ABSTRACT
Vaccines are available against human papillomavirus (HPV), the causal agent of cervical and other cancers. Efficacy data from the HPV-16/18 AS04-adjuvanted vaccine clinical trial program were reviewed. Six randomized, controlled phase II/III trials evaluating cervical endpoints enrolled women from diverse populations and geographical locations. The program analyzed extensively the cohorts most relevant from a public health perspective: the total vaccinated cohort (TVC), approximating a general population including those with existing or previous HPV infection, and TVC-naïve, approximating a population of young women before sexual debut. Results show that the vaccine reduces HPV-16/18 infection and associated cervical endpoints in women regardless of age, location, or sexual experience. It provides cross-protection against some non-vaccine oncogenic HPV types and types causing genital warts, and may be effective against vulvar, oral, and anal HPV infection. Early epidemiology data following its introduction suggest a decline in the prevalence of vaccine and some non-vaccine HPV types.
Background
As per the latest data from the International Agency for Research on Cancer, there were an estimated 528,000 new cases of cervical cancer in 2012 and 266,000 deaths, making it the fourth most common cancer in women worldwide.[Citation1] Most of the burden occurs in emerging countries, where cervical cancer accounts for 12% of all cancers.[Citation1] Most developed nations have cervical cancer screening programs in place, although their efficiency and coverage are variable. Effective screening, along with diagnosis and treatment of pre-cancerous lesions, is costly for any country, which limits its widespread introduction.[Citation2–Citation6]
Human papillomavirus (HPV) is a necessary cause of cervical cancer.[Citation7] Numerous HPV types exist, approximately 15 of which are considered to be oncogenic.[Citation8] HPV-16 is the most prevalent type in cervical cancer, followed by HPV-18. Together, these two types are estimated to account for approximately 71% of all cervical cancers, with a further 14% accounted for by HPV-31, 33, and 45.[Citation9] The vast majority of adenocarcinoma (94%) is caused by HPV-16, 18, and 45.[Citation9]
HPV infection is frequently detected in adolescent girls and young women, often shortly after sexual debut.[Citation10–Citation12] Although the risk is the greatest in this age group, women over 25 years are also vulnerable to new infection, particularly in relation to new sexual partners.[Citation13,Citation14] Most HPV infections are transient or persist for only a few months. However, infections that have apparently cleared may reappear after a period of non-detection.[Citation15] Reinfection with the same HPV type is also possible.[Citation13] Cervical cancer and pre-cancer are almost always preceded by a persistent HPV infection.[Citation16,Citation17]
Two HPV vaccines are now licensed in many countries worldwide, the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, GSK group of companies) and the HPV-6/11/16/18 vaccine (Gardasil®, Merck). Both vaccines are used in public health vaccination programs, with the main target population being adolescent girls before sexual debut. Catch-up programs up to the age of 26 years have been offered in some countries, and both vaccines are licensed for use in women beyond this age in many countries. A 9-valent vaccine (HPV-6/11/16/18/31/33/45/52/58, Gardasil® 9, Merck) has been recently approved in some countries.
In this article, we review the published efficacy data for the HPV-16/18 AS04-adjuvanted vaccine against HPV infection and associated disease. As data on the vaccine have now been accumulating for more than a decade, we aimed to provide a comprehensive, up-to-date overview of its efficacy, its likely impact through population immunization programs, and its relevance to individual women.
Inclusion criteria for articles and search strategy
We included articles if they reported a randomized, controlled clinical trial describing the efficacy of the HPV-16/18 AS04-adjuvanted vaccine in female or male human participants of any age at any geographical location. Articles were eligible if they were in English and included any efficacy outcome associated with any HPV type. Efficacy parameters considered were HPV infection at any location (cervical, vulvar, vaginal, anal, oral), genital warts, cervical cancer or pre-cancer, and cancer or pre-cancer at any other location. We excluded articles if they were a review or commentary-type article, reported only the immunogenicity or safety of the vaccine, reported a health economic, benefit, or uptake analysis, reported HPV epidemiology, reported data for another HPV vaccine, or described the methodology, mode of action, adjuvant, laboratory, or animal studies.
We conducted a PubMed search on 3 October 2014 using the following search terms in the title or abstract: (HPV AND vaccine AND AS04) OR (HPV AND vaccine AND bivalent) OR (HPV-16/18 AND vaccine) OR (HPV16/18 AND vaccine). No date restrictions were applied. The term AS04 was used because this is the name of the GSK proprietary Adjuvant System that is unique to Cervarix. Because Cervarix is sometimes described as a bivalent vaccine, the term bivalent was also applied to identify any papers not referring to the Adjuvant System. The titles and abstracts of articles were reviewed to identify articles meeting the inclusion criteria described above. In addition, the reference lists of systematic reviews of vaccine efficacy were scanned to identify articles missed in the primary search.
The PubMed search identified 465 articles (; Appendix). Following a review of the titles and abstracts, we included 25 articles ().[Citation18–Citation42] The search was updated on 18 April 2015, and 27 additional articles were identified, of which two met the inclusion criteria ().[Citation43,Citation44] The reasons for exclusion are shown in and Appendix. We reviewed the reference lists of four systematic reviews,[Citation45–Citation48] but did not identify any additional articles. The authors identified two further articles from their own knowledge of the literature.[Citation49,Citation50]
Description of studies included
Study design & participants
We identified six blinded, randomized, controlled phase II or phase III trials and their extension phases (). All trials assessed three doses of the HPV-16/18 AS04-adjuvanted vaccine, administered at 0, 1, and 6 months. Each 0.5 mL dose of the HPV-16/18 vaccine contained 20 μg each of HPV-16 and HPV-18 L1 capsid protein. Controls were aluminum hydroxide or licensed hepatitis A vaccine. All trials were conducted in women aged at least 15 years. Although the main target population for vaccination is adolescent girls before sexual debut, efficacy studies gathering data on cervical HPV infection and associated abnormalities are not feasible in this age group and may raise ethical concerns. Such studies would require long-term follow-up in order to collect these data, and to date, this has not been undertaken. Immuno-bridging studies are generally accepted to infer efficacy in this young age group. However, this raises the importance of duration of protection, as young adolescents would require well over 10 years of protection to be afforded the same protection as that seen in the trials of young adult women.
Table 1. Overview of studies.
