ABSTRACT
Background: Hepatitis A and B are two of the most common vaccine-preventable diseases and vaccination for Hepatitis A virus (HAV) and hepatitis B virus (HBV) is recommended for those at risk of contracting HAV and/or HBV through their occupation, travel or lifestyle.
Objective: To describe the vaccine efficacy, immunogenicity, effectiveness and safety of the combined vaccine against hepatitis A and hepatitis B.
Methods: A systematic review of the literature published between 1990 and 2015.
Results: Anti-HAV seropositivity rates ranged from 96.2% to 100% and anti-HBs seroprotection rates from 82% to 100%. Antibodies persisted up to 15 years and geometric mean concentration (GMC) remained above the seropositivity cut-off value for both.
Anti-HAV and anti-HBs immune responses were lower in less immunocompetent individuals one month after completion of the immunization schedule.
The safety profiles of TwinrixTM and monovalent hepatitis A and B vaccines were similar.
Conclusion: The vaccine offers satisfactory long-term immunogenicity rates, expected duration of protection and safety profile similar to the monovalent hepatitis A or B vaccines.
Introduction
Hepatitis A and B are two of the most common vaccine-preventable diseases and are a serious global public health problem [Citation1–Citation3].
Hepatitis A virus (HAV) is primarily spread through ingestion of food or water that is contaminated with the faces of an infected individual. In 2005, 126 million cases of acute hepatitis A occurred, according to the World Health Organization (WHO) [Citation4]. HAV infections do not cause chronic liver disease and are often asymptomatic, especially in young children. However, the disease can be severe in adolescents and adults, and can cause debilitating symptoms and acute liver failure, which is associated with high mortality [Citation1,Citation4]. Currently, no treatment for acute hepatitis A is available, except for supportive care or liver transplantation in the rare cases with liver failure [Citation5].
Most children (90%) from areas with high endemicity levels of hepatitis A infections are infected before the age of 10 [Citation1]. In countries with intermediate endemicity, higher susceptibility and disease rates occur in older age groups. In low endemicity areas, disease may occur mainly among adolescents and adults in high-risk groups, such as injecting-drug users, men who have sex with men (MSM) and people traveling to areas of high endemicity [Citation1,Citation4].
In these countries, a relatively large part of the disease burden is attributable to infection acquired due to importation of contaminated food and infected returning travelers. In these settings, catch-up strategies targeted at adolescents and targeted vaccination for hepatitis A of high-risk groups should be considered in low and very low endemic countries to provide individual health benefits according to the WHO [Citation4].
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). HBV spreads through perinatal transmission, horizontal transmission (exposure to infected blood), percutaneous or mucosal exposure to infected blood, saliva, menstrual, vaginal, or seminal fluids, and sexual transmission [Citation2,Citation6]. Most acute infections are asymptomatic. Chronic HBV infections occur in 80–90% of infants infected during the first year of life and in about 30% of children infected before the age of 6 years. In adults, infection leads to chronic hepatitis in <5% of the cases [Citation6]. Cirrhosis and/or liver cancer will develop in 20–30% of chronically infected adults. Worldwide, 240 million people are thought to be chronically infected with hepatitis B and approximately 780,000 persons die each year from hepatitis B infection. Prevalence of hepatitis B is highest in sub-Saharan Africa and East Asia, where 5–10% of the adult population is chronically infected. High rates of chronic infections are also found in the southern parts of eastern and central Europe [Citation2].
Effective and safe vaccines are available for both hepatitis A and B [Citation3,Citation7,Citation8]. There is no recognized correlate of protection for hepatitis A. However, anti-HAV antibody levels ranging from 10 to 33 mIU/ml, depending on the assay, are usually considered a marker of protection against HAV infection [Citation4]. However, clinical experience suggests that protection following vaccination may be present even if there are no detectable HAV antibodies [Citation3,Citation4]. Anti-Hepatitis B surface antigen (HBs) antibody levels of 10 mIU/ml are the threshold for protection against HBV infection [Citation6].
Hepatitis B vaccination is recommended by the WHO for all infants as soon as possible after birth, preferably within 24 h. As of 2014, 184 Member States vaccinate infants against hepatitis B as part of their vaccination schedules and 81% of children received the completed hepatitis B vaccine course [Citation9]. This is a major increase compared with 31 countries in 1992.
Catch-up vaccination for hepatitis B includes people at increased risk for HBV infections, including injecting drug users, health-care workers and travelers who have not completed their hepatitis B vaccination series [Citation6].
There is considerable overlap in groups at risk of HAV and HBV infections, including persons traveling to areas of high or intermediate endemicity, patients with chronic liver disease, patients coinfected with HCV, and persons at increased risk of disease due to sexual contact (especially MSM) [Citation3,Citation10,Citation11]. Since the estimated case fatality rate for hepatitis A was 23-fold higher in patients with chronic liver disease, as compared to patients without chronic liver disease [Citation12], hepatitis A and B vaccination has been recommended for this group [Citation13].
Three presentations of the combined vaccine against hepatitis A and B (TwinrixTM, Twinrix PediatricTM, and AmbirixTM; GSK Vaccines, Belgium) are available [Citation3,Citation14], see . These vaccines are able to confer concurrent protection against the two infections while reducing the number of injections, associated costs, and other logistical issues. This has offered greater convenience to the vaccinee and has facilitated higher compliance to the schedule [Citation15,Citation16].
