Abstract
The use of pneumococcal conjugate vaccine (PCV) in childhood pneumococcal immunization programs successfully reduced the incidence of pneumococcal disease in children. Nonetheless, there remains a high burden of pneumococcal disease in adults, especially the elderly, and children/adults with chronic medical conditions. Two pneumococcal vaccines are currently available for adults at risk of pneumococcal disease: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the more recently licensed PCV13. As it is not possible to determine vaccine efficacy in all populations at risk of pneumococcal disease, immunogenicity studies, measuring pneumococcal-specific antibody concentrations and/or the opsonophagocytic activity of serum can provide valuable comparative data for PPV and PCV immunization. This article provides consolidated data on the immunogenicity of PCVs (largely PCV7, and a few studies with PCV9 or PCV13) based on a review of immunogenicity/safety studies in populations (mainly pediatric) at increased risk of pneumococcal disease.
Streptococcus pneumoniae (pneumococcus) is a leading cause of serious illness and death worldwide, with invasive manifestations including sepsis, meningitis and bacteremic pneumonia Citation[1–3]. The successful use of pneumococcal conjugate vaccine (PCV7) (comprising serotypes 4, 6B, 9V, 14, 18C, 19F, 23F) in childhood pneumococcal immunization programs worldwide has led to a reduced frequency of vaccine-type invasive pneumococcal disease (IPD) in vaccine-eligible children (aged <2 or <5 years, depending on the study) Citation[4,5]. Nevertheless, there remains a high burden of pneumococcal disease, primarily pneumococcal community-acquired pneumonia in adults, especially the elderly (aged >65 years) Citation[2,6,7] and in individuals of all ages with certain medical conditions Citation[8,9]. These conditions include chronic heart, hepatic or pulmonary disease, and diabetes mellitus, in immunocompetent individuals; immunosuppression due to acquired immunodeficiency syndrome or other conditions or to treatments that increase risk, including malignancies (solid tumor or hematologic) or transplantation (stem cell or organ); or asplenia, including sickle cell disease Citation[8,10–13]. There are also data suggesting that children with asthma characterized as chronic and/or with recurrent exacerbations, despite optimal treatment, are at increased risk of IPD, although many health authorities consider asthma to be a risk factor only in children receiving oral corticosteroid therapy Citation[14].
Until 2011, the 23-valent pneumococcal polysaccharide vaccine (PPV23) was the only vaccine licensed for active pneumococcal immunization in adults at risk of pneumococcal disease Citation[15]. PCV13 (Prevenar 13®, Pfizer; comprising serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F) has been licensed in Europe since 2011 for adults aged ≥50 years, with the indication expanded in 2013 to include children and adults aged 6–49 years. PCV13 is indicated in Europe for the prevention of IPD, pneumonia and acute otitis media (AOM) caused by S. pneumoniae in infants and children from 6 weeks up to the age of 17 years, and for the prevention of IPD and pneumonia caused by S. pneumoniae in adults aged ≥18 years Citation[16]. In the USA, PCV13 is indicated for the prevention of IPD and AOM in children aged 6 weeks to ≤5 years, for the prevention of IPD in those aged 6–17 years and for the prevention of IPD and pneumonia in those aged ≥50 years of age Citation[17]. PCV13 is also available for use in adults in other countries worldwide, such as Argentina, Australia, Brazil, Indonesia, Malaysia, Philippines and Russia, with vaccination age for routine cohorts and at-risk group recommendations differing between countries. PCV10 (Synflorix®, GlaxoSmithKline; comprising the additional serotypes: 1, 5, and 7F) is also available in many countries for active immunization against IPD, AOM and pneumonia in infants and children from 6 weeks up to 5 years of age Citation[18].
Pneumococcal vaccination for children and adults at risk of pneumococcal disease is recommended in many countries, as exemplified by Europe Citation[19,20]. Administration of PPV23 above the age of 2 years is recommended following PCV vaccination, to broaden serotype coverage Citation[11,21]. PPV23 should be administered at least 2 months following PCV vaccination Citation[22]. Evidence for the effectiveness of PPV23, including against IPD in adults with chronic illnesses or for the prevention of all-cause pneumonia in the general population, was reviewed in 2013 Citation[23].
Given that the clinical trials to establish vaccine efficacy do not include all possible variations (e.g., by age group, by risk group, by schedule or by concomitant vaccine[s]), available safety and immunogenicity study data may be acceptable as comparative evidence. The WHO has provided a standardized ELISA protocol to ensure comparable results within and between laboratories with respect to the quantification of S. pneumoniae serotype-specific human IgG antibodies following PCV immunization Citation[24]. The WHO ELISA protocol includes an absorption stage prior to the ELISA assay to inhibit the binding of non-specific antibodies in serum samples to serotype-specific pneumococcal polysaccharide. Originally, this involved a single pre-adsorption step with pneumococcal C-polysaccharide (C-PS), but the protocol now includes pre-adsorption with both C-PS and 22F polysaccharide. This protocol also specifies a standard human antipneumococcal reference serum (89-SF).
The responses by ELISA antibody concentration following a primary series vaccination in young children have been used to determine a correlate of protection against IPD, but are only applicable to pediatric populations. The IgG ELISA also does not take into account other isotypes such as IgM Citation[25]. The WHO has recommended measuring opsonophagocytic activity of serum (OPA, also referred to as opsonophagocytic killing, OPK) to corroborate the functional activity of ELISA antibodies Citation[26]. As OPA is posited to mimic natural host immune responses, it may act as a surrogate marker for vaccine efficacy Citation[25]. Serum OPA can be assessed in vitro using a phagocytic cell line in the presence of a complement source and measuring the killing of a specific serotype of S. pneumoniae Citation[27]. However, there is currently no standardized protocol for OPA, and no correlate of protection has been established for adults.
We performed a literature review of publications of immunogenicity and safety trials worldwide of PCVs in populations that are at higher risk of pneumococcal disease than the general population due to underlying chronic or immunocompromised conditions, to document immunogenicity as well as to provide a comprehensive summary of the methodologies used in these trials. The studies reviewed included both single-arm (non-comparative) and those that compared PCV with PPV23.
Search methodology & publication selection
This review was based on peer-reviewed articles rather than conference abstracts. A search of the PubMed database was conducted using the search term ‘PCV’. Search results were limited to papers published between 1 January 1999 and 1 July 2014. Papers were identified for inclusion in the review if they were published in a European Union language, and they reported immunogenicity data following immunization with a PCV in adults or children defined as being at elevated risk for pneumococcal infections. The following immunocompetent risk-group categories were included in the initial search and selection process: chronic or cyanotic heart disease; chronic liver or renal disease; chronic respiratory or lung disease (e.g., cystic fibrosis, bronchopulmonary dysplasia, recurrent pulmonary infections, chronic/severe asthma); pre-organ transplant recipients; central nervous system malformations, cerebrospinal leaks or liquor shunt; cochlear implant recipients; metabolic disease (e.g., diabetes); care home resident/permanent institutional care because of illness; smoking/exposure; alcoholism; and previous IPD. Children and adults with asplenia were also included. A range of individuals (of all ages) who were immunocompromised were represented: those with congenital or primary immunodeficiency (e.g., agammaglobulinemia, severe combined immunodeficiency, common variable immune deficiency, complement [C] deficiency [particularly early component deficiencies: C1, C2, C3, C4]); secondary immunodeficiency (e.g., HIV); nephrotic disease; bone marrow, hematopoietic stem cell and solid organ transplant recipients; neoplastic disease (e.g., Hodgkin lymphoma, non-Hodgkin lymphoma, lymphomas, leukemias, other diseases of the blood-forming organs); malignancies (immunosuppression due to malignancy or treatment); iatrogenic immunosuppression and chromosomal aberration (e.g., Down syndrome). Down syndrome and low birth weight were both classified as immunocompromising conditions. Down syndrome is associated with defects in the adaptive and innate immune system, and as a consequence these individuals may have a lower response to vaccines Citation[28]. Children with low birth weight have a greater risk of pneumococcal disease in early life compared with full-term, age-matched children, possibly due to an immune system that has not completely matured and lack of maternal antibodies Citation[29].