The HPV-001 trial (NCT00689741) was conducted in the US, Canada, and Brazil, in healthy women aged 15–25 years with no history of HPV infection or disease (N = 1113).[Citation18] Vaccine efficacy was assessed at 18 and 27 months after first vaccination. The extension study, HPV-007 (NCT00120848), assessed efficacy at up to 6.4 years after the first vaccination (N = 776).[Citation19,Citation20] Women initially enrolled in Brazilian centers from the HPV-001/007 studies were enrolled in a further extension phase, HPV-023 (NCT00518336), which assessed efficacy up to 9.4 years after the first vaccination (N = 437).[Citation21–Citation23]
The HPV-008 PApilloma TRIal against Cancer In young Adults (PATRICIA) (NCT00122681) was conducted in 14 countries in Europe, North and South America, Asia, and Australia (N = 18644). Healthy women aged 15–25 years were enrolled regardless of HPV DNA status, HPV serostatus, or cervical cytology at baseline. Three analyses were performed: an event-driven interim analysis,[Citation24] an event-driven final analysis, [Citation25,Citation43] and an end-of-study analysis at 4 years.[Citation26–Citation29,Citation44]
The HPV-009 Costa Rica HPV-16/18 Vaccine Trial (CVT) (NCT00128661) was conducted in healthy women aged 18–25 years, irrespective of past sexual behavior, HPV status, or cytology (N = 7466). An initial analysis of clearance of HPV infection was conducted at 6 and 12 months after the first vaccination, demonstrating that the vaccine had no effect on existing HPV infection.[Citation30] Further analyses were conducted 4 years after the first vaccination.[Citation31–Citation37,Citation49] One further article has reported a combined analysis of data from PATRICIA and the CVT.[Citation50]
The HPV-032 study (NCT00316693) (N = 1040) and the extension follow-up study, HPV-063 (NCT00929526) (N = 752), were conducted in healthy Japanese women aged 20–25 years, enrolled regardless of serological, cytological, or HPV DNA status. An event-driven interim analysis and an end-of-study analysis at 24 months were conducted for the initial study [Citation38,Citation39] and a 4-year analysis for the extension study.[Citation40] The HPV-039 trial (NCT00779766) is an ongoing trial conducted in China in healthy women aged 18–25 years (N = 6051), and the event-driven final analysis per protocol has been reported.[Citation41]
The HPV-015 Human PapillomaVIrus: Vaccine Immunogenicity ANd Efficacy (VIVIANE) trial (NCT00294047) was conducted in 12 countries in Europe, Australia, Southeast Asia, and North and South America (N = 5752) and enrolled women aged more than 25 years irrespective of past sexual behavior, cytology, HPV serostatus, or DNA status. It included a subset of up to 15% of women with a history of HPV infection/disease in each age stratum (26–35, 36–45, and >45 years). An interim analysis 4 years after the first vaccination has been reported.[Citation42]
Endpoints
All studies evaluated incident and persistent cervical infection, cytological abnormalities, and cervical intraepithelial neoplasia (CIN). Six-month persistent infection (6MPI) and 12-month persistent infection (12MPI) were defined as detection of the same HPV type in two consecutive samples collected over a 6-month or a 12-month period, respectively. The most common cytological endpoint reported was atypical squamous cells of undetermined significance or worse (ASC-US+), which included ASC-US, low-grade squamous intraepithelial lesion (LSIL), atypical glandular cells of undetermined significance (AGC-US), atypical squamous cells/high-grade ASC-US, does not exclude HSIL (ASC-H), and high-grade squamous intraepithelial lesion (HSIL). All studies reported CIN grade 1 or worse (CIN1+) and CIN grade 2 or worse (CIN2+); some studies reported CIN grade 3 or worse (CIN3+) and adenocarcinoma in situ (AIS). CIN1+ included CIN1, CIN2, CIN3, AIS, and cervical cancer. No study accumulated sufficient cases to report vaccine efficacy against cervical cancer. The PATRICIA study and the CVT reported the number of colposcopy referrals and cervical excision procedures. The CVT reported efficacy against cervical HPV infection after one or two doses of the HPV-16/18 vaccine, as well as data on vulvar, oral, and anal HPV infection. A combined analysis of data from PATRICIA and the CVT reported efficacy after one or two doses. Within each study, a standard colposcopy referral algorithm was followed. Across trials, there was some variation in the algorithms used in order to reflect the different age groups studied and, for single-country studies, reflecting individual country differences. A quality assurance and training program for study colposcopists was in place in all trials.
Broad-spectrum and multiplex type-specific polymerase chain reaction (PCR) techniques were used to detect HPV DNA in tissue and cytology samples.[Citation51,Citation52] Fourteen oncogenic HPV types were evaluated: HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. The genotyping method (SPF10 PCR-DEIA-LiPA25) could not distinguish between types 68 and 73. Eleven non-oncogenic HPV types were evaluated: HPV-6, 11, 34, 40, 42, 43, 44, 53, 54, 70, and 74. Endpoints associated with a composite of HPV types included at least one of those types (e.g. HPV-16/18 included either HPV-16 or HPV-18, or both). Vaccine efficacy against cytological and histological endpoints irrespective of the presence of HPV DNA or the HPV DNA type was also reported in some studies.
For histopathological endpoints, cases were assigned to a specific HPV type based on the detection of HPV DNA in tissue biopsies in all studies except for the CVT. In the CVT, HPV-type assignment was based on the HPV DNA detected in the preceding cytological sample. Cases with multiple HPV types were counted in each relevant analysis (e.g. a case associated with HPV-16, HPV-18, and HPV-31 was counted once each in the analysis of HPV-16 alone, HPV-18 alone, HPV-31 alone, HPV-16/18, the composite of oncogenic HPV types, and irrespective of HPV DNA).
Analysis cohorts
Vaccine efficacy was evaluated across the studies in the total vaccinated cohort (TVC), the TVC for efficacy (TVC-E), and the according-to-protocol (ATP) cohort. The PATRICIA and HPV-032/063 (Japan) studies included an additional cohort, the TVC-naïve. Case counting in the TVC, TVC-E, and TVC-naïve began from the day after the first vaccination; in the ATP cohort, it began from the day after the third vaccination.
The ATP cohort included women who received all three vaccine doses within protocol-defined windows, complied with the protocol, and had negative or low-grade cytology at baseline (Panel A). The primary analysis of vaccine efficacy in the ATP cohort excluded women who were DNA-positive for the HPV type under analysis at baseline or who became DNA-positive for the type under analysis before the vaccine course was completed (Panel B). For HPV-16/18-related endpoints, women who were seropositive at baseline for the type under analysis were also excluded in all studies except the CVT (Panel B), although analyses stratified by initial serostatus were also performed.
The TVC-E included women who had received at least one vaccine dose and had negative or low-grade cytology at baseline (Panel A). The primary analysis of vaccine efficacy in the TVC-E was also of women who were uninfected at baseline with the HPV type under consideration (Panel B).
The TVC included all women who received at least one vaccine dose, regardless of previous or current infection and cytology status (Panel A). The primary analysis of the TVC included all women, irrespective of their HPV DNA status or serostatus at baseline (Panel B). Other analyses reported for the TVC included a population of women uninfected at baseline in the HPV-001/007/023 study (this study enrolled only women who were HPV DNA-negative and seronegative during prior screening that took place up to 90 days prior to study start), a population of DNA-negative, seropositive women in PATRICIA, and a population of women with a history of previous infection or disease in VIVIANE.
The TVC-naïve included women who had negative cytology, were DNA-negative for all 14 oncogenic HPV types evaluated, and were seronegative for HPV-16 and HPV-18 at baseline (Panels A and B). Although the CVT did not include a TVC-naïve cohort, the cohort was simulated according to criteria defined in the PATRICIA trial and the Females United To Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) trials of Gardasil.[Citation33] Data reported in this paper refer to the analysis performed according to the PATRICIA criteria.
The ATP cohort was key to understanding how well the vaccine works across all endpoints in ideal conditions, and to meeting vaccine licensure requirements. The main aim of the ATP analysis was to assess prophylactic efficacy in women who were uninfected at baseline with the HPV type under consideration. From a public health perspective, the most relevant study cohorts were the TVC irrespective of the woman’s HPV status and the TVC-naïve, as these cohorts resemble most the cohorts in the general population who are likely to be considered for general vaccination programs. The TVC approximated a general population of women, including those with existing HPV infection that the vaccine does not impact upon, and those with previous HPV infection, which may promote a level of endogenous immunity. In contrast, the TVC-naïve approximated, from an immunological standpoint, a population of young women prior to sexual debut with limited or no genital HPV exposure, who are the primary target for HPV vaccination programs. Vaccine efficacy in this population represents the potential impact of the vaccine against new infections and lesions when administered to populations prior to sexual debut.
Efficacy findings
Vaccine efficacy against HPV-16/18-associated cervical infection, cytological abnormalities, and CIN in women without evidence of previous HPV type-specific infection
Vaccine efficacy against persistent infection was seen in all studies in the ATP cohort in women with no evidence of infection with the HPV-type under analysis, with point estimates ranging from 90.9% to 100% in women aged ≤25 years (). Vaccine efficacy was 100% in the analysis up to 9.4 years of the HPV-001/007/023 trial.[Citation23] In the VIVIANE study in women aged >25 years, vaccine efficacy against persistent infection was 82.9% in the ATP cohort ().[Citation42] Vaccine efficacy against 6MPI was also reported in PATRICIA in the TVC-E (80.4% in the interim analysis) [Citation24] and in the HPV-001/007/023 study in the TVC (94.4% at 4.5 years) ().[Citation19]
Table 2. Vaccine efficacy against persistent cervical infection and ASC-US+ associated with HPV-16/18 in women with no baseline evidence of infection with the HPV type under analysis.