Table 1. Three presentations of combined vaccine against hepatitis A and B.
The objective of this systematic review was to summarize data on the efficacy, immunogenicity, effectiveness, and safety of TwinrixTM, Twinrix PediatricTM, and AmbirixTM.
Methods
Search strategy
The PubMed and Cochrane databases were searched for literature published between 1 January 1990 and 13 October 2015 (date of search). A search string combining terms on hepatitis A, hepatitis B, and vaccines was built (see Appendix 1).
Inclusion and exclusion criteria
Only primary studies, such as trials and cohort studies, relevant for the research objective were included. Review articles were excluded in this review; however, the reference lists of systematic reviews were checked to identify additional relevant primary studies. No additional relevant articles were retrieved from systematic reviews. Articles comparing TwinrixTM, Twinrix PediatricTM, or AmbirixTM with other hepatitis A or B vaccines were also included; articles that focused only on other vaccines were excluded. Other exclusion criteria were studies with a sample size less than 50, studies on treatment, vaccination coverage, case reports, economic evaluations, biochemistry or molecular studies, and animal studies.
Selection process
Relevant articles were selected by a three-step selection procedure. First, titles and abstracts identified through the search strategy were assessed on relevancy for the objectives. Forty percent of the titles and abstracts were screened in duplicate by two independent researchers. Second, the full text articles, selected in the first selection step, were screened. It was determined whether the paper indeed answered the review question. Only in that case critical appraisal of full text articles was done. A checklist was used to avoid selection of references with poor reporting or critical quality issues. During these two selection steps the above mentioned in- and exclusion criteria were kept in mind. Third, further scrutiny of articles during the data-extraction phase took place. Articles presenting data for the same study population were combined in one data-extraction table.
Definitions
Cut-off levels for HAV- and HBs antibodies were established as a reliable marker of immediate and long-term protection against infection. The lowest limit of anti-HAV antibodies required to prevent HAV infection has not been accurately defined, but levels of 10, 20, or 33 mIU/ml are used in clinical studies [Citation3,Citation4]. Seroconversion for HAV infection was defined as anti-HAV antibody levels above these cut-off levels (depending on assay used) after full vaccination. Seroconversion following full hepatitis B immunization was defined as an anti-HBs antibody concentration ≥1 mIU/ml, an anti-HBs antibody concentration ≥10 mIU/ml after full vaccination was considered protective against HBV infection [Citation3,Citation6]. Throughout this publication, seroprotection against hepatitis B is therefore defined as titer higher than 10 mIU/ml. Recent publications, however, show that individuals with non-zero anti-HBs levels show an anamnestic response to a challenge dose and indicate the persistence of immune memory to HBsAg even when anti-HBs are well below the estimated protective threshold [Citation17].
Results
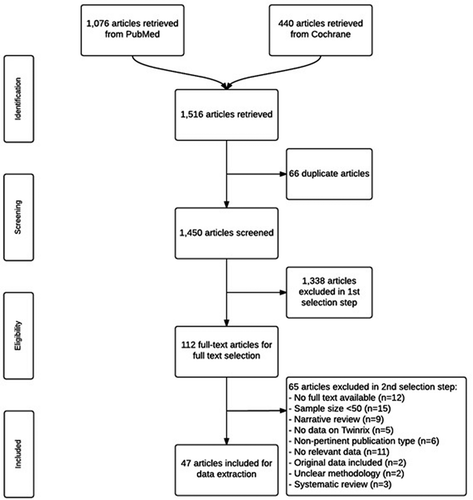
The search resulted in 1450 unique hits, of which 47 were included in this systematic review (). In total, none of the articles described the efficacy of TwinrixTM, Twinrix PediatricTM, or AmbirixTM, 42 reported data on immunogenicity, one on effectiveness, and 38 on safety. Two articles reported data retrieved from the Vaccine Adverse Event Reporting System (VAERS) database. The majority of the articles presented data for more than one outcome. Study characteristics of all included articles are presented in Appendix 2.
Immunogenicity
TwinrixTM
Twenty-four articles provided data on the immunogenicity of TwinrixTM (–).
Table 2. Immunogenicity of TwinrixTM in adults.
Table 3. Immunogenicity of TwinrixTM in children.
Table 4. Immunogenicity of TwinrixTM following an alternative schedule.
Anti-HAV seropositivity 1 month after full vaccination
After full vaccination, the rate of seropositivity for anti-HAV ranged from 96.2% to 100%, regardless of the schedule used or whether the study was done in adults or children (–) [Citation11,Citation12,Citation14,Citation18–Citation20,Citation22–Citation25,Citation27–Citation30,Citation32,Citation34–Citation37].
Two articles reported lower seropositivity rates at month 7. In hepatitis C virus (HCV)-positive adults, the anti-HAV seropositivity was 88% after full vaccination [Citation12]. The other study reported a seropositivity rate of anti-HAV of 71.2% following vaccination in a general population in which the frequency of seropositivity significantly decreased with increasing age; in subjects younger than 40 years, the seropositivity rate was 92%, which decreased to 63% in persons over 60 years [Citation27,Citation33].