During the selection process, trials measuring vaccine immunogenicity outside a 4- to 8-week post-vaccination period were excluded, as were those not reporting geometric mean concentration or titer (GMC or GMT) values for ELISA or OPA and/or those only reporting the ratio of pre- to post-vaccination antibody concentrations and/or responder rates based on fold-rises in antibody concentrations. Publications were also excluded if they were interim analyses, sub-studies or follow-up studies, if there was only partial randomization, or the data were not split into separate vaccine groups. Only those publications reporting immunological data from PCV formulations that led to licensure were selected, and thus studies of investigational PCV formulations were excluded.
Immune response data (GMC for ELISA and GMT for OPA) from the selected publications have been tabulated within this review, where available. Reactogenicity and safety findings from these publications have also been summarized.
Results
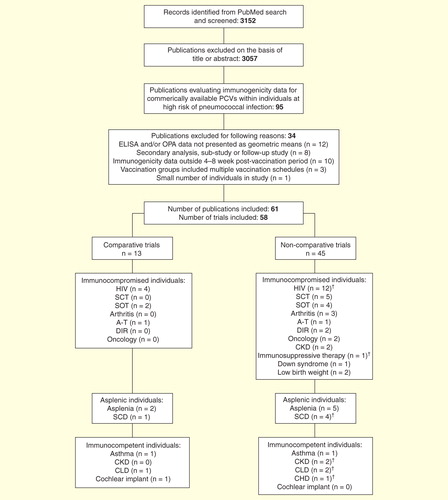
In total, 3152 references were identified, of which 3057 were excluded on the basis of their title or abstract. Of the remaining references, 95 reported immunogenicity data for commercially available PCVs in children and/or adults at high risk of pneumococcal infection, while 61 of these articles (58 separate trials) met the inclusion/exclusion criteria and are summarized within this review.
Overview of included clinical trials
provides an overview of the trials included in this review. Only 13 of the 58 trials were comparative; the other 45 were non-comparative and, with the exception of one trial of PCV9 Citation[30] and one trial of PCV13 Citation[31], all evaluated PCV7. Twenty-eight studies were conducted in children, 21 in adults and 9 in both children and adults. The studies involved individuals with a variety of at-risk conditions, with the HIV risk factor accounting for 15 of the 58 trials.
Table 1. Summary of trials: immunocompromised, asplenic, and immunocompetent individuals.
Comparative trials
PCV7 was compared with PPV23 in 13 trials; seven involved vaccine-naive children and/or adults, five involved children and/or adults who had previously received PPV23 and one involved both vaccine-naive and pre-vaccinated adults. Most (10/13) of these comparative trials involved asplenic or immunocompromised children and/or adults, and the majority (8/13) compared one dose of PCV7 with one dose of PPV23. In one study, the effect of sequential PCV7 and PPV23 vaccination schedules, PCV7/PCV7/PPV23 versus PCV7/PPV23/PPV23, on PCV-induced immunologic priming was assessed in asplenic children and adults with β-thalassemia (i.e., two doses of PCV7 at 0 and 1 month followed by a final PPV23 dose at 12 months [PCV7/PCV7/PPV23] vs one dose of PCV7 at 0 month followed by two doses of PPV23 at 1 and 12 months[PCV7/PPV23/PPV23]) Citation[32]. Similarly, PCV7/PCV7/PPV23 (administered at 0, 8 and 16 weeks) was compared with a single dose of PPV23 in children and adults with sickle cell disease Citation[33]. The immunogenicity of PCV7/PPV23 was evaluated in two trials that involved adults with HIV infection and in one trial involving adult renal transplant recipients: one trial (in HIV-infected adults) included the administration of placebo (PCV7/PPV23 vs PCV7/PCV7 or placebo/PPV23 or placebo/placebo), the other HIV trial compared PCV7/PPV23 with PPV23 alone, and the trial in renal transplant recipients compared PCV7/PPV23 with PPV23/PPV23 Citation[34–36].
Non-comparative trials
Of the 45 non-comparative trials, 32 assessed primary vaccination and 9 assessed PPV23/PCV7, the PPV23 administered within the past 5 years in children and/or adults with immunocompromising conditions (e.g., chronic kidney disease or asplenia). Another four trials, all of which involved asplenic or otherwise immunocompromised children or adults, included previously vaccinated individuals as well as vaccine-naive individuals. A variety of vaccination schedules was used across the non-comparative studies. Within immunocompetent children, these schedules included PCV7/PCV7 administered 2 months apart in children with chronic kidney disease Citation[37]; a 3 + 1 PCV7 schedule (at 2, 4, 6, 12–24 months of age) in children with chronic or recurrent lung disease <2 years of age, or one dose of PCV7 in those aged >2 years Citation[38] and PCV7/PPV23 (2- or 10-month dosing interval) in children with asthma Citation[39]. Within trials of individuals infected with HIV, vaccination schedules ranged from one dose of PCV7, either in previously vaccinated or unvaccinated adults and children Citation[40,41], through to three PCV7 doses at 8-week intervals, followed by a booster PCV7 dose at 15 months and then PPV23 at 24 months in infants with HIV infection Citation[42]. PCV7 (one to three doses) was administered prior to PPV23 in all but one of the trials involving children and/or adults with asplenia (including those with sickle cell disease) Citation[32,43–47]. One dose of PCV13 was administered to adults with β-thalassemia who had previously received PCV7/PPV23 Citation[31]. In most (4/5) of the trials of stem cell transplant recipients (children and/or adults), PCV7 was administered as three or four doses Citation[48–51]; in 2/5 trials PCV7 administration was followed by a final PPV23 vaccination Citation[49,52]. Solid organ recipients (children or adults) in three trials also received PCV7 (one to two doses) Citation[53–55], followed by PPV23 Citation[53–55].
Variability in immunologic assessments
An overview of the immunogenicity methodology used in the 58 trials included in this review is presented in . All 58 trials reported pneumococcal antibody concentrations as measured by ELISA, 33 of which used the WHO protocol (utilizing absorbent containing C-PS and/or 22F capsular polysaccharide and standard human antipneumococcal reference serum). The majority of the trials (n = 47) presented antibody concentrations as GMC values, including 41 trial publications that provided tabulated GMC data (summarized in ). In addition, 52 trials reported serotype-specific and/or overall response rates based on thresholds and/or fold-rises; these response rates were based on antibody concentration thresholds in 29 trials, on fold-rises in eight trials and on combined thresholds and/or fold-rises in 15 trials. Nineteen of 58 studies used an antibody concentration threshold of ≥0.35 µg/ml, which the WHO recommends as a correlate of protection (applied 1 month after the final doses of the primary series) for the licensure of new PCVs for children. Thirteen of the trials reported OPA data, with five of these trials using a ≥1:8 responder criterion and seven reporting tabulated GMT OPA data .
Table 2. Summary of immunologic assessments and responder criteria utilized in the 58 separate studies summarized in this review.
Table 3. Immunologic findings following primary and secondary pneumococcal vaccination (comparative and non-comparative studies).
In this review, we have focused on GMCs for ELISA and GMTs for OPA data rather than response rates because there are no established correlates of protection for adults and immunocompromised individuals. There was wide variability in the protective response criteria applied in the trials identified in this review . One of the most frequently applied, the WHO-recommended threshold of 0.35 µg/ml, is a licensing criterion applied to a population of vaccinated individuals that was derived from pediatric data and thus is not applicable to other populations Citation[52].