High efficacy against cytological abnormalities (ASC-US+) associated with HPV-16/18 was reported in most studies in the ATP cohort, with significant vaccine efficacy estimates of between 80.6% and 100% (). The HPV-032/063, HPV-039, and VIVIANE studies also reported significant vaccine efficacy in the TVC-E in women with no evidence of infection with the HPV type under consideration with, respectively, point estimates of 93.3%, 88.5%, and 86.1%.[Citation39,Citation41,Citation42] In the HPV-001/007/023 study, which enrolled only HPV DNA-negative and seronegative women, 97.1% vaccine efficacy against ASC-US+ was seen up to 9.4 years of follow-up in the TVC.[Citation23]
Protection against CIN was evaluated in different cohorts across the studies (). In the PATRICIA, CVT and 6.4-year analysis of the HPV-001/007 studies, which had adequate participant numbers and follow-up, significant vaccine efficacy against HPV-16/18-associated CIN2+ was observed: 94.9% and 89.8% in the ATP cohorts of the 4-year analysis of the PATRICIA and CVT studies,[Citation26,Citation32] and 100% in the TVC of the HPV-001/007 6.4-year analysis ().[Citation20] CIN3+, which includes CIN3, AIS, and invasive cervical cancer, is the most stringent endpoint for the estimation of vaccine efficacy against cervical cancer. At the PATRICIA end-of-study analysis after 4 years of follow-up, vaccine efficacy against HPV-16/18-associated CIN3+ was 91.7% (95% confidence interval 66.6–99.1) and 100% (85.5–100) in the ATP and TVC-naïve cohorts, respectively ().[Citation26] Corresponding values for AIS were 100% (−8.6–100) and 100% (15.5–100).[Citation26]
Table 3. Vaccine efficacy against CIN1+, CIN2+, and CIN3+ associated with HPV-16/18 in women with no baseline evidence of infection with the HPV type under analysis.
Vaccine efficacy against cervical infection and CIN associated with non-vaccine HPV types (cross-protection)
Protection against HPV-31 and HPV-45 was seen consistently (); however, the low number of cases associated with non-vaccine types limited the statistical power of the analysis in most studies. The long-term follow-up studies HPV-001/007/023 were not powered to evaluate cross-protection. Cross-protective vaccine efficacy against non-vaccine oncogenic HPV types has been evaluated most extensively in the end-of-study analysis of PATRICIA, which had the most statistical power to examine cross-protection against less common types.[Citation27] Significant vaccine efficacy was seen in the ATP analysis against CIN2+ associated with HPV-31 (87.5%), HPV-33 (68.3%), HPV-45 (81.9%), and HPV-51 (54.4%) (). There was also significant vaccine efficacy against 6MPI with these types (). HPV-33 is now recognized as a highly oncogenic type; the risk of developing carcinoma in situ or AIS in women infected with HPV-33 is as high as in women infected with HPV-16.[Citation53] Efficacy against HPV-45 is also important, as HPV-45 causes approximately 12% of adenocarcinoma (approximately 50% is caused by HPV-16 and 32% by HPV-18).[Citation9,Citation54] The prevalence of adenocarcinoma is rising in many countries.[Citation55] Significant efficacy against CIN2+ associated with HPV-39 was also observed; however, no efficacy against 6MPI was observed.
Table 4. Cross-protective vaccine efficacy against infection and CIN2+ in women with no baseline evidence of infection with the HPV type under analysis (ATP cohort).
The broad-spectrum L1-based SPF10 PCR DNA immunoassay line probe assay system (SPF10 PCR-DEIA-LiPA25) was the protocol-specified testing algorithm used in PATRICIA.[Citation51] Broad-spectrum assays may underestimate the prevalence of genotypes present in relatively low concentrations in multiple infections.[Citation51] To increase the sensitivity of HPV DNA detection for a larger number of HPV types, a post hoc analysis of the end-of-study data was performed that combined the results from the SPF10 PCR-DEIA-LiPA25 system with a novel E6-based multiplex type-specific PCR and reverse hybridization assay.[Citation44] Using this algorithm, higher estimates of vaccine efficacy against persistent infection were obtained against some non-vaccine oncogenic HPV types ().
As discussed by Wheeler and colleagues,[Citation27] assignment of an individual HPV type as the cause of a CIN lesion poses a considerable challenge because DNA from several HPV types may be detected. The analysis of cross-protection is particularly complicated by the frequent detection of coinfection with vaccine and non-vaccine types. The PATRICIA analysis of cross-protection addressed this issue by performing two analyses of vaccine efficacy against CIN2+ and CIN3+ associated with a composite of 12 non-vaccine types: an analysis with or without coinfection with HPV-16/18 and an analysis excluding coinfection with HPV-16/18.[Citation27] Women were included in this analysis if they were DNA-negative for at least one of the HPV types included in the composite in the ATP cohort, irrespective of HPV DNA status in the TVC, and DNA-negative for all oncogenic HPV types tested in the TVC-naïve. Vaccine efficacy was substantially higher against CIN3+ than CIN2+, and, as expected, was higher in the analysis with or without HPV-16/18 coinfection ().[Citation27] Vaccine efficacy against CIN3+ excluding HPV-16/18 coinfection, the most conservative analysis, was 62.1% in the ATP cohort and 81.9% in the TVC-naïve (). Efficacy remained substantial in the TVC (40.0%) despite inclusion of women with evidence of previous HPV infection ().[Citation27]
Figure 2. Vaccine efficacy against CIN2+ and CIN3+ associated with a composite of 12 non-vaccine HPV types, with or without HPV-16/18 coinfection and excluding HPV-16/18 coinfection (PATRICIA).[Citation27] ATP: according to protocol – women DNA-negative for HPV type under analysis at baseline; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline;
![Figure 2. Vaccine efficacy against CIN2+ and CIN3+ associated with a composite of 12 non-vaccine HPV types, with or without HPV-16/18 coinfection and excluding HPV-16/18 coinfection (PATRICIA).[Citation27] ATP: according to protocol – women DNA-negative for HPV type under analysis at baseline; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline;](/cms/asset/8dbf975a-78b4-497c-8807-4aa4fe0e71b2/ierv_a_1124763_f0002_b.gif)
The analysis with or without coinfection is likely to overestimate cross-protective efficacy because some of the cases were undoubtedly caused by HPV-16/18. However, the analysis excluding coinfection is very conservative and could underestimate cross-protection for two reasons. First, if two independent lesions were found in the same woman, one infected with HPV-16/18 and the other infected with a non-vaccine type, both lesions were excluded from the analysis, reducing the overall number of cases associated with a non-vaccine type. Second, because fewer cases in the vaccine group than in the control group were coinfected with HPV-16/18, substantially more control cases than vaccine cases were excluded from the analysis, including some that might have been caused by a non-vaccine type. The true cross-protective vaccine efficacy likely lies between the two estimates.
Moderate vaccine efficacy against infection with non-oncogenic HPV types associated with genital warts has also been observed post hoc in the PATRICIA trial. In the TVC-naïve, vaccine efficacy against 6MPI was 34.5% (11.3–51.8) for HPV-6/11 and 49.5% (21.0–68.3) for HPV-74, which cause the vast majority of genital warts.[Citation29]
Vaccine efficacy against CIN irrespective of HPV
The PATRICIA, HPV-001/007/023, VIVIANE, Japanese (HPV-032/063), and CVT studies evaluated vaccine efficacy against CIN irrespective of HPV DNA (i.e. included all histopathologically diagnosed CIN cases without consideration of the HPV PCR result in the lesions). This endpoint was generally reported for the TVC and TVC-naïve. In the TVC, it was evaluated regardless of women’s HPV DNA status or serostatus at baseline. By definition, the TVC-naïve excluded women with any evidence of previous HPV infection.
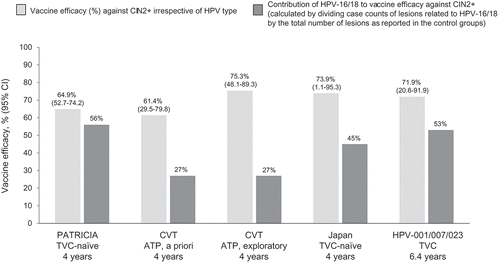
Vaccine efficacy irrespective of HPV was evaluated most extensively in the event-driven final analysis and end-of-study analysis of PATRICIA.[Citation26,Citation43] In the end-of-study analysis, in both the TVC and TVC-naïve, vaccine efficacy increased with greater lesion severity: 33.1% against CIN2+ and 45.6% against CIN3+ in the TVC, and 64.9% and 93.2% in the TVC-naïve ().[Citation26] Corresponding values in the event-driven final analysis were 30.4% and 33.4% in the TVC and 70.2% and 87.0% in the TVC-naïve.[Citation43] The increasing efficacy likely reflects the rising prevalence in more severe lesions of HPV types belonging to the A7 species (including HPV-18 and HPV-45) and A9 species (including HPV-16, HPV-31, and HPV-33), whilst the prevalence of other HPV types declines.[Citation56] In the TVC at the end-of-study analysis, 158 CIN3+ cases were reported in the control group, compared with 86 in the vaccine group, of which 36 cases were associated with HPV-16/18 only (). In the TVC-naïve, 44 CIN3+ cases were reported in the control group compared with only three cases in the vaccine group, none of which were associated with HPV-16/18 (). Vaccine efficacy against AIS was also high (76.9% [16.0–95.8] in the TVC and 100% [31.0–100] in the TVC-naïve) ().
