakeholders. Multi-pronged interventions have already benefited millions of people, including the expansion of malaria interventions, which have helped to reduce malaria incidence by 30% globally and by 34% in Africa. This interdisciplinary approach included vector control through insecticide-treated mosquito nets and indoor residual spraying, public awareness strategies, symptomatic treatment, as well as seasonal malaria chemoprevention. Since 2000, the prevalence of malaria parasite infection (including both symptomatic and asymptomatic infections) has decreased significantly in Africa, falling from 173 million to 128 million in 2013 – a reduction of 26%. This has occurred despite a 43% increase in the African population living in malaria transmission areas [Citation75].
Similar work to control and eliminate other parasites has been conducted, including a sustained national schistosomiasis control program in many countries, such as China, which has interrupted transmission in most endemic areas through a comprehensive multi-faceted strategy including the construction of dams, delivery of potable water and providing basic sanitation [Citation19]. In 2014 Ecuador became the second country in the world after Colombia in 2013 to be declared free of onchocerciasis after successfully implementing elimination activities for decades, which included community-directed treatment with ivermectin [Citation76].
Many World Health Assembly (WHA) resolutions have provided a mandate for countries, as well as other stakeholders, to become more active in this area. These include the adoption of an updated resolution on the NTDs in 2013 strengthening efforts to prevent, control, eliminate or eradicate NTDs. In addition, there are specific resolutions addressing the policies and technical assistance required by countries to deal with many parasitic diseases that affect the CNS [Citation77]. Likewise, many disease-specific resolutions have also been adopted and have highlighted that further investments are required regarding preventing parasitic-related neurological diseases, including the epilepsy resolution endorsed in 2015, which includes reference to NCC and its prevention [Citation78].
While there are barriers, it is entirely possible to control or eliminate or eradicate parasitic diseases. During the past decade, there has been an improved recognition that human-, animal- and ecosystem-health are inextricably linked. The international community, as well as local community partners, have committed to bringing resources and expertise to the task of overcoming parasitic and other vector-borne diseases. This has included the concept of ‘One Health,’ and the corresponding approach, which aims to bring together human health care practitioners, veterinarians, public health and environmental health professionals. This collaboration reduces the gaps between institutions and disciplines that can cause costly delays, and even failures, in disease detection and control [Citation79]. The use of an intersectoral approach in many settings indicates improved cost–effectiveness and ensures treatment coverage reaches neglected populations [Citation70].
Overcoming parasite-related disease makes sense both for economies and for development. Without a significant reduction in the burden of these diseases, the achievement of other health-related goals as well as those in education, gender equality, poverty reduction and economic growth will be jeopardized. Improving the control and elimination of parasitic disease is therefore key to achieving the Sustainable Development Goals (SDGs). By tackling parasitic diseases, more than one billion people living in low-income endemic areas have a chance for improved health and wellbeing. These diseases should be neglected no more.
Expert commentary
Parasitic diseases of the CNS that were previously labeled as ‘exotic’ diseases are a serious global health issue. Parasites specific to humans might accidently be located in abnormal or ectopic locations, including the brain or spinal cord. However, precise estimates of prevalence and burden of many of these diseases are lacking, and information on the pathogenesis of CNS lesions is limited. Parasitic infections in the CNS can present with a wide range of clinical manifestations, which range from fulminant to insidious onset and chronic progressive to relapsing-remitting courses. Most of these diseases have specific findings in relation to areas of involvement of the CNS and can sometimes have overlapping imaging features. Based on a high clinical index of suspicion, patient demographics, and specific laboratory investigations, a proper diagnosis can be made. Diagnosis of parasitic infections relies on the demonstration of an offending organism using laboratory investigations. Nevertheless, neuroimaging studies play an important role in reliable and early diagnosis. In terms of treatment, substantial progress has been made in developing an evidence base for existing drugs and treatment approaches. However, new therapeutic agents are needed for some infections, particularly in light of the suboptimal efficacy and high toxicity of some agents. Ultimately, prevention and control are needed to progress towards eradicating these diseases.
Five-year view
The genome of some parasites, particularly Taenia solium, will soon be sequenced, and this may improve our knowledge of mechanisms of pathogenesis and the genetic role in disease heterogeneity. CNS parasitic diseases, especially NCC, are probably a risk factor for both acute seizures and acquired epilepsy in endemic countries. An interesting theory to consider NCC as a human model of epileptogenesis has been proposed; however, prospective cohort studies are needed to assess the association of different evolutionary phases of the parasite, potential genetic predisposition, and the role of precipitating factors in the development of seizures and epilepsy. In this context, use of International League Against Epilepsy guidelines for epidemiological studies to standardized concepts of classification of epilepsy is mandatory.