Immunogenicity of PCVs
Overview
PCV7 was immunogenic, as assessed by ELISA and/or OPA, for the risk groups included in this review, with the exception of one trial in children and adults with ataxia-telangiectasia (A-T) Citation[56]. There was also evidence of improved immunogenicity from PCV7 compared with PPV23. Six of the 11 comparative trials with tabulated ELISA data demonstrated significantly greater increases in GMC values (or fold-rises) between pre-vaccination and post-vaccination assessments with PCV7 than with PPV23 for ≥2 serotypes Citation[33–35,57–59], whereas in three trials, PPV23 was as immunogenic as PCV7 (two trials) or PCV7/PPV23 (one trial) Citation[32,36,60]. For two of the four comparative trials reporting OPA data, PCV7 generated significantly greater OPA response than PPV23 Citation[33,58].
HIV, transplantation and asplenia (including sickle cell disease) accounted for majority of the risk groups assessed in trials in this review, and thus are the focus of the following sections.
Human immunodeficiency virus
Trials in HIV-infected individuals varied in terms of design, vaccination schedules and population (i.e., children or adults; previously vaccinated or vaccine-naive); however, PCV7 was generally found to be immunogenic.
Two trials in adults with HIV compared PCV7 with PPV23 primary vaccination Citation[34,35]. Felkin et al. compared the following two-dose vaccination sequences, PCV7/PCV7, PCV7/PPV23, placebo/PPV23 and placebo/placebo, all administered 8 weeks apart, in HIV-infected adults using ELISA and OPA measurements Citation[34]. Eight weeks following the second vaccination, significantly greater antibody concentrations were observed in adults receiving PCV7/PCV7 (serotypes 4, 6B, and 9V) or PCV7/PPV23 (serotype 23F) than in those receiving placebo/PPV23 (p <0.05). OPA responses in these two study groups were also significantly greater than in those receiving placebo/PPV23 (PCV7/PCV7, serotypes 4, 6B, 9V; PCV7/PPV23, serotypes 9V and 23F) (p <0.05). PCV7/PCV7 or PCV7/PPV23 (administered 8 weeks after the first dose of PCV7) provided no additional increase in antibody concentration or OPA beyond that observed after the first dose of vaccine. In this PCV study in HIV-infected adults, vaccine-specific immunologic response did not appear to be affected by CD4 counts, degree of virologic control or use of highly active antiretroviral therapy (HAART). In a separate study, also in HIV-infected adults, PCV7/PPV23 (PCV7 followed 4 weeks later by PPV23 vaccination) resulted in significantly greater antibody concentrations for 6 of 7 serotypes (4, 9V, 14, 18C, 19F, and 23F) than with PPV23 alone Citation[35].
In a study conducted before the introduction of antiretroviral therapy (ART), primary vaccination with a single dose of PCV7 also resulted in significant increases in IgG GMCs and OPK titers (for serotypes 4 and 14) 2 months post-vaccination in both HIV-infected and HIV-uninfected adults, with serotype 14 IgG levels and OPK titers being dependent on CD4+ cell counts in the HIV-infected adults Citation[61]. In a trial demonstrating that zinc or vitamin A supplementation had no effect on immune response to PCV7, significant fold-rises in IgG GMCs for all PCV7 serotypes were observed in a HIV-infected adult population, most of whom were not receiving HAART Citation[62]. Also, in both these trials, PCV7/PPV23, with the PPV23 administered 2 Citation[61] or 6 months Citation[62] following PCV7, did not increase IgG concentrations beyond those observed post-PCV7. A single dose of PCV7 was also shown to be immunogenic 1 month post-vaccination in HIV-infected (none receiving ART) and HIV-uninfected adults within a placebo-controlled trial Citation[40]. A more recent trial in pneumococcal vaccine-naive HIV-infected adults, in which data were provided only as response rates, found that the immunogenicity of a single dose of PCV7 or PPV23 was improved by delaying vaccination until after reconstitution of the immune system (i.e., CD4 count >200 cells/mm3) (data not shown) Citation[63]. Within this trial, no differences were observed between the immunogenicity of the two vaccines.
The effect of HAART on the immune response to PCV7/PCV7/PPV23 (two doses of PCV7 administered 3 months apart, followed by PPV23 administered 6 months later) in HIV-infected adults has also been evaluated in an observational cohort study (a pre-planned sub-study of Søgaard et al. Citation[64]) Citation[65]. There was some variability in antibody IgG GMC values between HAART-treated adults and those not receiving HAART (both following the second PCV7 and the PPV23 dose), although these differences were non-significant: GMC values for serotypes 9V, 14 and 18C tended to be higher in HAART-treated adults, while those for serotype 6B were lower. PPV23 vaccination did not increase IgG GMCs beyond those observed 1 month following the second PCV7 dose. OPA GMTs (1 month following the second PCV7 dose) were significantly greater among those who received HAART than in those who were HAART-naive (serotypes 14, 19F, and 23F), and these differences remained significant following adjustment for baseline CD4+ cell count. PPV23 vaccination increased OPA titers for serotypes 6B, 19F and 23F to above those found after the second PCV7 dose.
In a comparison of PCV7/PCV7 with PPV23/PCV7/PCV7, past administration (42–79 months previously) of PPV23 was not shown to affect the immune response of two doses of PCV7 (administered 4 weeks apart) in HIV-infected adults Citation[66]. In both groups, significant increases in IgG GMCs were observed for all serotypes after the first PCV7 dose (p <0.01) and for all serotypes except 14 and 9V after the second dose, independent of whether individuals had a past history of PPV23 or no previous vaccination.
The immunogenicity of primary PCV vaccination has also been demonstrated in children with HIV infection Citation[30,42,67,68]. In a placebo-controlled trial, Madhi et al. evaluated a primary vaccination with PCV9 (administered at 6, 10 and 14 weeks of age) in HIV-infected (mostly not receiving ART) and HIV-uninfected infants Citation[30]. In both HIV-infected and HIV-uninfected groups, they found significantly greater IgG GMCs in PCV9-vaccinated children than in placebo recipients for all nine serotypes following the third PCV9 dose. However, HIV-uninfected infants had a superior functional response as demonstrated by the proportion of infants with a measurable OPA titer (≥1:8) for the three serotypes (6B, 19F and 23F) studied. In a follow-up trial Citation[41], these children received a single PCV7 dose 5 years following the primary (PCV9 or placebo) vaccination series. After the PCV9 primary series, the immune response to PCV7 was lower in HIV-infected than in HIV-uninfected children. Following the PCV7 booster, HIV-infected children (both the PCV9 and placebo recipients) were less likely to have OPA responses than HIV-uninfected children, suggesting that HIV-infected vaccinees experienced a partial loss of anamnestic responses to PCV priming.
Madhi et al. Citation[68] have also evaluated the effect of timing of ART in HIV-infected children on immune responses to a primary series of PCV7 (three doses administered at 6–12, 9–18, and 12–24 weeks of age), as well as the effect of perinatal HIV exposure in HIV-uninfected infants. Antibody concentrations were similar between HIV-infected infants receiving ART (HIV+/ART+) and HIV-infected infants not receiving ART (HIV+/ART−). Surprisingly, antibody concentrations were lower in HIV-uninfected infants born to HIV-seronegative mothers (M−/I−) than in HIV-uninfected infants born to HIV-infected mothers (M+/I−) (significant differences only observed for serotypes 6B and 23F). However, M−/I− infants had superior OPA responses compared with those in HIV+/ART− infants (p <0.01), and better OPA responses were observed in HIV+/ART+ than in HIV+/ART− infants (p <0.05). A more recent publication reporting the secondary end points from this trial has demonstrated similarity between the immune responses following the second and third doses of PCV7, although there were significantly higher proportions of children with antibody ≥0.35 µg/ml to serotypes 6B (all four groups) and 23F (M−/I− and HIV+/ART+ infants), and with OPA ≥8 to serotypes 23F (M−/I−, HIV+/ART− and HIV+/ART+ infants) following three doses than following two doses Citation[69].