The anti-HAV antibody geometric mean concentration (GMC) increased after the first two doses and decreased in all studies before the third dose. After the third dose, the GMC ranged from 2747 to 8895 mIU/ml [Citation11,Citation12,Citation14,Citation19,Citation20,Citation22–Citation25,Citation27–Citation30,Citation34,Citation35], which is well above the seropositivity cut-off levels, inferring seroprotection. However, GMC decreased with increasing age and body mass index (BMI) [Citation23,Citation27,Citation33] was lower in HCV-positive adults [Citation12], and it was lower in smokers compared to nonsmokers [Citation24,Citation27]. One study did not find any factors significantly influencing the anti-HAV seropositivity rates [Citation32]. Since almost all individuals become anti-HAV seropositive after vaccination, the number of nonresponders is very low, making it difficult finding significant risk factors for seropositivity. However, risk factors for GMC can be found due to the large range of GMC after vaccination.
Long term anti-HAV seropositivity
Long-term data showed that 97.3–100% of the adults were seropositive for anti-HAV up to year 4 or 15. The GMC showed a 10-fold decrease (to 373.9 and 674.6 mIU/ml) at year 10, but remained above the seropositivity cut-off values [Citation30].
Anti-HBs seropositivity/seroprotection 1 month after full vaccination
After full vaccination, the rate of seropositivity (i.e. ≥1 mIU/ml) for anti-HBs ranged from 98.4% to 100% [Citation11,Citation19,Citation21,Citation25,Citation27]. In general, the seroprotection rate for anti-HBs ranged from 82% to 100% [Citation11,Citation12,Citation18–Citation28,Citation30,Citation32,Citation34–Citation37]. Three articles reported, however, lower seroprotection rates at month 7. The seroprotection 30–60 days after full vaccination in residents of nursing facilities was only 34% [Citation31]. In another population of relatively old participants (mean age 54 years), the seroprotection rate was 37.5% [Citation33]. Seroprotection for anti-HBs was 75.4% in human immunodeficiency virus (HIV)-infected youth at month 7 [Citation16]. Anti-HBs antibody GMC ranged from 749 to 9454 mIU/ml after the third dose [Citation11,Citation12,Citation14,Citation19–Citation30,Citation34,Citation35].
Risk factors anti-HBs seroprotection
Seroprotection and GMC for anti-HBs decreased with increasing age [Citation23,Citation26,Citation27,Citation32,Citation33,Citation36] or BMI [Citation27,Citation32]. One article found that smokers had significantly lower GMC for anti-HBs antibodies compared to nonsmokers [Citation24], but, two other articles did not find such an effect [Citation27,Citation32]. In residents of nursing homes, no statistically significant differences in anti-HBs response by demographic or clinic factors (i.e. age, gender, diabetes, BMI, or smoking) were found. However, the study was limited by a sample size of 86 participants, which likely decreased the ability to detect statistically significant differences [Citation31]. One article reported on the immunogenicity of TwinrixTM in HIV-infected youth. Predictors of vaccine response in univariate analyses were female gender, higher nadir cluster of differentiation 4 (CD4), higher baseline CD4 count or percentage, lower baseline viral load, enrollment at a US site, and perinatally infected participants (compared to those infected by high risk behavior) [Citation16]. The effect of underlying chronic diseases on anti-HBs seroprotection rates was inconclusive [Citation32,Citation33]. Interestingly, GMC following full vaccination was significantly lower if the third dose was performed in the summer or autumn, compared to the winter of spring. Seroprotection rates were not influenced by seasonality [Citation26].
Long-term persistence of circulating anti-HBs antibodies
One article on follow-up up to 10 years showed approximately a 20-fold decrease in GMC from 2637.3 or 6601 mIU/ml (depending on study center) at month 7 to 103.8 and 320.4 mIU/ml at year 10, respectively [Citation30]. However, these levels are still well above the seroprotection cut-off values. After 15 years, this study population showed a decrease from 100% seroprotection at month 7 to 89.3% or 92.9% (depending on study center) [Citation14]. One article reported a larger decrease from 92.8% at month 7 to 76.9% at year 4 for seropositivity and from 91.7% to 57.1% at year 4 for seroprotection. The study population was relatively old (>40 years old), which may explain the low seropositivity and seroprotection rates [Citation29]. Subgroup analysis showed lower seropositivity rates at year 4 in subjects aged ≥61 years old, with a BMI ≥30 kg/m2, receiving concomitant medication or with a current concomitant medical condition [Citation29].
Twinrix pediatricTM
Ten articles reported the immunogenicity of Twinrix pediatricTM in children and/or adolescents ( and ).
Table 5. Immunogenicity of Twinrix PediatricTM in children and adolescents.
Table 6. Immunogenicity of Twinrix PediatricTM in children following an alternative schedule.
Seropositivity/seroprotection after full vaccination
From month 7 to year 10 after the first vaccine dose, all subjects (100%) remained seropositive for anti-HAV antibodies [Citation10,Citation15,Citation38-Citation45]. After the third dose (month 7), the anti-HAV antibody GMC ranged from 4174 to 9257 mIU/ml. After 10 years, GMC decreased to 335.5 and 680 mIU/ml [Citation15,Citation43]. However, these levels are still well above the seropositivity cut-off values, inferring seroprotection.