Many of current diagnostic strategies for detecting parasites are labor intensive, insensitive and in the case of serology, not necessarily indicative of current active infection. Clearly, additional research into the safety, practicality, and feasibility of immunological and molecular diagnosis is warranted. Molecular methods with high sensitivity and specificity for the detection of parasites that provide prognostic information to the clinician are an aspect of care that has been absent in the field of parasitology. Management of parasitic infections will necessarily be advanced by improved diagnostic capabilities. Molecular methods are also likely to play an increasingly important role in determination of drug resistance.
Regarding prevention and control, despite the gains obtained by the WHO programs, continuing challenges remain for the future. These are predominantly related to:
Quantification of burden among neglected populations;
Urgent development, improvements and accessibility of diagnostic tools;
Provision of treatment and other interventions to communities in need;
Procurement and supply of medicines and a system for delivery of these to cover at-risk populations;
Innovation of more effective medicines, vaccines and insecticides;
Promotion of integrated vector management;
Early protection of children and other special populations, including those with HIV;
Surveillance and monitoring.
Future field interventions should meet basic methodological requirements: (i) an adequate study design and use of validated surveys in community-based studies; (ii) case–control studies with high levels of exposure to parasites, (iii) sufficient statistical power by recruiting adequate numbers of people with epilepsy and their control, (iv) matching of controls by sex, age, location and confounders, and (v) neuroimaging, molecular (PCR), and serological assays (Ag-ELISA and EITB) and CSF should be performed for all included participants.
Key issues
CNS parasitic infections can be life-threatening, but are often preventable and treatable; however, clinical outcomes largely depend on early diagnosis and treatment.
Host and parasite factors involved in clinical heterogeneity and brain inflammation are beginning to be deciphered and will provide new clues to better management of these diseases in the future.
Evidence-based recommendations for management of parasitic infections warrant much additional investment and research in the coming years.
Anti-parasite antibodies are synthesized soon after host invasion and so their detection is still the most frequently employed diagnostic tool. However, their detection is not definitive proof of a current, viable infection as antibodies can persist for months, even years, after elimination of the parasite.
The development of specific and sensitive serodiagnostic and molecular biological (PCR) assays for viable parasites is an urgent priority that will complement and confirm clinical examination.
Neuroimaging studies (CT scan and MRI) play an important role in early diagnosis; however, there is a wide range of neuroimaging findings in CNS parasite infections, often with considerable overlap, which makes diagnosis difficult.
Many large-scale programs for control, elimination or eradication are being implemented by WHO and other partners and stakeholders.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Walker M, Kublin JG, Zunt JR. Parasitic central nervous system infections in immunocompromised hosts: malaria, microsporidiosis, leishmaniasis, and African trypanosomiasis. Clin Infect Dis. 2006;42:115–125.
- Graeff-Teixeira C, Da Silva AC, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22:322–348.
- Nash TE. Parasitic diseases that cause seizures. Epilepsy Curr. 2014;14:29–34.
- Abdel Razek AA, Watcharakorn A, Castillo M. Parasitic diseases of the central nervous system. Neuroimaging Clin N Am. 2011;21:815–841.
- Vezzani A, Fujinami RS, White HS, et al.. Infections, inflammation and epilepsy. Acta Neuropathol. 2016; 131:211–234.
- Serem GK, Newton CR, Kariuki SM. Incidence, causes and phenotypes of acute seizures in Kenyan children post the malaria-decline period. BMC Neurol. 2015;15:180.
- Ngugi AK, Bottomley C, Kleinschmidt I, et al.. Prevalence of active convulsive epilepsy in Sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–263.
- Kamuyu G, Bottomley C, Mageto J, et al. Exposure to multiple parasites is associated with the prevalence of active convulsive epilepsy in sub-Saharan Africa. PLoS Negl Trop Dis. 2014;8:e2908.
- Showler AJ, Wilson ME, Kain KC, et al.. Parasitic diseases in travelers: a focus on therapy. Expert Rev Anti Infect Ther. 2014;12:497–521.
- Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5:e1152.
- Carpio A, Fleury A, Hauser WA. Neurocysticercosis: five new things. Neurol Clin Pract. 2013;3:118–125.
- Carpio A, Romo ML. The relationship between neurocysticercosis and epilepsy: an endless debate. Arq Neuropsiquiatr. 2014;72:383–390.
- Cárdenas G, Jung H, Ríos C, et al.. Severe cysticercal meningitis: clinical and imaging characteristics. Am J Trop Med Hyg. 2010;82:121–125.
- Fleury A, Garcia E, Hernandez M, et al. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS Negl Trop Dis. 2013;7:e2096.