As with adults, PCV vaccination has been evaluated in HIV-infected children previously vaccinated with PPV23. Spoulou et al. reported greater (although non-significant) increases in GMCs in age-matched healthy controls than in children (aged 20–163 months) with symptomatic HIV infection 1 month following an initial PCV7 dose Citation[70]. In the HIV-infected children who had previously received PPV23 (18–39 months earlier), PPV23/PCV7/PCV7, a second dose of PCV7 (administered 1 month after the first dose) failed to increase GMCs.
In contrast, Abzug et al. Citation[71] observed incremental gains in GMCs with PCV7/PCV7/PPV23 after each vaccine dose (two doses of PCV7 followed by PPV23; all at 8-week intervals) in children aged 2 to <19 years, receiving stable HAART for ≥3–6 months, 75% of whom had received previous PPV23 vaccination. They also reported the following predictors of response: greater antibody concentration at entry, higher immune stratum (based on nadir CD4% before HAART and CD4% at screening), lower entry viral RNA, longer duration of the entry HAART regimen and age <7 years. Individuals from this study were subsequently randomized to receive one dose of PCV7 or PPV23 4–5 years after PCV7/PCV7/PPV23 vaccination (age range at revaccination: 8–22 years). This revaccination with PCV7 resulted in GMCs for serotypes 6B and 14 that were 1.5- to 3.0-fold higher than after the original PCV7/PCV7/PPV23 vaccination, whereas the GMC for serotype 1 following PPV23 was similar to that following PCV7/PCV7/PPV23 Citation[72].
Transplantation
Findings from two trials of PCV7 vaccination in children and adults undergoing stem cell transplantation demonstrate the benefits of a PCV7 schedule administered prior to stem cell collection and then early after transplantation. PCV7 vaccination administered 6–17 days before stem cell collection improved subsequent immunological responses to a three-dose PCV7 schedule (at 3, 6, and 12 months) following transplantation compared with responses observed in those not receiving PCV7 prior to stem cell collection, with significantly greater IgG GMC values observed for three of the PCV7 serotypes (18C, 19F and 23F) following the third PCV7 dose Citation[48].
In a separate trial, PCV7 vaccination only initiated 3 months after stem cell transplantation resulted in an immunological response similar to PCV7 vaccination initiated at 9 months following vaccination, which suggests that PCV7 can be administered early following stem cell transplantation to ensure protection against early life-threatening pneumococcal disease Citation[49].
The immunogenicity of single doses of PCV7 or PPV23 has been compared in adult renal transplant recipients based on both ELISA and OPA. PCV7 vaccination resulted in a significantly greater fold-rise in antibody level at 8 weeks for serotype 23F Citation[60]. However, no significant differences were observed between the two vaccines with respect to fold-rises in OPA titer. In a separate retrospective observational study, a sequential schedule of PCV7/PPV23 (administered 8 weeks apart) was evaluated in adults following heart or lung transplantation (median time lapse between transplantation and vaccination: 58.5 months) Citation[54]. PCV7 was found to be immunogenic, as demonstrated by significant increases from baseline in GMCs for six of the PCV7 serotypes; however, IgG concentrations remained at similar levels as those post-PCV7 following subsequent PPV23 vaccination. Similarly, in a PCV7/PPV23 versus placebo trial involving adult liver transplant recipients, PCV7 resulted in significant increases in IgG GMTs compared with placebo; likewise, there were no differences between these two groups following subsequent PPV23 vaccination (administered 8 weeks later) Citation[53].
The immunogenicity of PCV7 has been assessed in two additional trials involving pediatric solid organ transplant recipients Citation[55,73]. Barton et al. Citation[73] reported increases in GMCs with PCV7/PCV7/PCV7 (three doses, administered at 8-week intervals), which reached significance for serotypes 19F, 14, 6B, 23F and 9V. Following subsequent PPV23 vaccination (administered 8 weeks later), increases in GMCs were observed only for serotype 19F. Both heart or lung recipients derived additional benefit from the third dose of PCV7, whereas heart recipients demonstrated further responses to the subsequent PPV23 vaccination. In a separate case–controlled trial Citation[55], the immunogenicity of sequential vaccination, PCV7/PCV7/PPV23 (pre-vaccination vs 6–8 weeks post-vaccination), was evaluated in children who had received solid organ (mostly heart or liver) transplants. Significant increases in IgG GMCs (except serotype 19F) were observed in the transplant group following the initial PCV7 dose, with similar GMCs observed between the transplant and control groups for serotypes 6B, 14, 18C and 19F. However, antibody concentrations in the transplant recipients did not significantly increase further following the second PCV7 dose or subsequent PPV23 dose. Heart transplant recipients were also reported to have lower antibody responses than liver transplant recipients.
Other immunocompromised risk groups
PCV7 has also been assessed in other immunocompromised risk groups, including those with arthritis, A-T, congenital or primary immunodeficiency, malignancies, Down syndrome, and low birth weight. These trials suggest that PCV7 is immunogenic in these immunocompromised children and adults, although the responses may be lower than in healthy individuals, and the immune response may be affected by immunomodulators. For example, within trials in children with juvenile idiopathic arthritis Citation[74] and adults with chronic arthritis Citation[75,76], a single dose of PCV7 resulted in a lower immune response in some of the children with juvenile idiopathic arthritis receiving TNF inhibitors, whereas in the adults, PCV7 immunogenicity was impaired by methotrexate, abatacept and rituximab, but not by TNF inhibitors or tocilizumab. In the adults with established rheumatoid arthritis, the immunogenicity was lower with rituximab than with abatacept and even lower when rituximab was combined with methotrexate Citation[76]. Advanced disease activity and longer disease duration were also found to be predictors of an impaired antibody response (based on ≥twofold increase in antibody levels).
Two trials have also investigated the immunogenicity of PCV7/PCV7, with PCV7 administered as a primary dose Citation[77] and then as a booster dose Citation[78] (12–14 months apart), in children with idiopathic nephrotic syndrome. Each dose of PCV7 was found to be immunogenic 1 month after vaccination, with ≥94% individuals reaching the 0.35 µg/ml antibody cut-off for five or more serotypes. However, in both trials, those treated with mycophenolate mofetil and/or cyclosporine A in addition to prednisolone had a lower immune response to serotypes 4, 9V and 18C than those receiving no therapy or prednisolone-only therapy.
Conflicting findings were also observed in two trials involving children and adults with A-T. In a trial involving 14 individuals with A-T Citation[56], significant increases in antibody GMCs (as measured by ELISA) were observed following a single dose of PCV7; however, the authors concluded that the individuals failed to produce an immune response based on their response criterion of a GMT >10 U/ml. In another trial of individuals with A-T, increases in antibody GMCs were reported for the PCV7 serotypes, although these increases were less than in healthy controls Citation[79].
Similarly, a single dose of PCV7 produced a lower immune response in adults with chronic lymphocytic leukemia than in controls Citation[80]. Nonetheless, the immunogenicity of PCV7 was improved by vaccinating individuals before the commencement of chemotherapy with development of hypogammaglobulinemia. The immunogenicity of PCV7/PCV7 (two doses administered 1 month apart) has also been demonstrated in children with hematological cancer or with solid tumors receiving maintenance chemotherapy, as well in those who had discontinued chemotherapy 3–12 months before vaccination Citation[81].
A PCV7/PCV7/PPV23 schedule has also been shown to provide immunogenicity (demonstrated by ELISA and OPA) in children and adults with Down syndrome Citation[82].