At month 7, all subjects (100%) were seroprotected for anti-HBs antibodies [Citation38-Citation44]. Follow-up data were available up to 10 years after the primary vaccination; the percentage of seroprotected subjects decreased slightly, but remained above 85% [Citation10,Citation15,Citation43]. After the third dose (month 7), the anti-HBs antibody GMC ranged from 4865 to 13683 mIU/ml. GMC decreased over time after vaccination. After 10 years, GMC was reported to be 60.1 and 162 mIU/ml, which is above the cut-off value for seroprotection [Citation15,Citation43].
Risk factors
Anti-HAV and anti-HBs antibody GMCs tended to be higher in children vaccinated when they were 6–15 years old compared to children vaccinated when they were 1–6 years old [Citation42,Citation43]. However, the sample size was too small to make any definite conclusion [Citation43].
Alternative schedule
One study reported immunogenicity after 2 doses of Twinrix pediatricTM at 0 and 6 months and a challenge dose after 7 years (). After the second dose, the seropositivity rates were 100% and 97.1% for anti-HAV and anti-HBs, respectively [Citation44]. Seven years after primary vaccination seropositivity rates slightly decreased to 97.8% and 92%, respectively. The anti-HBs seroprotection rate decreased from 96.5% after the second dose to 75.1% after 7 years. After the challenge dose, 100% of the children seroconverted for anti-HAV and anti-HBs [Citation44,Citation45].
AmbirixTM
Thirteen articles reported the immunogenicity of AmbirixTM in children and/or adolescents ( and ).
Table 7. Immunogenicity of AmbirixTM in children and adolescents.
Table 8. Immunogenicity of AmbirixTM in adolescents following an alternative schedule.
Seropositivity/seroprotection after full vaccination
After full vaccination following the regular immunization schedule, all children were seropositive for anti-HAV [Citation38,Citation41,Citation48,Citation50–Citation54]. Five to 10 years after primary vaccination, all children (100%) remained seropositive for anti-HAV [Citation10,Citation15,Citation47,Citation53]. After the second dose (month 7), GMC was 3701–14790.1 mIU/ml [Citation10,Citation15,Citation38,Citation41,Citation47,Citation48,Citation50–Citation54]. Long-term follow-up data showed that GMC decreased over the years, but remained above the seropositivity cut-off up to 10 years follow-up [Citation10,Citation15,Citation47,Citation53].
In one study, anti-HBs seropositivity was measured and was 93.9% after full vaccination [Citation50], and seroprotection rates ranged from 91.8% to 100% [Citation38,Citation41,Citation48–Citation54]. Seroprotection rates decreased to at least 81.7% 10 years after primary vaccination [Citation10,Citation15,Citation47,Citation53]. GMC ranged from 1524 to 32540 mIU/ml 7 months after primary vaccination [Citation38,Citation41,Citation48,Citation50–Citation54]. After 10 years GMC decreased to 50.6–80.7 mIU/ml [Citation15,Citation53].
Risk factors
One article reported the immunogenicity of AmbirixTM in children with HIV or children receiving immunosuppressive medication (IM) for rheumatic diseases [Citation46]. Seroprotection rates after full vaccination were 99% and 100% for anti-HAV and 97% and 93% for anti-HBs in children with HIV or receiving IM, respectively. The GMCs found after full vaccination in HIV-infected children and children on IM were 327 and 288 mIU/ml for anti-HAV antibodies and 483 and 321 mIU/ml for anti-HBs antibodies, respectively. In children infected with HIV, no statistical differences in anti-HAV antibody GMC were found regarding CD4 count, viral load, or use of combination antiretroviral therapy (cART). For HBV, a significantly higher anti-HBs antibody GMC was found in children with lower viral load and children on cART [Citation46].
Alternative schedule
One study described the immunogenicity following two doses of AmbirixTM at 0 and 12 months (). Seropositivity/protection rates at month 13 were 99.0% and 97.0% for anti-HAV and anti-HBs, respectively. Six years after primary vaccination the seropositivity/protection rates were 100% and 92.9%, respectively. The study demonstrated long-term persistence of immunity to hepatitis A and B, following two doses of AmbirixTM, when given either at 0 and 6 months or 0 and 12 months [Citation47].
TwinrixTM vs. monovalent hepatitis A and B vaccines
Seventeen articles described a direct comparison of the immunogenicity of TwinrixTM and monovalent hepatitis A and/or B vaccines (–). The anti-HAV seropositivity rates were equal for both vaccines in all studies. Anti-HAV antibody GMC was equal or higher following full vaccination with TwinrixTM or AmbirixTM compared to full vaccination with HavrixTM or unspecified hepatitis A vaccines [Citation35,Citation50,Citation51]. TwinrixTM was equal or higher than other monovalent hepatitis B vaccines with regard to the anti-HBs response (–).
Table 9. Comparison between TwinrixTM and concomitant administration of monovalent hepatitis A and B vaccines.
Table 10. Comparison between Twinrix pediatricTM and concomitant administration of monovalent hepatitis A and B vaccines.