Figure 3. Vaccine efficacy against CIN1+, CIN2+, CIN3+, and AIS irrespective of HPV DNA (4-year, end-of-study analysis of PATRICIA).[Citation26] AIS: adenocarcinoma in situ; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline.
![Figure 3. Vaccine efficacy against CIN1+, CIN2+, CIN3+, and AIS irrespective of HPV DNA (4-year, end-of-study analysis of PATRICIA).[Citation26] AIS: adenocarcinoma in situ; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline.](/cms/asset/84d1a68e-b838-40ad-b738-976ca02a9dc2/ierv_a_1124763_f0003_b.gif)
Figure 4. Number of cases of CIN3+ associated with vaccine and non-vaccine HPV types in the TVC and TVC-naïve (4-year, end-of-study analysis of PATRICIA).[Citation26] CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline. Reprinted from The Lancet Oncology, Lehtinen et al, Vol 13, Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomized, double-blind PATRICIA trial, pages 89–99, copyright 2012 with permission from Elsevier.
![Figure 4. Number of cases of CIN3+ associated with vaccine and non-vaccine HPV types in the TVC and TVC-naïve (4-year, end-of-study analysis of PATRICIA).[Citation26] CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline. Reprinted from The Lancet Oncology, Lehtinen et al, Vol 13, Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomized, double-blind PATRICIA trial, pages 89–99, copyright 2012 with permission from Elsevier.](/cms/asset/6a248b9a-8b1f-4605-a474-f9c1084b08ab/ierv_a_1124763_f0004_b.gif)
In the 9.4-year analysis of the HPV-001/007/023 study, vaccine efficacy against CIN irrespective of HPV DNA could not be assessed in a meaningful way because only the Brazilian cohort of the original study was included in the analysis at that stage, and few CIN cases were observed.[Citation23] However, at the 6.4-year analysis of the trial, vaccine efficacy irrespective of HPV DNA was 50.3% (12.5–72.6) against CIN1+ and 71.9% (20.6–91.9) against CIN2+ in the TVC.[Citation20] In the HPV-001/007/023 study, only women with no history of HPV infection or disease were enrolled; hence the vaccine efficacy estimate in the TVC was considerably higher than that in PATRICIA. In the interim analysis of VIVIANE (women aged >25 years), vaccine efficacy against CIN1+ irrespective of HPV DNA in the lesion was 14.9% (−10.1–34.2) in the TVC.[Citation42] Vaccine efficacy against CIN1+, CIN2+, and CIN3+ irrespective of HPV DNA in the 4-year analysis of the Japanese trial (HPV-032/063) was 56.7% (32.8–72.6), 54.9% (20.5–75.3), and 36.4% (−57.8–75.7), respectively, in the TVC, and 61.0% (11.8–84.2), 73.9% (1.1–95.3), and 100% (−417.0–100), respectively, in the TVC-naïve.[Citation40]
Analysis of vaccine efficacy against CIN2+ irrespective of HPV was performed in the ATP cohort for the CVT.[Citation32] Two definitions of the endpoint (a priori and exploratory), different from that in the aforementioned trials, were used, as described by Hildesheim and colleagues.[Citation32] Vaccine efficacy was 61.4% (29.5–79.8) for the a priori definition and 75.3% (48.1–89.3) for the exploratory definition. Considering evidence from all studies, high efficacy against CIN2+ irrespective of HPV type has been shown consistently ().
Figure 5. Vaccine efficacy against CIN2+ irrespective of HPV type in the lesion versus the contribution of HPV-16/18 to the lesion: a cross-trial comparison. In the CVT, the a priori analysis included all CIN2+ cases; the exploratory analysis considered evidence of HPV persistence preceding referral to colposcopy. Cohorts considered in the analyses: ATP – in the CVT, the analysis considered outcomes that occurred in the absence of HPV during the vaccination period; TVC – in the HPV-001/007/023 study, only women who were DNA-negative for all HPV types tested were enrolled; TVC-naïve: women DNA-negative for all HPV types tested at baseline. ATP: according to protocol; CI: confidence interval; CIN: cervical intraepithelial neoplasia; CVT: Costa Rica Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort.
The PATRICIA and CVT studies also showed that the HPV-16/18 vaccine reduces the number of colposcopy referrals and cervical excision procedures,[Citation26,Citation49] which reflects the reduction in pre-cancerous lesions due to vaccination. In the PATRICIA TVC end-of-study analysis, vaccine efficacy was 14.8% (8.9–20.3) against colposcopy referrals and 33.2% (20.8–43.7) against cervical excision procedures. Corresponding values in the TVC-naïve were 29.0% (21.6–35.8) and 70.2% (57.8–79.3). In the CVT, vaccine efficacy was 7.9% (−0.5–15.5) against colposcopy referrals and 11.3% (−8.9–27.8) against excision procedures in the TVC. Efficacy was higher in the TVC-naïve population: 21.3% (3.9–35.7) and 45.6% (−9.3–73.9) against colposcopy referrals and excision procedures, respectively. Considerable morbidity is associated with pre-cancerous lesions. Women with abnormal Pap smears may be referred for colposcopy and many pre-cancerous lesions require surgery. Treatment is associated with a higher risk of preterm births and perinatal mortality.[Citation57,Citation58] In some countries with effective cervical cancer screening programs, the cost of managing pre-cancerous lesions is similar to or higher than the cost of treating cervical cancer.[Citation2,Citation3] Therefore, this demonstration from PATRICIA and the CVT has the potential to reduce both costs – direct and due to loss of productivity – and anxiety associated with diagnosis and treatment of pre-cancer.
Vaccine efficacy against cervical infection and CIN in different subpopulations
Efficacy in different age groups
The vaccine appears to be effective in women with no evidence of previous exposure regardless of age at vaccination. In the CVT, efficacy against HPV-16/18 12MPI in the ATP cohort was 95.9% (78.5–99.8) for women aged 18–19 years, 86.6% (59.2–96.8) for 20–21 years, 95.7% (77.4–99.8) for 22–23 years, and 82.2% (43.9–95.9) for 24–25 years.[Citation31] Data on age-stratified vaccine efficacy have not been presented for the ATP cohort in PATRICIA, but vaccine efficacy against HPV-16/18-associated CIN3+ in the TVC-naïve was 100% (69.4–100) and 100% (67.8–100) in 15–17 and 18–25-year-olds, respectively.[Citation26] Efficacy was lower in the older age group in the TVC, likely due to the high baseline prevalence of infection in these young adult women (56.3% [13.6–79.1] in 18–20-year olds and −10.1% [−90.5–36.1] in 21–25-year olds).[Citation26] The VIVIANE study demonstrated efficacy of the vaccine in women aged >25 years. Efficacy against the combination of HPV-16/18 6MPI and CIN1+ in the ATP cohort (the primary endpoint of the study) was 83.5% (45.0–96.8) in women aged 26–35 years and 77.2% (2.8–96.9) in women aged 36–45 years.[Citation42] Although some women older than 45 years were included in the study, insufficient cases occurred for meaningful evaluation of vaccine efficacy.[Citation42]
Efficacy in women with evidence of previous infection
An early analysis of the CVT showed that the vaccine has no therapeutic efficacy and does not promote clearance of existing infection.[Citation30] However, it has been shown to be effective in preventing new infections in women who have had previous infections. In PATRICIA, vaccine efficacy against 6MPI with HPV-16 was 70.3% (46.0–84.7) in women in the TVC who were DNA-negative and seropositive at baseline for HPV-16.[Citation28] In women who were DNA-negative and seropositive at baseline for HPV-18, vaccine efficacy against 6MPI with HPV-18 was 76.3% (32.6–93.5). The VIVIANE study evaluated a subset of women with a history of previous infection or disease, defined as two or more abnormal smears in sequence, abnormal colposcopy, or biopsy or treatment of the cervix after abnormal smear or colposcopy findings.[Citation42] Although the study was not powered for this group, vaccine efficacy was 49.9% (−0.3–76.2) against HPV-16/18 6MPI/CIN1+ and 66.9% (1.9–91.0) against HPV-16/18 ASC-US+. Nonsignificant efficacy was seen against CIN1+ and CIN2+.