A few trials have evaluated the immunogenicity of PCV7 in infants with low birth weight Citation[83–85]. Within a trial involving infants with low birth weight (split into two groups: birth weight <1000 or ≥1000 g), three doses of PCV7, administered at 2, 4 and 6 months of age, resulted in significant increases in PCV7 serotype antibody GMCs compared with pre-vaccination antibody concentrations, with no differences observed between the two groups Citation[83]. Antibody concentrations increased again following a subsequent PCV7 dose at 16 months of age, with GMCs after the fourth dose being significantly greater than those following the third dose. Immunogenicity following the primary series was lowest for serotypes 6B and 23F in both groups. Similarly, infants in another trial with a birth weight ≤1000 g were found to have immune responses to all PCV7 serotypes (except 6B and 23F) following three doses of PCV7 that were similar to those in heavier premature infants, although lower birth weight as well as post-natal glucocorticoid exposure and lower current weight were found to be risk factors for poorer immunogenicity, especially to serotypes 6B and/or 23F Citation[84]. A sub-analysis of a large PCV7 trial also found that immune response to the PCV7 serotypes did not differ significantly between infants with low birth weight and those with normal birth weight Citation[85].
Asplenia/sickle cell disease
Prior PPV23 vaccination has been found to influence immune response to subsequent PCV7 vaccination in children and/or adults with asplenia. The immunogenicity of two vaccine strategies (PCV7/PCV7/PPV23 or PCV7/PPV23/PPV23 – first two doses administered 1 month apart followed by the third dose 12 months later) was compared in asplenic individuals (aged 12–41 years) who, historically, either had previously received PPV23 or who were PPV23-naive Citation[32]. PCV7/PCV7/PPV23 and PCV7/PPV23/PPV23 both resulted in similar increases in antibody GMCs. However, individuals who had previously received two or more PPV23 doses had significantly lower fold-rises (baseline to 1 month) in antibody GMCs following the first dose of PCV7 for all serotypes except 6B as compared with vaccine-naive individuals (including age-matched PPV-naive, β-thalassemics), after adjusting for baseline values. Immune responses following the 12-month PPV23 dose were also inferior in those who had previously received two or more PPV23 doses historically, compared with vaccine-naive individuals. These findings are also supported by those from two other studies involving asplenic adults (the majority of whom had previously received PPV23); in these studies, increases in IgG GMCs were observed following an initial PCV dose but not following an initial PPV23 dose Citation[43,44].
A separate study involving asplenic male adults with β-thalassemia who had received a PCV7 dose 7 years prior to the trial also demonstrated the ability of prior PPV23 vaccination to blunt subsequent PCV13 immunogenicity. Prior to PCV13 vaccination, antibody GMCs were >0.35 µg/ml in all individuals. Although there were significant increases in antibody concentrations for all serotypes 28 days after vaccination (data not shown), antibody concentrations (at day 28) correlated negatively with the number of previous PPV23 doses but correlated positively with the time since last PPV23 vaccination. Prior PPV23 was also found to have a negative effect on PCV13 immunological memory as measured by antigen-specific B-cell memory responses Citation[31].
The immunogenicity of primary PCV7 vaccination (in a schedule of one to three doses followed by a final PPV23 vaccination) has also been evaluated in children with sickle cell disease Citation[33,45–47]. In one study, Vernacchio et al. compared the sequence PCV7/PCV7/PPV23 (all administered 8 weeks apart) with PPV23-only vaccination (single dose) in children with sickle cell disease, measuring both OPA and ELISA Citation[33]. A greater OPA response was observed with the PCV7/PCV7/PPV23 sequence than with PPV23 alone, as demonstrated by significantly greater OPA antibody GMTs for five of seven vaccine serotypes (6B, 14, 18C, 19F and 23F) after PPV23 vaccination. Antibody GMCs (as measured by ELISA) following PPV23 vaccination were significantly greater, however, for only two of seven serotypes (14 and 19F). Assessment of OPA titers showed increases following the second PCV7 dose and/or following the PPV23 dose for serotypes 4, 6B, 9V, 18C and 19F, which were not observed by ELISA.
Another trial evaluated PCV7/PCV7/PCV7/PPV23 (administered at 2, 3, 4 and 15–18 months of age) in infants with sickle cell disease Citation[47]. Primary PCV7 immunization was found to be highly immunogenic as demonstrated by increases in GMCs throughout the PCV7 series, and the PCV7 series also primed for immune memory as indicated by increases in GMCs following the PPV23 final dose. Finally, O’Brien et al. Citation[46] demonstrated that children with sickle cell disease achieved an immunological response that is comparable with individuals without sickle cell disease, following primary PCV7 vaccination administered according to age (infants aged <2 months: PCV7/PCV7/PCV7 at 2, 4 and 6 months; infants enrolled between 2 and 12 months of age: PCV7 at 12 months). Significant increases in antibody concentrations for the PCV7 serotypes were also observed following a final PPV23 vaccination (at 24 months), irrespective of the primary schedule. The immunological responses to pneumococcal vaccination observed in these two studies may have been due to the fact the children were not yet asplenic.
Immunocompetent risk groups
PCV7 has been evaluated in children and adults with chronic diseases (lung [including asthma] and kidney) as well as in cochlear implant recipients . A single dose of PCV7 was compared with a single dose of PPV23 in three studies involving immunocompetent individuals (i.e., children with asthma Citation[57] or a cochlear implant Citation[59], and adults with chronic lung disease Citation[58]). In each of these populations, significantly greater immune responses were observed (as demonstrated by pre- vs post-vaccination increases in antibody GMC values) in PCV7-vaccinated individuals than in those receiving PPV23 – for two serotypes (serotypes 14 and 23F Citation[57]), for four serotypes (serotypes 4, 9V, 18C and 23F Citation[58]), and for five serotypes (all PCV7 serotypes except serotypes 9V and 14 Citation[59]). Within the trial of adults with chronic lung disease, PCV7 also demonstrated superior OPA response, with significantly greater OPK index values seen for five serotypes (4, 6B, 9V, 18C and 23F) 1 month post-vaccination in those receiving PCV7 compared with those receiving PPV23 Citation[58]. Another trial compared two dosing schedules for PCV7/PPV23 vaccination in pre-school children with asthma Citation[39]. A 10-month interval between the two vaccines was associated with significantly greater immunogenicity (GMCs for all serotypes except serotypes 5 and 7; p < 0.01) compared with a 2-month interval.
Safety of PCVs
Local and systemic reactogenicity data following PCV7 vaccination were reported in 31 of the 58 studies. Local reactions following PCV7 vaccination mostly comprised erythema, induration, pain and, in a few studies, movement impairment. Reactogenicity did not appear to increase with subsequent PCV7 doses Citation[42,46,47], although frequencies of erythema and swelling were significantly lower after the first dose than after the third dose in a study of children undergoing stem cell transplantation Citation[50]. Fever was the most commonly reported systemic reaction across the studies. Other systemic reactions included decreased appetite, headache, gastrointestinal reactions (nausea, vomiting and diarrhea), fatigue, myalgia/arthralgia and flu-like symptoms.
Adverse event data were reported in 30 of the 58 studies, although most focused on the likelihood of the exacerbation of the patient’s underlying chronic medical condition (e.g., organ rejection in transplant recipients). Serious adverse events after PCV7 were reported in three studies: gastroenteritis (7/49 individuals) and bronchiolitis (4/49 individuals), none of which were considered vaccine related, in infants with sickle cell disease during primary PCV7 vaccination (at 2, 3 and 4 months of age) Citation[47]; foot infection and pneumonia (in one patient each and requiring hospitalization [relationship to vaccine not reported]) in adults with established arthritis vaccinated with a single PCV7 dose Citation[75]; and mostly disease-related or transplant-related serious adverse events (not considered to be vaccine-related and including 24 fatal events) in stem-cell transplant recipients (children and adults) receiving three doses of PCV7 followed by PPV23 Citation[49]. Deaths were reported in two HIV trials: four deaths (considered to be due to HIV-related complications) among 109 HIV-infected adults; in HIV-infected infants, there were two deaths and one death, respectively, in the PCV7 (n = 30) and placebo (n = 15) arms (relationship to vaccine not reported) Citation[42,66]. Four deaths were also reported following a single dose of PCV7 (n = 91) in a study of adults with chronic lung disease (vs 7/90 deaths in those receiving PPV23; relationship to vaccine not reported) Citation[58].