- Ngoungou EB, Bhalla D, Nzoghe A, et al. Toxoplasmosis and epilepsy–systematic review and meta analysis. PLoS Negl Trop Dis. 2015;9:e0003525.
- Groer MW, Yolken RH, Xiao JC, et al. Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am J Obsetet Gynecol. 2011;204:433.
- Brooks JM, Carrillo GL, Su J, et al. Toxoplasma gondii infections alter gabaergic synapses and signaling in the central nervous system. Mbio. 2015;6:e01428-15.
- Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16.
- World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases; a roadmap for implementation (executive summary). [2012; cited 2015 Dec 29]. Available from: http://apps.who.int/iris/bitstream/10665/70809/1/WHO_HTM_NTD_2012.1_eng.pdf.
- Böttcher D, Bangoura B, Schmäschke R, et al. Diagnostics and epidemiology of alveolar echinococcosis in slaughtered pigs from large-scale husbandries in Germany. Parasitol Res. 2013;112:629–636.
- Davidson RK, Romig T, Jenkins E, et al. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–247.
- Newton CR, Preux P-M, Singhi P. Parasitic disorders. Handb Clin Neurol. 2013;112:1139–1152.
- Ferrari TC, Moreira PR. Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol. 2011;10:853–864.
- Carod-Artal FJ. Neuroschistosomiasis. Expert Rev Anti Infect Ther. 2010;8:1307–1318.
- Katchanov J, Nawa Y. Helminthic invasion of the central nervous system: many roads lead to Rome. Parasitol Int. 2010;59:491–496.
- Pittella JE. Pathology of CNS parasitic infections. Handb Clin Neurol. 2013;114:65–88.
- White NJ, Pukrittayakamee S, Hien TT, et al. Malaria. Lancet. 2014;383:723–735.
- Quattrocchi G, Nicoletti A, Marin B, et al. Toxocariasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1775.
- Kaiser C, Pion SD, Boussinesq M. Case-control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2147.
- Colebunders R, Post R, O’Neill S, et al. Nodding syndrome since 2012: recent progress, challenges and recommendations for future research. Trop Med Int Health. 2015;20:194–200.
- Winkler AS, Friedrich K, Velicheti S, et al. MRI findings in people with epilepsy and nodding syndrome in an area endemic for onchocerciasis: an observational study. Afr Health Sci. 2013;13:529–540.
- Perez CJ, Lymbery AJ, Thompson RC. Reactivation of Chagas disease: implications for global health. Trends Parasitol. 2015;31:595–603.
- Leon-Sarmiento FE, Mendoza E, Torres-Hillera M, et al. Trypanosoma cruzi-associated cerebrovascular disease: a case-control study in Eastern Colombia. J Neurol Sci. 2004;217:61–64.
- Wilkins PP. Immunodiagnosis of CNS parasitic infections. Handb Clin Neurol. 2013;114:23–36.
- Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26:137–144.
- Ferrer E, Bonay P, Foster-Cuevas M, et al. Molecular cloning and characterisation of Ts8B1, Ts8B2 and Ts8B3, three new members of the Taenia solium metacestode 8 kDa diagnostic antigen family. Mol Biochem Parasitol. 2007;152:90–100.
- Harrison LJ, Joshua GW, Wright SH, et al.. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989;11:351–370.
- Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–477.
- Garcia HH, Parkhouse RM, Gilman RH, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans Royal Soc Trop Med Hyg. 2000;94:673–676.
- Nguekam JP, Zoli AP, Ongolo-Zogo P, et al. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite. 2003;10:65–68.
- Rodriguez S, Dorny P, Tsang VC, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199:1345–1352.
- Hernández M, Gonzalez LM, Fleury A, et al. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann Trop Med Parasitol. 2008;102:317–323.
- Blanchard N, Dunay IR, Schlüter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 2015;37:150–158.
- Slemenda SB, Maddison SE, Jong EC, et al.. Diagnosis of paragonimiasis by immunoblot. Am J Trop Med Hyg. 1988;39:469–471.
- Mishra SK, Newton CR. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5:189–198.
- Vidal JE, Sztajnbok J, Seguro AC. Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg. 2003;69:341–343.
- Umezawa ES, Nascimento MS, Kesper N Jr., et al. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol. 1996;34:2143–2147.
- Saini J, Gupta RK, Jain KK. Intracranial infections: key neuroimaging findings. Semin Roentgenol. 2014;49:86–98.
- Mullins ME. Emergent neuroimaging of intracranial infection/inflammation. Radiol Clin North Am. 2011;49:47–62.
- Shih RY, Koeller KK. Bacterial, fungal, and parasitic infections of the central nervous system: radiologic-pathologic correlation and historical perspectives. Radiographics. 2015;35:1141–1169.