Efficacy against infection in women who received only one or two doses of vaccine
A post hoc analysis of the CVT has suggested that vaccination with two or possibly even one dose of the HPV-16/18 vaccine might be effective in preventing infection.[Citation34] All women were included in the analysis unless they were DNA-positive for both HPV-16 and HPV-18 at baseline. Significant vaccine efficacy against 12MPI with HPV-16/18 was seen regardless of whether women received one, two, or three doses: 100% (66.5–100), 84.1% (50.2–96.3), and 80.9% (71.1–87.7), respectively.
Summary data from PATRICIA and the CVT were combined in a post hoc analysis of the efficacy of fewer than three doses of the HPV-16/18 vaccine.[Citation50] The analyses were conducted in the TVC-naïve and in a modified TVC (m-TVC), which excluded women who were DNA-positive at baseline for the HPV type under evaluation. Significant vaccine efficacy (76–100%) against incident one-time detection of HPV-16/18 and HPV-16/18 6MPI was seen in both the m-TVC and TVC-naïve after one, two, or three doses (). Vaccine efficacy against the composite of HPV-31/33/45 was also evaluated in the m-TVC (but was not reported for the TVC-naïve). Vaccine efficacy against incident one-time detection was 36.6% (−5.4–62.2) after one dose, 37.7% (12.4–55.9) after two doses, and 59.7% (56.0–63.0) after three doses. Corresponding values for HPV-31/33/45 6MPI were 48.8% (−16.9–78.5), 30.7% (−27.9–63.0), and 60.1% (54.0–65.4). When the analysis of women who received two vaccine doses was stratified according to timing of the second dose, efficacy against incident one-time detection of HPV-16/18 was significant regardless of whether the second dose was administered 1 month or 6 months after the first dose (75.3% [54.2–87.5] and 82.6% [42.3–96.1], respectively). Vaccine efficacy was significant for HPV-31/33/45 when the second dose was given 6 months after the first dose (68.1% [27.0–87.0]), but not 1 month after the first dose (10.2% [−42.0–43.3]).
Figure 6. Vaccine efficacy against HPV-16/18 incident one-time detection and 6-month persistent infection with three, two, and one dose of HPV-16/18 vaccine (combined analysis of PATRICIA and the CVT).[Citation50] Women were excluded if they had less than 12 months of follow-up. 6MPI: 6-month persistent infection; CI: confidence interval; CVT: Costa Rica HPV-16/18 Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; m-TVC: modified total vaccinated cohort – excluding women DNA-positive at baseline for the HPV type under evaluation; TVC-naïve: women DNA-negative for all HPV types tested at baseline.
![Figure 6. Vaccine efficacy against HPV-16/18 incident one-time detection and 6-month persistent infection with three, two, and one dose of HPV-16/18 vaccine (combined analysis of PATRICIA and the CVT).[Citation50] Women were excluded if they had less than 12 months of follow-up. 6MPI: 6-month persistent infection; CI: confidence interval; CVT: Costa Rica HPV-16/18 Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; m-TVC: modified total vaccinated cohort – excluding women DNA-positive at baseline for the HPV type under evaluation; TVC-naïve: women DNA-negative for all HPV types tested at baseline.](/cms/asset/20aff9d1-d57a-40f6-9bb2-a7f1410dcc2e/ierv_a_1124763_f0006_b.gif)
Several studies have also shown high immunogenicity after one or two doses of the HPV-16/18 vaccine [Citation59–Citation61] and after two doses of the HPV-6/11/16/18 vaccine.[Citation62] A two-dose schedule in girls under 15 years of age is now recommended by the WHO.[Citation63] The vaccines are now licensed in a 2-dose schedule in over 100 countries.
Vaccine efficacy against vulvar, oral, and anal HPV infection
Efficacy against non-cervical HPV infection has been evaluated in the 4-year analysis of the CVT. Evaluation of oral and anal infection was based on one-time detection at 4 years of follow-up; no data on infection at the oral and anal sites were collected at baseline. In an analysis of oral HPV infection (irrespective of cervical HPV DNA status at baseline), vaccine efficacy was 93.3% (62.5–99.7) against infection with HPV-16/18 and 45.7% (6.9–69.0) against infection with any oncogenic HPV type.[Citation37] Vaccine efficacy against anal infection with HPV-16/18 was 83.6% (66.7–92.8) in a population of women who were HPV-16/18 DNA-negative at the cervix and seronegative at baseline and received all three vaccine doses, and 62.0% (47.1–73.1) in all women from whom an anal sample was obtained regardless of DNA and serostatus at baseline.[Citation36] Cross-protection against anal infection was also observed, with values in the corresponding populations of 61.8% (42.8–75.0) and 49.4% (30.3–63.6) for HPV-31/33/45.[Citation36] A reduction in the number of vulvar HPV-16/18 infections was also seen, with vaccine efficacy of 54.1% (4.9–79.1) in all women with vulvar samples collected.[Citation35]
Expert commentary
Strengths of the clinical trial program of the HPV-16/18 AS04-adjuvanted vaccine include its large size, geographical reach, inclusion of women both previously exposed and unexposed to HPV infection, and analysis of different populations of women (in the different study cohorts) to allow comparison of vaccine efficacy. The studies used similar data collection procedures, although the CVT and HPV-039 studies used different laboratories for analysis of cytology and histopathology specimens, and the CVT used a different case definition for the histopathological endpoints. Despite these differences in methodology, efficacy was consistent across studies. The data show that the vaccine reduces infection with HPV-16/18 and associated cervical endpoints in women regardless of age, geographical location, or sexual experience. Based on the cumulative evidence to date, the data indicate that the HPV-16/18 AS04-adjuvanted vaccine protects against HPV infections at the anatomic sites where HPV causes cancer in women, including the cervix, vulva, anus, and oral region. It also provides cross-protection against some non-vaccine oncogenic HPV types and moderate cross-protection against types causing genital warts.
Both the level of efficacy against vaccine and non-vaccine HPV types and the duration of protection contribute toward the overall protection offered by an HPV vaccine. The duration of vaccine protection influences cost-effectiveness in economic models.[Citation64,Citation65] It is noteworthy in the context of duration of protection that the HPV-001/007/023 study provides the longest follow-up of any HPV vaccine study to date, with evidence of sustained efficacy against HPV-16/18-persistent infection and CIN1+ up to almost 10 years.[Citation18–Citation23] Substantial cross-protection up to 4 years was shown in the PATRICIA study [Citation27] and in the combined analysis of the PATRICIA and CVT studies.[Citation50] As discussed by Wheeler and colleagues,[Citation27] the analysis of cross-protection against CIN lesions attributable to individual non-vaccine HPV types is particularly demanding. Large sample sizes and extensive follow-up periods are needed for meaningful analysis of CIN lesions, which are less often associated with non-vaccine HPV types than HPV-16/18. Furthermore, CIN lesions are not infrequently associated with more than one oncogenic HPV type, including HPV-16 and -18, which complicates attribution of the vaccine’s efficacy against non-vaccine HPV types.
Efficacy against CIN irrespective of HPV DNA in the lesion is a product of efficacy against lesions associated with both vaccine and non-vaccine HPV types. It is an important clinical measure as it provides an overall indication of efficacy, without regard to the HPV type or types in the lesion, and is therefore not confounded by multiple infections or limitations of HPV typing. Furthermore, it provides a conservative estimate of likely protection in the real-world setting.[Citation66] Vaccine efficacy against CIN irrespective of HPV is recognized as the clinical trial endpoint most relevant from a public health perspective.[Citation67,Citation68] We believe, therefore, that the demonstration of strong and significant efficacy of the HPV-16/18 vaccine against CIN irrespective of HPV is of great public health importance.