Expert commentary
Based on the 58 trials summarized in this review, PCV7 is immunogenic in children and adults at higher risk of pneumococcal disease than the general population, whether immunocompetent (n = 9), immunocompromised (n = 39) or asplenic (n = 12). Most of the trials were in individuals with HIV or asplenia (including sickle cell disease) or in transplantation recipients, while data are still lacking in many risk groups. Most (45/58) of the studies identified were non-comparative.
Cordonnier et al. recently reviewed pneumococcal immunization in immunocompromised individuals focusing on trials involving transplant recipients, or those with HIV infection, cancer or hematological malignancies Citation[10]. They concluded that while there are data demonstrating the benefit of pneumococcal vaccination in individuals with HIV infection or those undergoing stem cell transplantation, more trials were required to define the optimal timing of vaccination in patients undergoing solid organ transplantation.
Our review also demonstrates the benefits of early PCV7 vaccination in individuals undergoing stem cell transplantation. However, the immunogenicity of PCV7 in solid organ transplant recipients was found to vary according to organ, with heart or lung recipients having the lowest responses. In adults with HIV, the functional immunogenicity of PCV7 appears to be significantly greater in those receiving HAART than in those not receiving HAART. Similarly, there is some evidence that the immune response in adults with HIV is improved by delaying vaccination until after reconstitution of the immune system. Furthermore, case–control studies suggest that certain immunocompromised individuals (e.g., children and adults with A-T, or adults with chronic lymphocytic leukemia) may have an immune response following PCV7 vaccination that is lower than healthy individuals. Additional trials in immunocompromised populations may be required to determine the optimal vaccination schedule.
Prior PPV23 vaccination also appears to blunt the immune response to subsequent PCV7 vaccination in asplenic children and adults, with the number of previous PPV23 doses inversely correlating with PCV immune response and length of time since the previous PPV23 correlating with superior response.
Comparative data from our review suggest that PCV7 may be more immunogenic than PPV23 in adults with HIV, children with asthma or a cochlear implant and in adults with chronic lung disease. Furthermore, immune response to a final dose of PPV23 after one or more initial doses of PCV7 varied across studies. PPV23 vaccination after PCV7 primary vaccination did not stimulate immune responses in adults with HIV and in children and adults with asplenia, although the timing between doses was only a few weeks to a few months, whereas increases in antibody concentrations were reported in young children (≤2 years of age) with sickle cell disease following a final PPV23 vaccination.
One potential reason for the differences observed between PCV and PPV immunogenity may be due to the ability of PCV to stimulate memory B-cell responses, whereas PPV has been shown to deplete peripheral B cells Citation[86]. These distinct B-cell responses have been observed in adults, and may explain the hyporesponsiveness observed in some of the above populations following PPV23 vaccination. In adults aged 50–70 years vaccinated with PCV7/PCV7/PPV23, PPV23/PCV7/PCV7 or PCV7/PPV23/PCV7 (administered 6 months apart), PPV23 resulted in depletion of the peripheral B cell population, attenuating memory B-cell responses to subsequent PCV7 vaccination Citation[86]. In contrast, PCV7 induced peripheral memory B-cell production following both initial and subsequent doses Citation[86]. Similarly, a B-cell response was observed in adults aged 28–44 years following PCV7 vaccination 12–18 months following primary PCV7 vaccination Citation[87]. The induction of a memory B-cell response following PCV7 vaccination suggests that it still could be used in individuals previously vaccinated with PPV23 in spite of hyporesponsiveness although timing and dosing need to be determined Citation[86]. Data reviewed here suggest that a superior response is achieved by increasing the time between PPV23 and subsequent PCV7 vaccination.
It is important to select the correct immunological assessment for the population. Measurement of OPA titers is a useful complement to ELISA IgG concentrations to support the registration of new PCV formulations and, as it reflects functional immune responses in the host, it may be an ideal surrogate for protective responses in individuals Citation[25,26]. With recent technical advances, OPA has become more frequently used in vaccine trials, and OPA data seem to correlate with disease protection, especially in immunocompromised patients Citation[25]. However, there is a need to standardize the OPA protocol, so that results are comparable across different laboratories, and also to define a protective threshold. The 1:8 OPA threshold has so far been used at the population level in pediatric trials among healthy children but this threshold may not apply to other age groups or to high-risk groups Citation[25].
Serotype-specific antibody concentrations as measured by ELISA can provide valuable information regarding the immunogenicity of PCV7 in populations at risk of pneumococcal disease. We found, however, that the ELISA methodology chosen for each study varied, with only around half of the trials adopting a protocol consistent with WHO recommendations Citation[24]. The WHO has recommended a threshold antibody concentration of 0.35 µg/ml for protection against invasive disease in infants 1 month after a three-dose primary series. This threshold was derived from a pooled analysis of immunogenicity and efficacy data from completed PCV trials with invasive disease end points, but cannot be used with other clinical end points (e.g., pneumonia or otitis media). Also, this threshold may not be applicable to indications in other populations, such as adults and immunocompromised patients Citation[52], or in substantiating protective immune responses in individuals vaccinated with PPV23 Citation[57]. The response criteria chosen for anti-pneumococcal antibody concentrations varied across trials and included a variety of protective thresholds as well as fold-rises. Consequently, for the purposes of this review, we focused on antibody concentration (GMC) data.
Although the immunogenicity of PCV vaccination has been studied in parallel with its effectiveness in two trials involving individuals with HIV Citation[30,88], further research is required to understand the appropriate thresholds for at-risk populations.
Five-year view
This review consolidates evidence on the immunogenicity of PCV7 in populations at high risk of pneumococcal disease and should help increase our understanding of the optimal approach to pneumococcal vaccination in these populations. Research is required to determine the best method for PCV vaccination, in terms of priming and induction of memory. The persistence of serum antibodies and the ability to revaccinate will provide insights into long-term protective immune responses. More studies will need to focus on higher-valent PCVs, such as PCV10 and PCV13.
The majority of safety and immunogenicity trials of PCV7 in children and adults at increased risk of pneumococcal disease were single arm (non-comparative)
The methodology used to assess serotype-specific IgG and S. pneumoniae varied across trials, as did the immunogenic response criteria; there is currently no standardized protocol for OPA and no protective thresholds have been established.
Immunogenicity of PCV7, as assessed by pneumococcal-specific antibody concentrations and/or OPA activity, has been demonstrated in immunocompetent, immunocompromised and asplenic children and adults at risk of pneumococcal disease.
In HIV-infected adults, PCV7 primary vaccination resulted in significantly greater antibody concentrations and/or functional activity than PPV23, and subsequent PPV23 vaccination did not appear to enhance the PCV7 immune response.
PCV7 or PCV9 vaccination was also immunogenic in children with HIV, although functional responses tended to be lower in HIV-infected children than in HIV-uninfected children.
The benefits of early PCV7 vaccination were demonstrated in children and adults undergoing stem cell transplantation (either prior to or early after transplantation).
PCV7 immunogenicity was demonstrated in adults and children undergoing solid organ transplants with some variability being reported according to the organ involved (e.g., heart or lung recipients appear to have the lowest responses).