Table 11. Comparison between AmbirixTM and concomitant administration of monovalent hepatitis A and B vaccines.
Twinrix pediatricTM or AmbirixTM co-administered with other vaccines
Co-administration of Twinrix pediatricTM or AmbirixTM with other vaccines (i.e. quadrivalent conjugate meningococcal vaccine [MenACWY-TT], human papilloma virus [HPV], diphtheria, tetanus, acellular pertussis – inactivated poliovirus/Haemophilus influenzae type b vaccine [DTPa-IPV/Hib] or measles, mumps, rubella vaccine [MMR]) was immunologically noninferior to administration of the vaccine alone [Citation39,Citation40,Citation52].
Effectiveness
One article reported data on the effectiveness of TwinrixTM. The incidence rate of hepatitis A in Catalonia decreased from 5.44 per 100,000 person-years before universal adolescent vaccination (1992–1998) to 3.02 per 100,000 person-years after universal adolescent vaccination (2001–2006) (p < 0.001). The decrease in incidence rates of hepatitis A after introduction of universal adolescent vaccination was statistically significant in male (6.85 vs. 3.89 per 100,000 person-years, p < 0.001) and female participants (4.10 vs. 2.18 per 100,000 person-years, p < 0.001) [Citation55].
Safety
TwinrixTM
The most reported local solicited reaction was soreness/pain at injection site (10.5–65.6%; ). The most reported systemic solicited reaction differed between articles (i.e. fatigue, headache, and malaise). Subjects receiving TwinrixTM alone at a travel clinic had significantly lower relative risk for experiencing any general symptoms compared to subjects receiving one or more vaccines concomitantly (p > 0.05) [Citation56]. In HCV-infected patients, the most reported local reaction was soreness at injection site and the most reported systemic reaction was headache [Citation12].
Table 12. Solicited and unsolicited adverse events following immunization with TwinrixTM, Twinrix pediatricTM, or AmbirixTM.
The percentage of individuals experiencing unsolicited adverse events (AEs) ranged between 0% and 20.8% [Citation19,Citation22,Citation35,Citation37,Citation56]. Reported unsolicited events included tonsillitis, pharyngitis, cold, influenza, otitis, diarrhea, dizziness, upper respiratory tract infection, and local reactions or bruising at the injection site.
The percentage of individuals experiencing serious adverse events (SAEs) ranged between 0% and 9.5% [Citation11,Citation12,Citation14,Citation16,Citation18,Citation21,Citation23,Citation27,Citation28,Citation30,Citation32,Citation34,Citation35,Citation37]. Only one article reported a SAE related to the vaccine: a participant developed simultaneous headache, numbness of the face, and vomiting, each of moderate intensity [Citation37].
Between May 2001 and September 2003, VAERS received 305 reports of possible AEs after vaccination with TwinrixTM. Of these reports, 67.9% listed TwinrixTM as the sole vaccine. After vaccination with only TwinrixTM, the reported AEs were related to neurologic conditions (34.7%), liver and gastrointestinal tract (18.2%), integument (9.1%), flu-like syndrome (7.4%), musculoskeletal disorders (7.4%), allergy or anaphylaxis (6.6%), infections (4.1%), or other (12.4%) [Citation57]. Pregnant women filed 35 reports following TwinrixTM vaccination between 1996 and 2013, 40% of the reports did not describe an AE, 16 were maternal events, and 5 were infant/neonatal events. Reported AE included pregnancy specific AE (spontaneous abortion, elective termination, premature delivery, oligohydramnios, abruption placentae, uterine, and abdominal cramps), nonpregnancy specific AE (pelvic pain, sinusitis, nausea and dizziness, paleness, and injection site pain), and infant AE (jaundice, fetal distress, and growth under lower lip). Two serious reports after TwinrixTM in pregnant women included oligohydramnios and neonatal jaundice [Citation58].
Twinrix pediatricTM
The most reported local solicited reaction was soreness/pain at injection site (). The most reported systemic reactions were fatigue and headache. Two articles reported the number of unsolicited AEs; in one study, 14.8% of the adolescents experienced an unsolicited AE [Citation39]. In the other study, 17.4% of the children reported at least one unsolicited AE related to vaccination [Citation41]. The nature of these AEs was not reported.
SAEs were reported in 0.4% and 0.8% of the participants following immunization with Twinrix pediatricTM [Citation39,Citation41]. Only one SAE was considered to be associated with vaccination: vomiting, fever with symptoms of rhinorrhea, and cough occurring four days after receiving the first vaccine dose. The subject was hospitalized and diagnosed with viral upper respiratory tract infection and exacerbation of eczema [Citation41].
AmbirixTM
The most reported local solicited reactions were soreness/pain at injection site () and redness. The most reported solicited systemic reactions were fever, fatigue, and headache (). The percentage of participants experiencing unsolicited AEs ranged between 0% and 30.2% [Citation41,Citation48,Citation50,Citation54]. The kind of unsolicited event was not reported.