The extent and the duration of cross-protection provided by the HPV-16/18 vaccine have been questioned by other authors.[Citation47,Citation69] Cuzick noted that the 9-valent HPV vaccine would be expected to provide approximately 90% protection against cervical cancer, and observed that the HPV-16/18 vaccine has demonstrated a similar level of protection against CIN3+ irrespective of HPV, in HPV-naïve women.[Citation69] He attributed the high level of protection offered by the HPV-16/18 vaccine to chance rather than a real effect because lower levels of protection against persistent infection with non-vaccine HPV types were observed. However, data on cross-protective efficacy of the HPV-16/18 vaccine contradict his hypothesis. In the TVC-naïve cohort of the PATRICIA study, we see that vaccine efficacy against 6MPI with HPV-31, −33, and −45 was 77.1%, 43.1%, and 79.0%, respectively, while the vaccine efficacy observed against CIN2+ was 89.4%, 82.3%, and 100%, respectively.[Citation27] Thus, vaccine protection against infection is lower than against CIN lesions. This is probably because the definitions of incident infection and persistent infection are imperfect, as they are inferred by positive HPV DNA testing at specific time points: incident and persistent infection may represent deposition of HPV DNA rather than actual infection or recurrence of latent infection present before vaccination. This is likely to cause misclassification of endpoints and dilution of vaccine efficacy.
It is recognized that the added value of using persistent infection as an endpoint is that it avoids the complexity of causality attribution in the presence of multiple infections, particularly to evaluate protection against individual HPV types other than 16 and 18.[Citation70] However, it is important to note that persistent infection is a surrogate of CIN2+ but a more distant surrogate of cancer; therefore, estimates of vaccine efficacy against persistent infection may not be fully informative about likely vaccine efficacy against cervical cancer.[Citation70] Despite this limitation, persistent infection is not biased by multiple infections, and hence it provides a useful but conservative estimate of protection against CIN endpoints.
A review by Malagón and colleagues [Citation47] of cross-protection based on data from the HPV-001/007/023 and PATRICIA trials of the HPV-16/18 vaccine and the FUTURE I and II trials of the HPV-6/11/16/18 vaccine concluded that the HPV-16/18 vaccine offers higher levels of cross-protection than the HPV-6/11/16/18 vaccine. However, it was also suggested that cross-protective efficacy of the HPV-16/18 vaccine may wane over time, based on vaccine efficacy estimates from the phase II HPV-007 and -023 studies, including data from the GSK Clinical Study Register.[Citation71,Citation72] As described earlier, the HPV-007 and HPV-023 studies were extensions of the phase II HPV-001 study. The HPV-001 study was conducted in the US, Canada, and Brazil in 1113 women and assessed efficacy up to 4.5 years. The first extension, HPV-007, retained 776 women and assessed efficacy up to 6.4 years. HPV-023 was the second extension phase, included 437 women from Brazil, and assessed efficacy up to 9.4 years. When considering all available evidence, the conclusion of waning efficacy based on data from the HPV-007 and HPV-023 studies should be challenged for two reasons. First, the studies were not designed or powered to evaluate cross-protection. Sample size calculations were done for the HPV-001 study and were based on an assumption of a 6% cumulative incidence rate of both HPV-16 and HPV-18 infections over 12 months.[Citation18] Incidence rates of non-vaccine type infections are considerably lower, and therefore much larger sample sizes would have been required to demonstrate cross-protection. Large sample sizes are essential for the evaluation of cross-protection.[Citation27] Second, Malagón and colleagues base their conclusion of waning cross-protective efficacy on the heterogeneity in vaccine efficacy against 6MPI observed between the HPV-007, HPV-023, and PATRICIA trials, and suggest that time of follow-up was the key driver. However, it is unclear whether the analysis of heterogeneity accounted for the fact that HPV-001, HPV-007, and HPV-023 were not independent studies. Furthermore, the vaccine efficacy estimates reported by Malagón and colleagues were in fact based on the HPV-001/007/023 combined analysis, calling into question the value of the analysis of heterogeneity.
It should also be noted that there was no decline in the HPV-001/007/023 studies in cross-protective vaccine efficacy against incident infection. As mentioned above, the small sample size, in particular the low number of women remaining by the end of the study, led to low case counts, which made evaluation of cross-protection problematic. Many more cases of incident infection than 6MPI were observed. Point estimates for vaccine efficacy against incident infection were similar at the 6.4-year and 9.4-year analyses for HPV-31, HPV-33, and HPV-45, with narrower confidence intervals than seen for 6MPI.[Citation20,Citation23,Citation72] Importantly, immunogenicity data from this study demonstrated sustained and stable antibody responses against HPV-31 and HPV-45 up to 9.4 years.[Citation73] In view of this, there is no biologically plausible mechanism to explain waning cross-protection against 6MPI but sustained cross-protection against incident infection, other than the low sample size and low number of 6MPI events. Indeed, in the combined analysis of the CVT and PATRICIA trials, including more than 24,000 women, vaccine efficacy against incident infection with HPV-31/33/45 was similar to that against 6MPI.[Citation50] In addition, efficacy estimates for HPV-16/18 appear to be more conservative for incident infection than for 6MPI.[Citation74] It has been suggested that even a one timepoint detection of HPV infection might be predictive of protection against CIN2+. Together, these data suggest that if efficacy wanes, then it would be expected to be also observed for incident infection.
In summary, we contend that the waning cross-protective efficacy reported by Malagón and colleagues [Citation47] has resulted from the small sample size and resulting low case counts of 6MPI. Together, the observation of continued cross-protection against incident infection [Citation20,Citation23,Citation72] and immunogenicity data demonstrating long-term persistence of cross-reactive antibody responses [Citation73,Citation75,Citation76] suggest that the HPV-16/18 vaccine will provide sustained cross-protective efficacy.
Based on the vaccine efficacy seen in the PATRICIA trial in the TVC-naïve, a modeling exercise assuming lifetime protection has estimated a worldwide annual reduction of 246,086 cervical cancer cases assuming 50% HPV-16/18 vaccine coverage and a reduction of 442,955 cases assuming 90% coverage.[Citation77] Several epidemiological studies have evaluated the prevalence of HPV infection and associated cervical abnormalities following introduction of the vaccine. In the UK, the HPV-16/18 AS04-adjuvanted vaccine was used for the national HPV immunization program between 2008 and 2012. Girls aged 12–13 years were offered the vaccine in a school-based program, and a catch-up campaign for those under 18 years was conducted between 2008 and 2011. A survey from 2010 to 2012 in 16–24-year-old sexually active women in England showed that the prevalence of HPV-16/18 infection among 16–18-year olds was 6.5% compared with 19.1% in the period before vaccination was introduced.[Citation78] Among women aged 19–21 years, some were eligible for vaccination whilst others were not; however, HPV-16/18 prevalence declined in both groups. There was no reduction in HPV-16/18 prevalence among women aged 22–24 years. Overall, there was a slightly lower prevalence of HPV-31/33/45 in the post-immunization period. Altogether, the data suggest high vaccine effectiveness and some herd-protection benefits.[Citation78] Similarly, surveillance in Scotland of women at age 20 years attending for cervical screening has shown a decline in the prevalence of HPV-16/18 infection from 29.8% to 13.6% following the introduction of vaccination, as well as cross-protection evidenced by significant reduction in the prevalence of HPV-31/33/45.[Citation79] The Scottish surveillance also showed a significant reduction in the diagnosis of CIN1+, CIN2+, and CIN3+, with a relative risk of 0.45 (0.35–0.58) in women who received three vaccine doses compared with unvaccinated women for CIN3+.[Citation80]
Surprisingly, a 31% decrease in the prevalence of genital warts during the post-immunization period 2008–2014 has also been observed in England among women aged 15–19 years, with a decline of 10% in those aged 20–24 years.[Citation81] The greatest declines were seen among 15-, 16-, 17-, and 18-year-old females (51%, 47%, 37%, and 30%, respectively), who would have been eligible for school-based vaccination (with reported vaccination coverage of over 70%).[Citation81] A 24% decrease was also seen in non-vaccinated young men aged 15–19 years over the same period.[Citation81]
The clinical trial program has also shown that the HPV-16/18 vaccine reduces infection and associated cervical abnormalities in women aged >25 years and in those with previous HPV infection. This is a relevant finding with regard to catch-up vaccination programs that are being offered to women up to the age of 26 years in some countries, or where the vaccine is licensed to be used in women over this age. It is clear that young and mid-adult women have the potential to benefit from vaccination by protection from infection. A recent health economic modeling analysis has shown that inclusion of previously exposed women in the model substantially improves the cost-effectiveness of the vaccine in adults.[Citation82] The recent combined analysis of PATRICIA and the CVT confirms the efficacy of the HPV-16/18 vaccine given as a two-dose or possibly even a one-dose schedule, which also has implications for cost-effectiveness.[Citation34]
Five-year view
Over the next 5 years, epidemiological data will provide important information on the prevalence of infection and associated disease/outcomes (including non-cervical), as well as the duration of protection against vaccine and non-vaccine genotypes. The case for vaccination programs will become more compelling as these data emerge, particularly data demonstrating marked reductions in pre-cancerous cervical lesions in young women. We are likely to see substantial progress in the implementation of vaccination programs, including even the wider introduction of two-dose vaccination schedules, further investigation of one-dose schedules, expansion of vaccination in developing countries, and vaccination of boys.