The immunogenicity of PPV23 following PCV7 primary vaccination varied within asplenic individuals; sequential PCV7 and PPV23 vaccination was immunogenic in infants with sickle cell disease, whereas PPV23 did not further enhance immune responses in adults with asplenia following primary PCV7 vaccination.
There is also evidence from comparative trials that PCV7 may be more immunogenic than PPV23 in children with asthma or a cochlear implant and adults with chronic lung disease.
Acknowledgements
The authors take full responsibility for the content of this article and thank Neostar Communications Limited, Oxford, UK (funded by Pfizer, France) for their assistance in preparing the manuscript. The authors also wish to thank their Pfizer, USA colleagues, Rosalind Hollingsworth and Raul Isturiz, for their review of the manuscript.
Financial and competing interests disclosure
N Dartois and MA Fletcher are employees of Pfizer, France. E Bonnet was an employee of Pfizer, France. P Balmer is an employee of Pfizer, USA. The authors received writing assistance from Neostar Communications, and this was funded by Pfizer, France. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Advisory Committee on Immunization Practices (ACIP). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816-19
- Active bacterial core surveillance report, emerging infections program Network, Streptococcus pneumoniae, 2010. Centers for Disease Control and Prevention. 2011. Available from: www.cdc.gov/abcs/reports-findings/survreports/spneu10.pdf [Last accessed December 2012]
- Estimated Hib and pneumococcal deaths for children under 5 years of age. World Health Organization. 2013. Available from: www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/ [Last accessed December 2012]
- Myint TT, Madhava H, Balmer P, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther 2013;30:127-51
- Fitzwater SP, Chandran A, Santosham M, et al. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012;31:501-8
- Surveillance report: annual epidemiological report reporting on 2010 surveillance data and 2011 epidemic intelligence data. European Centre for Disease Prevention and Control, 2012. Available from: http://ecdc.europa.eu/en/publications/Publications/Annual-Epidemiological-Report-2012.pdf [Last accessed 13 May 2013]
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012;67:71-9
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012;65:17-24
- Rose MA, Christopoulou D, Myint TT, et al. The burden of invasive pneumococcal disease in children with underlying risk factors in North America and Europe. Int J Clin Pract 2014;68:8-19
- Cordonnier C, Averbuch D, Maury S, et al. Pneumococcal immunization in immunocompromised hosts: where do we stand? Expert Rev Vaccines 2014;13:59-74
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013;62:521-4
- Wong WY, Overturf GD, Powars DR. Infection caused by Streptococcus pneumoniae in children with sickle cell disease: epidemiology, immunologic mechanisms, prophylaxis, and vaccination. Clin Infect Dis 1992;14:1124-36
- Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer 2010;55:401-6
- Esposito S, Musio A, Principi N. Paediatric asthma and pneumococcal vaccination. Vaccine 2013;31:5015-19
- Pneumovax 23 summary of product characteristics. Sanofi Pasteur MSD, 2012. Available from: www.medicines.org.uk/emc/medicine/1446 [Last accessed 14 June 2013]
- Prevenar 13: summary of product characteristics. European Medicines Agency. 2015. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf [Last accessed 4 June 2015]
- Prevnar 13 prescribing information. Pfizer Inc, 2012. Available from: www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM201669.pdf [Last accessed 3 March 2014]
- Synoflorix summary of product characteristics. GlaxoSmithKline Biologicals S.A. 2013. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf [Last accessed 4 March 2013]
- Pneumococcal vaccination (PCV) overview in European countries. EUVAC.NET, 2014. Available from: www.euvac.net/graphics/euvac/vaccination/pcv.html [Last accessed 15 January 2014]
- Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther 2014;31:1011-44
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 Years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822-5
- World Health Organization. Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine 2012;30:4717-18
- Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013;1:CD000422
- Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 2003;10:514-19
- Song JY, Moseley MA, Burton RL, et al. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013;19:412-25
- WHO expert committee on biological standardization: fifty-fourth report. World Health Organization. 2005. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_927_eng.pdf [Last accessed 17 October 2013]
- Romero-Steiner S, Libutti D, Pais LB, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997;4:415-22
- Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol 2011;164:9-16
- Saari TN. Immunization of preterm and low birth weight infants. American Academy of Pediatrics Committee on Infectious Diseases. Pediatrics 2003;112:193-8
- Madhi SA, Kuwanda L, Cutland C, et al. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J 2005;24:410-16
- Papadatou I, Piperi C, Alexandraki K, et al. Antigen-Specific B-Cell Response to 13-valent pneumococcal conjugate vaccine in asplenic individuals with beta-thalassemia previously immunized with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis 2014;59:862-5
- Orthopoulos GV, Theodoridou MC, Ladis VA, et al. The effect of 23-valent pneumococcal polysaccharide vaccine on immunological priming induced by 7-valent conjugate vaccine in asplenic subjects with beta-thalassemia. Vaccine 2009;27:350-4
- Vernacchio L, Romero-Steiner S, Martinez JE, et al. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. J Infect Dis 2000;181:1162-6
- Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001;20:545-53
- Lesprit P, Pedrono G, Molina JM, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. Aids 2007;21:2425-34
- Tobudic S, Plunger V, Sunder-Plassmann G, et al. Randomized, single blind, controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in renal transplant recipients. PLoS One 2012;7:e46133
- Vieira S, Baldacci ER, Carneiro-Sampaio M, et al. Evaluation of antibody response to the heptavalent pneumococcal conjugate vaccine in pediatric chronic kidney disease. Pediatr Nephrol 2009;24:83-9
- Navarro D, Escribano A, Cebrian L, et al. Type-specific antibodies to pneumococcal capsular polysaccharide acquired either naturally or after vaccination with Prevenar in children with underlying chronic or recurrent lung diseases. Clin Vaccine Immunol 2006;13:665-70
- Rose MA, Gruendler M, Schubert R, et al. Safety and immunogenicity of sequential pneumococcal immunization in preschool asthmatics. Vaccine 2009;27:5259-64
- Gordon SB, Kayhty H, Molyneux ME, et al. Pneumococcal conjugate vaccine is immunogenic in lung fluid of HIV-infected and immunocompetent adults. J Allergy Clin Immunol 2007;120:208-10
- Madhi SA, Klugman KP, Kuwanda L, et al. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J Infect Dis 2009;199:1168-76
- Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics 2003;112:66-73
- Meerveld-Eggink A, de Weerdt O, van Velzen-Blad H, et al. Response to conjugate pneumococcal and Haemophilus influenzae type b vaccines in asplenic patients. Vaccine 2011;29:675-80
- Stanford E, Print F, Falconer M, et al. Immune response to pneumococcal conjugate vaccination in asplenic individuals. Hum Vaccin 2009;5:85-91
- Nowak-Wegrzyn A, Winkelstein JA, Swift AJ, et al. Serum opsonic activity in infants with sickle-cell disease immunized with pneumococcal polysaccharide protein conjugate vaccine. The Pneumococcal Conjugate Vaccine Study Group. Clin Diagn Lab Immunol 2000;7:788-93
- O’Brien KL, Swift AJ, Winkelstein JA, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM(197) among infants with sickle cell disease. Pneumococcal Conjugate Vaccine Study Group. Pediatrics 2000;106:965-72