The percentage of SAEs ranged from 0% to 1.7% [Citation38,Citation41,Citation48,Citation50,Citation54]. When AmbirixTM was co-administered with DTPa-IPV/Hib vaccine, 5% of the participants experienced a SAE, and 4.6% of the participants after had a SAE following co-administration with MMR vaccine [Citation52]. One SAE was reported unlikely to be associated with vaccination (acute diabetes mellitus) [Citation48] and one was reported to be associated with vaccination (febrile convulsions) [Citation52]. All other SAEs were not associated with vaccination.
Combined vs. separate hepatitis A and B vaccines
The safety profiles of the combined vaccines and unspecified concomitant hepatitis A and B vaccines, Engerix-BTM, simultaneous Engerix-BTM and HavrixTM, simultaneous Vaqta and Gen H-B-Vax and Recombivax-HB were similar [Citation16,Citation18,Citation23,Citation27,Citation35,Citation37,Citation49–Citation51]. However, the group receiving the mixed administration of HavrixTM and Engerix-BTM had a total local symptom score twice as high as the other two groups, probably due to the twofold volume of the vaccine and the twofold amount of aluminum hydroxide in the vaccine [Citation18]. Furthermore, while the percentage of recorded pain reactions, indurations, or swellings was similar after Engerix-BTM and TwinrixTM, it was lower after HavrixTM [Citation20]. The percentage of reported AEs was higher following TwinrixTM than in the group receiving concurrent Vaqta and Recombivax-HB/H-B-VaxII [Citation36].
Twinrix pediatricTM or AmbirixTM co-administered with other vaccines
Co-administration of Twinrix pediatricTM or AmbirixTM with other vaccines (i.e. MenACWY-TT, HPV, DTPa-IPV/Hib, or MMR) did not significantly alter the safety profile [Citation39,Citation40,Citation52]. However, the incidence of grade 3 pain was slightly higher when Twinrix pediatricTM was co-administered with HPV vaccine than when administered alone [Citation40]. Both local and general symptoms were more frequently reported after co-administered hepatitis A/B and MMR vaccination (48% and 46%, respectively) compared to co-administered hepatitis A/B and DTPa-IPV/Hib vaccination (18% and 32%, respectively) [Citation52].
Discussion
Summary of results
After full vaccination with TwinrixTM (regular and accelerated schedule), the rate of seropositivity for anti-HAV ranged from 96.2% to 100%. The rate of seroprotection for anti-HBs after full vaccination with TwinrixTM ranged from 82% to 100%. Increasing age was associated with decreasing immunogenicity response. Immunogenicity results were equal or higher for both anti-HAV and anti-HBs following TwinrixTM vaccination compared to monovalent hepatitis A or B vaccination. Co-administration of Twinrix pediatricTM or AmbirixTM with other routine childhood vaccines was immunologically noninferior to administration of the combined hepatitis A and B vaccine alone and did not significantly alter the safety profile. The safety profiles of the combined vaccines and monovalent hepatitis A and B vaccines were similar.
Risk groups for hepatitis A and/or B infection
Patients with chronic hepatitis C are at increased risk of complication once exposed to hepatitis A or B. Therefore, vaccination against these vaccine preventable diseases has been recommended for patients with chronic liver disease caused by hepatitis [Citation12]. The immunogenicity of both monovalent and combined hepatitis A and B vaccines was lower in patients with chronic hepatitis C infection [Citation12]. Unfortunately, the adherence to hepatitis vaccination guidelines for patients with chronic liver disease is low and there are significant variations among providers [Citation59].
Vaccination for both HAV and HBV is also recommended in HIV-infected subjects [Citation16]. Protection for both infections can be achieved by vaccination with TwinrixTM, which potentially improves the patient acceptance and reduces the costs [Citation16]. The immune response in children with HIV after full vaccination is satisfactory and comparable to the response in healthy children. However, a substantial proportion of these children was not protected after the first dose, which should be taken into account in travel health when full vaccination may not be possible due to time restrictions [Citation46].
Although there is a lot of attention on immunization of high risk groups, only three studies describing the immunogenicity of TwinrixTM, Twinrix pediatricTM, or AmbirixTM in high risk populations (i.e. HCV- or HIV-infected patients or patients on IM for rheumatic diseases) were found [Citation12,Citation16,Citation46]. In children infected with HIV or on IM, the immunogenicity of AmbirixTM was similar to healthy children. The GMC was lower but remained above the cut-off levels [Citation46]. In HIV-infected youth, the anti-HBs seroprotection rate was lower than in healthy participants (75.4%) [Citation16]. In HCV-infected adults, the anti-HAV seroprotection was slightly lower than in the general population (88%) [Citation12].
Another high risk group for hepatitis A and B infection is travelers. Hepatitis A vaccination is recommended for all travelers to areas of intermediate or high prevalence. Hepatitis B vaccination is recommended for travelers likely to be at risk for hepatitis B infection, traveling to areas of intermediate or high HBV prevalence. Elderly travelers are at particular risk for morbidity and mortality related to HAV and HBV, which underscores the need for vaccination of this group. For travelers to countries with intermediate or high prevalence of both HAV and HBV, TwinrixTM could be used to protect the traveler for both infections. An accelerated schedule, vaccination given at day 0, 7, and 21, and a booster at 12 months, could be considered for travelers [Citation60].