In 2014, the WHO recommended a two-dose vaccination schedule for girls under 15 years of age.[Citation63] This schedule has already been widely implemented. The two-dose schedule will become standard practice and will improve access to vaccination as well as coverage in countries with currently low vaccine uptake. In addition, optimal dose intervals to improve convenience and reduce implementation cost will be further explored.
Implementation of vaccination programs in developing countries will be facilitated through the introduction of one- and two-dose schedules. A reduction in the number of doses is particularly important in resource-constrained settings because of the cost and logistical difficulties in delivering the standard three-dose schedule. Many countries, including those with the highest risk for cervical cancer, have not yet introduced a vaccination program.[Citation83] The one-dose schedule could open the possibility of administering the vaccine in a single vaccine dose pulse campaign every 5–10 years.[Citation84] Such campaigns consume substantially less resource than annual vaccination programs and can be extremely useful in resource-poor settings. The efficacy of the HPV-16/18 vaccine given in a single dose is being further evaluated in a long-term follow-up of the CVT.[Citation85] Reductions in vaccine price will also facilitate adoption in developing countries. An economic model based in Peru showed that the cost of vaccinating preadolescent girls would be less than US$500 per year of life saved at a vaccine price of $7/dose, and $1300 at a cost of $20/dose.[Citation86]
Vaccination of boys is likely to be increasingly adopted in countries that can afford to offer the vaccine to this group. Price negotiations may be required because the cost-effectiveness of vaccination of males is lower than for females.[Citation87] An ongoing cluster randomized trial of best vaccination strategy is evaluating the overall and herd effects of vaccinating girls only versus both girls and boys.[Citation88] The case for equity of access to the vaccine is a compelling one, given the fact that those in high-risk groups, such as men who have sex with men, are unlikely to be identified before exposure to HPV.
Finally, high coverage of HPV vaccination will allow longer intervals for cervical cancer screening, and HPV testing will become more important. The optimal approach is likely to be a combination of vaccination, HPV testing, and Pap testing.
Trademark statement
Cervarix® is a registered trademark of GSK group of companies.
Gardasil® is a registered trademark of Merck and Co., Inc.
Financial & competing interests’ disclosure
GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and publication of this manuscript. N Burlet, A Mihalyi, and F Struyf report being employees by the GSK group of companies and own share/stock options in the GSK group of companies. R Skinner received funds through her institution to cover expenses involved in the collection of data and travel expenses to conferences to present data from the PATRICIA and VIVIANE clinical trials, advisory board membership honorarium, and grant support. R Skinner was also coinvestigator on an investigator-driven educational research grant funded by bioCSL. D Apter reports grants from the GSK group of companies for the conduct of HPV vaccination studies. R Konno reports lecture fees from MSD and the GSK group of companies. J Paavonen has received research grants from Merck & Co and the GSK group of companies through the University of Helsinki to conduct clinical trials of HPV vaccines, and has received consulting fees or lecture fees from both companies. B Romanowski received through her institution grant support from the GSK group of companies and personal fees to cover travel expenses for speaking engagement. C Roteli-Martins reports personal fees and grants from the GSK group of companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Key issues
Vaccines are available against HPV, the causal agent of cervical and certain other cancers.
Data from an extensive clinical trial program show that the HPV-16/18 AS04-adjuvanted vaccine reduces infection with HPV-16/18 and associated cervical endpoints in women regardless of age or sexual experience, and provides cross-protection against some non-vaccine HPV types.
Six randomized, controlled phase II/III trials enrolling women from different populations and locations, including Europe, North America, Latin America, Australia, South-East Asia, China, and Japan were identified in this review. The program included two cohorts of high relevance from a public health perspective: the TVC, approximating a general population including those with existing or previous HPV infection, and the TVC-naïve, approximating from an immunological standpoint a population of young women before sexual debut. CIN3+, irrespective of whether HPV is detected in the lesion, is the closest endpoint to cervical cancer.
In PATRICIA, efficacy against CIN3+ irrespective of HPV was 45.6% in the TVC and 93.2% in the TVC-naïve. High efficacy against CIN2+ irrespective of HPV was consistently reported in various trials.
In women with no evidence of infection with the HPV type under analysis, efficacy against persistent HPV-16/18 infection was 93.4–100% in women aged ≤25 years and 82.9% in women aged >25 years (ATP cohorts).
Analysis of the CVT suggested similar efficacy against persistent HPV-16/18 infection with one, two, or three vaccine doses.
Cross-protection against HPV-31 and HPV-45 was seen consistently throughout all the studies, and an extensive analysis in PATRICIA also showed efficacy against HPV-33 and HPV-51.
Efficacy against vulvar, oral, and anal HPV infection was observed in the CVT.
Early epidemiology data following introduction of the vaccine suggest that the prevalence of vaccine and certain non-vaccine HPV types is declining.
Acknowledgments
The authors would like to thank the patients for their participation in the respective studies. They also thank Walid Kandeil (GSK Vaccines, Wavre) for his input during the initial stages of the paper’s development, Mary Greenacre (An Sgriobhadair, Isle of Barra, UK) and Annick Moon (Moon Medical Communications Ltd, Oxford, UK) for publication writing assistance provided on behalf of GSK Vaccines, and Matthieu Depuydt (Business & Decision Life Sciences, Belgium) and Jean-Michel Heine (Keyrus Biopharma, Belgium) for publication coordination and management provided on behalf of GSK Vaccines.
References
- International Agency for Research on Cancer. Cervical cancer. Estimated incidence, mortality and prevalence worldwide in 2012, 2014 [cited 2014 Nov 16]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114–120.
- Brown RE, Breugelmans JG, Theodoratou D, et al. Costs of detection and treatment of cervical cancer, cervical dysplasia and genital warts in the UK. Curr Med Res Opin. 2006;22:663–670.
- Annemans L, Remy V, Lamure E, et al. Economic burden associated with the management of cervical cancer, cervical dysplasia and genital warts in Belgium. J Med Econ. 2008;11:135–150.
- Low JJ, Ko Y, Ilancheran A, et al. Health and economic burden of HPV-related diseases in Singapore. Asian Pac J Cancer Prev. 2012;13:305–308.
- Salo H, Leino T, Kilpi T, et al. The burden and costs of prevention and management of genital disease caused by HPV in women: a population-based registry study in Finland. Int J Cancer. 2013;133:1459–1469.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19.
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens. Part B: biological agents. Lancet Oncol. 2009;10:321–322.
- De Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056.
- Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–192.
- Moscicki AB. HPV infections in adolescents. Dis Markers. 2007;23:229–234.
- Collins S, Mazloomzadeh S, Winter H, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. BJOG. 2002;109:96–98.
- Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–8577.
- Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–2087.
- Insinga RP, Perez G, Wheeler CM, et al. Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19:1585–1594.
- Koshiol J, Lindsay L, Pimenta JM, et al. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–137.
- Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240.
- Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765.
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255.
- Romanowski B, De Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–1985.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255.
- Roteli-Martins CM, Naud P, De Borba P, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390–397.
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147–2162.
- Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170.
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314.
- Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99.
- Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–110.