- Reinert P, Benkerrou M, de Montalembert M, et al. Immunogenicity and safety of a pneumococcal conjugate 7-valent vaccine in infants with sickle cell disease. Pediatr Infect Dis J 2007;26:1105-9
- Antin JH, Guinan EC, Avigan D, et al. Protective antibody responses to pneumococcal conjugate vaccine after autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:213-22
- Cordonnier C, Labopin M, Chesnel V, et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis 2009;48:1392-401
- Meisel R, Kuypers L, Dirksen U, et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood 2007;109:2322-6
- Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood 2003;101:831-6
- Meerveld-Eggink A, van der Velden AM, Ossenkoppele GJ, et al. Antibody response to polysaccharide conjugate vaccines after nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2009;15:1523-30
- Kumar D, Chen MH, Wong G, et al. A randomized, double-blind, placebo-controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in adult liver transplant recipients. Clin Infect Dis 2008;47:885-92
- Gattringer R, Winkler H, Roedler S, et al. Immunogenicity of a combined schedule of 7-valent pneumococcal conjugate vaccine followed by a 23-valent polysaccharide vaccine in adult recipients of heart or lung transplants. Transpl Infect Dis 2011;13:540-4
- Lin PL, Michaels MG, Green M, et al. Safety and immunogenicity of the American Academy of Pediatrics–recommended sequential pneumococcal conjugate and polysaccharide vaccine schedule in pediatric solid organ transplant recipients. Pediatrics 2005;116:160-7
- Sanal O, Ersoy F, Tezcan I, et al. Antibody response to a seven-valent pneumococcal conjugated vaccine in patients with ataxia-telangiectasia. J Clin Immunol 2004;24:411-17
- Rose MA, Schubert R, Kujumdshiev S, et al. Immunoglobulins and immunogenicity of pneumococcal vaccination in preschool asthma. Int J Clin Pract 2006;60:1425-31
- Dransfield MT, Harnden S, Burton RL, et al. Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clin Infect Dis 2012;55:e35-44
- Rose M, Hey C, Kujumdshiev S, et al. Immunogenicity of pneumococcal vaccination of patients with cochlear implants. J Infect Dis 2004;190:551-7
- Kumar D, Rotstein C, Miyata G, et al. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis 2003;187:1639-45
- Chen M, Ssali F, Mulungi M, et al. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine 2008;26:4962-8
- Deloria-Knoll M, Steinhoff M, Semba RD, et al. Effect of zinc and vitamin A supplementation on antibody responses to a pneumococcal conjugate vaccine in HIV-positive injection drug users: a randomized trial. Vaccine 2006;24:1670-9
- Slayter KL, Singer J, Lee TC, et al. Immunization against pneumococcal disease in HIV-infected patients: conjugate versus polysaccharide vaccine before or after reconstitution of the immune system (CTN-147). Int J STD AIDS 2013;24:227-31
- Sogaard OS, Lohse N, Harboe ZB, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis 2010;51:42-50
- Sogaard OS, Schonheyder HC, Bukh AR, et al. Pneumococcal conjugate vaccination in persons with HIV: the effect of highly active antiretroviral therapy. Aids 2010;24:1315-22
- Miiro G, Kayhty H, Watera C, et al. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis 2005;192:1801-5
- Costa Ide C, Guilardi F, Kmiliauskis MA, et al. Evaluation of humoral response to heptavalent pneumococcal conjugate vaccine in HIV-infected children. Rev Saude Publica 2008;42:844-50
- Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis 2010;202:355-61
- Madhi SA, Izu A, Violari A, et al. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine 2013;31:777-83
- Spoulou VI, Tsoumas DL, Papaevangelou VG, et al. Immunogenicity and immunological memory induced by a 7-valent pneumococcal CRM197 conjugate vaccine in symptomatic HIV-1 infected children. Vaccine 2005;23:5289-93
- Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J 2006;25:920-9
- Abzug MJ, Song LY, Levin MJ, et al. Antibody persistence and immunologic memory after sequential pneumococcal conjugate and polysaccharide vaccination in HIV-infected children on highly active antiretroviral therapy. Vaccine 2013;31:4782-90
- Barton M, Wasfy S, Dipchand AI, et al. Seven-valent pneumococcal conjugate vaccine in pediatric solid organ transplant recipients: a prospective study of safety and immunogenicity. Pediatr Infect Dis J 2009;28:688-92
- Farmaki E, Kanakoudi-Tsakalidou F, Spoulou V, et al. The effect of anti-TNF treatment on the immunogenicity and safety of the 7-valent conjugate pneumococcal vaccine in children with juvenile idiopathic arthritis. Vaccine 2010;28:5109-13
- Kapetanovic MC, Roseman C, Jonsson G, et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 2011;63:3723-32
- Kapetanovic MC, Saxne T, Jonsson G, et al. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther 2013;15:R171
- Liakou CD, Askiti V, Mitsioni A, et al. Safety, immunogenicity and kinetics of immune response to 7-valent pneumococcal conjugate vaccine in children with idiopathic nephrotic syndrome. Vaccine 2011;29:6834-7
- Liakou CD, Askiti V, Mitsioni A, et al. Safety and immunogenicity of booster immunization with 7-valent pneumococcal conjugate vaccine in children with idiopathic nephrotic syndrome. Vaccine 2014;32:1394-7
- Schubert R, Reichenbach J, Rose M, et al. Immunogenicity of the seven valent pneumococcal conjugate vaccine in patients with ataxia-telangiectasia. Pediatr Infect Dis J 2004;23:269-70
- Sinisalo M, Vilpo J, Itala M, et al. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine 2007;26:82-7
- Cheng FW, Ip M, Chu YY, et al. Humoral response to conjugate pneumococcal vaccine in paediatric oncology patients. Arch Dis Child 2012;97:358-60
- Kusters MA, Manders NC, de Jong BA, et al. Functionality of the pneumococcal antibody response in Down syndrome subjects. Vaccine 2013;31:6261-5
- Szynczewska E, Chlebna-Sokol D. Immunogenicity of heptavalent conjugate vaccine against Streptococcus pneumoniae in premature babies with low birth weight. Pediatr Neonatol 2014;55:101-7
- D’Angio CT, Heyne RJ, O’Shea TM, et al. Heptavalent pneumococcal conjugate vaccine immunogenicity in very-low-birth-weight, premature infants. Pediatr Infect Dis J 2010;29:600-6
- Shinefield H, Black S, Ray P, et al. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J 2002;21:182-6
- Clutterbuck EA, Lazarus R, Yu LM, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012;205:1408-16
- Clutterbuck EA, Salt P, Oh S, et al. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology 2006;119:328-37
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010;362:812-22
- Rivera-Olivero IA, Del NB, Fuentes M, et al. Immunogenicity of a 7-valent pneumococcal conjugate vaccine (PCV7) and impact on carriage in Venezuelan children at risk of invasive pneumococcal diseases. Vaccine 2014;32:4006-11
- Uddin S, Borrow R, Haeney MR, et al. Total and serotype-specific pneumococcal antibody titres in children with normal and abnormal humoral immunity. Vaccine 2006;24:5637-44
- Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010;202:1114-25
- Shrimpton A, Duddridge M, Ziegler-Heitbrock L. Vaccination with polysaccharide-conjugate-vaccines in adult patients with specific antibody deficiency. Vaccine 2006;24:3574-80
- Cordonnier C, Labopin M, Jansen KU, et al. Relationship between IgG titers and opsonocytophagocytic activity of anti-pneumococcal antibodies after immunization with the 7-valent conjugate vaccine in allogeneic stem cell transplant. Bone Marrow Transplant 2010;45:1423-6
- Mikoluc B, Kayhty H, Bernatowska E, et al. Immune response to the 7-valent pneumococcal conjugate vaccine in 30 asplenic children. Eur J Clin Microbiol Infect Dis 2008;27:923-8
- Smets F, Bourgois A, Vermylen C, et al. Randomised revaccination with pneumococcal polysaccharide or conjugate vaccine in asplenic children previously vaccinated with polysaccharide vaccine. Vaccine 2007;25:5278-82
- Stoehr GA, Rose MA, Eber SW, et al. Immunogenicity of sequential pneumococcal vaccination in subjects splenectomised for hereditary spherocytosis. Br J Haematol 2006;132:788-90