Strengths and limitations
The main strengths of the methodology of this review are the systematic approach and the long search period used (1990–2015). To our knowledge there is no up-to-date systematic review available covering the immunogenicity, effectiveness, and safety of TwinrixTM, Twinrix PediatricTM, and AmbirixTM. However, only articles with a direct comparison between TwinrixTM and separate hepatitis A or B vaccines were included, which could have limited the comparison of the immunogenicity and safety of the separate vaccines.
A few publications with poor description of the methodology were excluded (see for more details). However, a number of the included publications lack some details describing, for example, the exact setting, study period, randomization process or whether or not intention-to-treat analyses or according to protocol analyses were done. Furthermore, there is a broad variety of study designs used in the included publications, each with strengths and limitations (see Table A1 for more details). Comparison of the studies with respect to their quality can be challenging, but all studies show consistent immunogenicity and safety data.
Another limitation with regard to the included studies was related to the heterogeneity of the assays used. In some studies, the assay used to determine serum concentrations changed during the study period. However, the equivalence of the used assays was validated and the change of kits had no significant effect on the outcomes.
Limited to no data were found on the effectiveness and efficacy of TwinrixTM, Twinrix pediatricTM, or AmbirixTM. The limited information found for the effectiveness can at least partially be explained by the fact that TwinrixTM is not included in any universal mass vaccination programs, as is it the case for Hepatitis B and to a lesser extend Hepatitis A monovalent vaccines, which makes it difficult to perform adequate effectiveness studies in a general population. Some articles reporting on the incidence of hepatitis A or B before and after implementation of vaccination were found; however, the vaccine used was not specified. The lack of efficacy data can be explained because immunologic correlates of efficacy were well established (or at least accepted) for each component of the combination vaccine. Therefore, data from immunogenicity studies and immune noninferiority studies versus the monovalent licensed vaccines could provide a basis for license approval without the need for additional evidence studies [Citation61]. Furthermore, there were only a few articles on the immunogenicity of safety of the vaccines in risk populations.
Conclusion
TwinrixTM is the only licensed combined hepatitis A and B vaccine largely available, outside of China, where another combined vaccine (Bilive) is available. A combined vaccine provides benefits over separate vaccine such as fewer injections, fewer office visits, lower costs, more convenience for vaccinees, simplify logistics and administration, and potentially increase compliance [Citation23,Citation27,Citation35–Citation37,Citation43,Citation50,Citation51]. This review shows that TwinrixTM, Twinrix pediatricTM, and AmbirixTM (conventional and accelerated schedules) offer satisfactory immunogenicity, sustained up to 15 years after vaccination, and show an expected duration of protection and safety profile similar to the monovalent vaccines.
Expert commentary
This review covers clinical trials and observational studies that have been published between 1990 and 2015 covering the immunogenicity, effectiveness, and safety of the combined Hepatitis A&B vaccines TwinrixTM, Twinrix PediatricTM, and AmbirixTM. To our knowledge, this is the first review with a systematic approach and the first summarizing more than two decades experience with the combined vaccine. Unlike monovalent Hepatitis A and Hepatitis B vaccines, A&B combination vaccines are usually not universally recommended at national level and not used in national immunization programs. Monovalent Hepatitis A vaccines are recommended and used in national childhood vaccination programs in countries with intermediate endemicity as recommended by WHO. Hepatitis B childhood vaccination is universally recommended and implemented in at least 183 WHO Member States (the Hepatitis B antigen being often used in combination with other childhood vaccines). On the other hand, the A&B combination vaccine is mostly recommended and used in individuals at risk of exposure, providing the opportunity to protect against Hepatitis A and B individuals that would suffer from higher morbidity and mortality, if infected. The impact of vaccination and long-term safety, immunogenicity, and effectiveness with this vaccine cannot be not measured in the context of national programs as it is the case for Hepatitis A [Citation62]or B [Citation63]. It is therefore key to continue monitoring how Hepatitis A&B combination vaccination performs from individual studies and to do so with and using a systematic and unbiased methodology seems to be the most appropriate approach. A combined vaccine provides benefits over separate vaccine such as fewer injections, fewer office visits, lower costs, more convenience for vaccinees, simplify logistics and administration, and potentially increase compliance. This review shows that TwinrixTM, Twinrix pediatricTM, and AmbirixTM (conventional and accelerated schedules) offer satisfactory immunogenicity, sustained up to 15 years after vaccination, and show a safety profile similar to the monovalent vaccines.
Five-year view
Systematic and unbiased reviews of published data are key to support regulatory authorities in their assessment of the benefit/risk of licensed vaccines over time. Additional long-term immunogenicity and safety data with TwinrixTM, Twinrix pediatricTM, and AmbirixTM will become available and should be systematically gathered and reviewed in order to illustrate the benefit/risk and also inform on the need to provide a booster vaccination for Hepatitis A, Hepatitis B, or both more specifically for individuals at increased risk of exposure to these viral hepatitis.
Key issues
TwinrixTM, Twinrix pediatricTM, and AmbirixTM offer satisfactory immunogenicity, sustained up to minimum 15 years after vaccination, and show a safety profile similar to the monovalent vaccines.