- Kumar GG, Mahadevan A, Guruprasad AS, et al. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31:1469–1472.
- Barcelo C, Catalaa I, Loubes-Lacroix F, et al. Interest of MR perfusion and MR spectroscopy for the diagnostic of atypical cerebral toxoplasmosis. J Neuroradiol. 2010;37:68–71.
- Jayakumar PN, Chandrashekar HS, Ellika S. Imaging of parasitic infections of the central nervous system. Handb Clin Neurol. 2013;114:37–64.
- Liu H, Lim CC, Feng X, et al. MRI in cerebral schistosomiasis: characteristic nodular enhancement in 33 patients. AJR Am J Roentgenol. 2008;191:582–588.
- Drugs for Parasitic Infections. Treatment guidelines from the medical letter. Vol. 8 (Suppl). New Rochelle (NY): The Medical Letter, Inc.; 2010.
- Garcia HH, Gonzales I, Lescano AG, et al.. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:687–695.
- Kaur S, Singhi P, Singhi S, et al.. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr Infect Dis. 2009;28:403–406.
- Morillo CA, Marin-Neto JA, Avezum A, et al.. Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295–1306.
- Alirol E, Schrumpf D, Amici Heradi J, et al.. Nifurtimox-eflornithine combination therapy for second-stage gambiense human African trypanosomiasis: Médicins Sans Frontièrs experience in the Democratic Republic of the Congo. Clin Infect Dis. 2013;56:195–203.
- Kuepfer I, Schmid C, Allan M, et al. Safety and efficacy of the 10-day melarsoprol schedule for the treatment of second stage Rhodesiense sleeping sickness. PLoS Negl Trop Dis. 2012;6:e1695.
- Debrah AY, Specht S, Klarmann-Schulz U, et al.. Doxycycline leads to sterility and enhanced killing of female onchocerca volvulus worms in an area with persistent microfilaridermia after repeated ivermectin treatment: a randomized, placebo-controlled, double-blind trial. Clin Infect Dis. 2015;61:517–526.
- Mohanty S, Mishra SK, Patnaik R, et al.. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis. 2011;53:349–355.
- Maude RJ, Hoque G, Hasan MU, et al. Timing of enteral feeding in cerebral malaria in resource-poor settings: a randomized trial. PLoS One. 2011;6:e27273.
- World Health Organization. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. [2010; cited 2015 Dec 29]. Available from: http://apps.who.int/iris/bitstream/10665/44440/1/9789241564090_eng.pdf.
- World Health Organization. Echinococcosis, fact sheet no.377. [2015; cited 2015 Dec 29]. Available from: http://www.who.int/mediacentre/factsheets/fs377/en/.
- World Health Organization. Malaria, fact sheet no.094. [2015; cited 2015 Dec 29]. Available from: http://www.who.int/mediacentre/factsheets/fs094/en/.
- World Health Organization. WHO estimates of the global burden of foodborne diseases. [2015; cited 2015 Dec 29]. Available from: http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf?ua=1.
- Hotez PJ, Alvarado M, Basanez MG, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865.
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223.
- World Health Organization. Investing to overcome the global impact of neglected tropical diseases; third WHO report on neglected tropical diseases. [2015; cited 2015 Dec 29]. Available from: http://apps.who.int/iris/bitstream/10665/152781/1/9789241564861_eng.pdf?ua=1.
- Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870.
- Torgerson PR, Macpherson CN. The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol. 2011;182:79–95.
- World Health Organization. Assembling a framework for intensified control of taeniasis and neurocysicercosis caused by taenia solium. [2013; cited 2015 Dec 29]. Available from: http://apps.who.int/iris/bitstream/10665/153237/1/9789241508452_eng.pdf?ua=1.
- Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375:239–247.
- World Health Organization. World malaria report. [2014; cited 2015 Dec 29]. Available from: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/.
- World Health Organization. Onchocerciasis, fact sheet no.374. [2015; cited 2015 Dec 29]. Available from: http://www.who.int/mediacentre/factsheets/fs374/en/.
- World Health Assembly (WHA). Resolutions on neglected tropical diseases: 1948–2013. [cited 2015 Dec 29]. Available from: http://www.who.int/neglected_diseases/mediacentre/resolutions/en/).
- Sixty-eighth World Health Assembly adopts resolution on epilepsy. [cited 2015 Dec 29]. Available from: http://www.who.int/mental_health/neurology/epilepsy/resolution_68_20/en/.
- The World Bank. People, pathogens, and our planet. Volume 2: the economics of one health. Report no. 69145-GLB. [2012; cited 2015 Dec 29]. Available from: https://openknowledge.worldbank.org/bitstream/handle/10986/11892/691450ESW0whit0D0ESW120PPPvol120web.pdf?sequence=1.