- Szarewski A, Poppe WA, Skinner SR, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15-25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106–116.
- Szarewski A, Skinner SR, Garland SM, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis. 2013;208:1391–1396.
- Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753.
- Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1:408–419.
- Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–5097.
- Lang Kuhs KA, Porras C, Schiller JT, et al. Effect of different human papillomavirus serological and DNA criteria on vaccine efficacy estimates. Am J Epidemiol. 2014;180:599–607.
- Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Nat Cancer Inst. 2011;103:1444–1451.
- Lang Kuhs KA, Gonzalez P, Rodriguez AC, et al. Reduced prevalence of vulvar HPV16/18 infection among women who received the HPV16/18 bivalent vaccine: a nested analysis within the Costa Rica vaccine trial. J Infect Dis. 2014;210:1890–1899.
- Kreimer AR, Gonzalez P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica vaccine trial. Lancet Oncol. 2011;12:862–870.
- Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PloS One. 2013;8:e68329.
- Konno R, Tamura S, Dobbelaere K, et al. Efficacy of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double-blind, randomized, controlled trial. Int J Gynecol Cancer. 2010;20:404–410.
- Konno R, Tamura S, Dobbelaere K, et al. Efficacy of human papillomavirus type 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double-blind, randomized controlled trial. Int J Gynecol Cancer. 2010;20:847–855.
- Konno R, Yoshikawa H, Okutani M, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women: open follow-up of a randomized clinical trial up to 4 years post-vaccination. Hum Vaccin Immunother. 2014;10:1781–1794.
- Zhu FC, Chen W, Ym H, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. Int J Cancer. 2014;135:2612–2622.
- Skinner SR, Szarewski A, Romanowski B, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014;384:2213–2227.
- Apter D, Wheeler CM, Paavonen J, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol. 2015;22:361–373.
- Struyf F, Colau B, Wheeler CM, et al. Post hoc analysis of the PATRICIA randomized trial of the efficacy of human papillomavirus type 16 (HPV-16)/HPV-18 AS04-adjuvanted vaccine against incident and persistent infection with nonvaccine oncogenic HPV types using an alternative multiplex type-specific PCR assay for HPV DNA. Clin Vaccine Immunol. 2015;22:235–244.
- Miltz A, Price H, Shahmanesh M, et al. Systematic review and meta-analysis of L1-VLP-based human papillomavirus vaccine efficacy against anogenital pre-cancer in women with evidence of prior HPV exposure. PloS One. 2014;9:e90348.
- La Torre G, De Waure C, Chiaradia G, et al. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine. 2007;25:8352–8358.
- Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–789.
- Medeiros LR, Rosa DD, Da Rosa MI, et al. Efficacy of human papillomavirus vaccines: a systematic quantitative review. Int J Gynecol Cancer. 2009;19:1166–1176.
- Rodriguez AC, Solomon D, Herrero R, et al. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. Am J Epidemiol. 2013;178:752–760.
- Kreimer A, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015 [cited 2015 Jun 9]. DOI:10.1016/S1470-2045(15)00047-9.
- Van Doorn LJ, Molijn A, Kleter B, et al. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:32–38.
- Van Alewijk D, Kleter B, Vent M, et al. A human papilloma virus testing algorithm comprising a combination of the L1 broad-spectrum SPF10 PCR assay and a novel E6 high-risk multiplex type-specific genotyping PCR assay. J Clin Microbiol. 2013;51:1171–1178.
- Wheeler CM, Hunt WC, Joste NE, et al. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Nat Cancer Inst. 2009;101:475–487.
- Pirog EC, Lloveras B, Molijn A, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–1567.
- Bray F, Carstensen B, Moller H, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199.
- Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84.
- Castanon A, Landy R, Brocklehurst P, et al. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in England: nested case-control study. BMJ. 2014;349:g6223.
- Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.
- Boxus M, Lockman L, Fochesato M, et al. Antibody avidity measurements in recipients of Cervarix vaccine following a two-dose schedule or a three-dose schedule. Vaccine. 2014;32:3232–3236.
- Romanowski B, Schwarz TF, Ferguson LM, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother. 2014;10:1155–1165.
- Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 2013;6:1242–1250.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–1802.
- World Health Organization. Immunization, vaccines and biologicals. Summary of the SAGE April 2014 meeting. [cited 2014 Nov 16]. Available from: http://www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en/
- Jit M, Chapman R, Hughes O, et al. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ. 2011;343:d5775.
- Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769.
- Vaccines and Related Biological Products Advisory Committee (VRBPAC). Cervarix. Human papillomavirus Bivalent (types 16 and 18) vaccine, recombinant. Briefing document, 2009 Sep 9. [cited 2015 Sep 15]. Available from: http://www.fda.gov/downloads/Adviso…uctsAdvisoryCommittee/UCM181371.pdf
- Di Mario S, Basevi V, Lopalco PL, et al. Are the two human papillomavirus vaccines really similar? A systematic review of available evidence: efficacy of the two vaccines against HPV. J Immunol Res. 2015;435141. DOI:10.1155/2015/435141.
- World Health Organization. Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89:465–492.
- Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines. 2015 [cited 2015 May 31]. DOI:10.1586/14760584.2015.1051470.
- Bosch FX, De Sanjosé S, Castellsagué X. The prospects of HPV vaccination in cervical cancer prevention: results of a new independent trial. Cancer Discov. 2011;1:377–380.
- GlaxoSmithKline. Clinical Study Register. Study ID: 580299/007. 2014 [cited 2015 Jun 20]. Available from: www.gsk-clinicalstudyregister.com/study/580299/007#rs
- GlaxoSmithKline. Clinical Study Register. Study ID: 109616 (Y7). 2014 [cited 2015 Jun 20]. Available from: http://www.gsk-clinicalstudyregister.com/study/109616%20(Y7)#rs
- Moscicki AB, Harper DM, Naud P, et al. HPV-16/18 AS04-adjuvanted vaccine: sustained immunogenicity against HPV-31 and HPV-45 non-vaccine oncogenic types up to 9.4 years. Presented at the 30th International Papillomavirus Conference and Public Health Workshops (HPV 2015); 2015 Sep 17–21; Lisbon, Portugal.
- Lowy DR, Herrero R, Hildesheim A. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol. 2015;16:e226–e233.
- Kemp TJ, Safaeian M, Hildesheim A, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix(R). Vaccine. 2012;31:165–170.
- Moscicki AB, Wheeler CM, Romanowski B, et al. Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine. 2012;31:234–241.
- Van Kriekinge G, Castellsague X, Cibula D, et al. Estimation of the potential overall impact of human papillomavirus vaccination on cervical cancer cases and deaths. Vaccine. 2014;32:733–739.
- Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32:26–32.
- Kavanagh K, Pollock KG, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–2811.
- Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer. 2014;111:1824–1830.
- Public Health England. Declines in genital warts since start of the HPV immunisation programme. Health Protect Rep – Wkly Rep. 2014 [cited 2015 Sep 15];9(22). Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/441554/hpr2215.pdf
- Turner HC, Baussano I, Garnett GP. Vaccinating women previously exposed to human papillomavirus: a cost-effectiveness analysis of the bivalent vaccine. PloS One. 2013;8:e75552.
- World Health Organization. Evidence based recommendations on human papilloma virus (HPV) vaccines schedules. Background paper for SAGE discussions. 2014 [cited 2014 Nov 16]. Available from: http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf
- Brotherton JM. Could one dose of bivalent HPV vaccine prevent cervical cancer? Lancet Oncol. 2015 [cited 2015 Jun 10]. DOI:10.1016/S1470-2045(15)00046-7.
- Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine. 2015;33:2141–2151.
- Goldie SJ, Levin C, Mosqueira-Lovon NR, et al. Health and economic impact of human papillomavirus 16 and 18 vaccination of preadolescent girls and cervical cancer screening of adult women in Peru. Rev Panam Salud Publica. 2012;32:426–434.
- Burger EA, Sy S, Nygard M, et al. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PloS One. 2014;9:e89974.
- Lehtinen M, Apter D, Baussano I, et al. Characteristics of a cluster-randomized phase IV human papillomavirus vaccination effectiveness trial. Vaccine. 2015;33:1284–1290.
Appendix
Panel A. Study cohorts.
Panel B. Primary analysis populations reported for each study according to cohort and endpoint.