Immunogenicity results were equal or higher for both anti-HAV and anti-HBs following TwinrixTM vaccination compared to monovalent hepatitis A or B vaccination.
Co-administration of Twinrix pediatricTM or AmbirixTM with other routine childhood vaccines was immunologically noninferior to administration of the combined hepatitis A and B vaccine alone and did not significantly alter the safety profile
Combined vaccines provide benefits such as fewer injections, fewer office visits, lower costs, more convenience for vaccinees, simplify logistics and administration, and potentially increase compliance compared to separate vaccination.
Limited data were found describing the immunogenicity of the combined vaccines in high risk populations, including patients with chronic liver disease or HIV-infected patients. Overall, the immunogenicity was lower in high risk patients compared to healthy subjects.
Trademarks
TwinrixTM, Twinrix pediatricTM, AmbirixTM, HavrixTM and Engerix-BTM are trademarks of the GSK group of companies.
Vaqta, Recombivax-HB and Gen H-B-Vax are trademarks of Merck & Co.
HB VAX PRO is trademark of Sanofi-Pasteur MSD.
Bilive is a trademark of SINOVAC Biotech Ltd.
Financial and competing interests disclosure
C Marano, M De Ridder and L De Moerlooze are employees of GSK group of companies and hold stock options/restricted shares from the sponsoring company. M Bakker and E Bunge report grants from GSK group of companies during the conduct of the study and outside the submitted work. This systematic review was sponsored and funded by GlaxoSmithKline Biologicals S.A., Belgium. GlaxoSmithKline Biologicals S.A. was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
The authors would like to thank Angeles Ceregido (from XPE Pharma & Science on behalf of GSK Vaccines) for editing and coordinating the publication development.
References
- World Health Organization. Hepatitis A - Fact sheet #328 [Internet]. World Health Organization. Updated July 2015. [cited 2016 Jan 14]. Available from: http://www.who.int/mediacentre/factsheets/fs328/en/
- World Health Organization. Hepatitis B - Fact sheet #204 [Internet]. World Health Organization. Updated July 2015. [cited 2016 Jan 14]. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
- Beran J. Bivalent inactivated hepatitis A and recombinant hepatitis B vaccine. Expert Rev Vaccines. 2007;6(6):891–902.
- World Health Organization. WHO position paper on hepatitis A vaccines - June 2012. Weekly Epidemiological Record. 2012;28-29(87):261–276.
- Burroughs AK, Westaby D. Chapter 7 - Liver, biliary tract and pancreatic disease. In: Kumar P, Clark M, editors. Clinical medicine. 6th ed. London: Elsevier Saunders; 2005. p. 362–364.
- World Health Organization. WHO position paper - hepatitis B vaccines. Weekly Epidemiological Record. 2009;40(84):405–420.
- Hendrickx G, Vorsters A, Van Damme P. Advances in hepatitis immunization (A, B, E): public health policy and novel vaccine delivery. Curr Opin Infect Dis. 2012;25(5):578–583.
- Ott JJ, Irving G, Wiersma ST. Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine. 2012;31(1):3–11.
- World Health Organization. Immunization coverage - Fact sheet #378 [Internet]. World Health Organization. Updated September 2015. [cited 2016 Jan 14]. Available from: http://www.who.int/mediacentre/factsheets/fs378/en/
- Marshall H, Nolan T, Diez Domingo J, et al Long-term (5-year) antibody persistence following two- and three-dose regimens of a combined hepatitis A and B vaccine in children aged 1-11 years. Vaccine. 2010;28(27):4411–4415.
- Thoelen S, Van Damme P, Leentvaar-Kuypers A, et al. The first combined vaccine against hepatitis A and B: an overview. Vaccine. 1999;17(13–14):1657–1662.
- Kallinowski B, Jilg W, Buchholz L, et al. Immunogenicity of an accelerated vaccination regime with a combined hepatitis a/b vaccine in patients with chronic hepatitis C. Z Gastroenterol. 2003;41(10):983–990.
- Henkle E, Lu M, Rupp LB, et al. Hepatitis A and B immunity and vaccination in chronic hepatitis B and C patients in a large United States cohort. Clin Infect Dis. 2015;60(4):514–522.
- Van Damme P, Leroux-Roels G, Crasta P, et al. Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined hepatitis A and B vaccine. J Med Virol. 2012;84(1):11–17.
- Beran J, Kervyn D, Wertzova V, et al. Comparison of long-term (10 years) immunogenicity of two- and three-dose regimens of a combined hepatitis A and B vaccine in adolescents. Vaccine. 2010;28(37):5993–5997.
- Flynn PM, Cunningham CK, Rudy B, et al. Hepatitis B vaccination in HIV-infected youth: a randomized trial of three regimens. J Acquir Immune Defic Syndr. 2011;56(4):325–332.
- Spradling PR, Kamili S, Xing J, et al. Response to challenge dose among young adults vaccinated for hepatitis B as infants: importance of detectable residual antibody to hepatitis B surface antigen. Infect Control Hosp Epidemiol. 2015;36(5):529–533.
- Ambrosch F, Wiedermann G, André FE, et al. Clinical and immunological investigation of a new combined hepatitis A and hepatitis B vaccine. J Med Virol. 1994;44(4):452–456.
- Bruguera M, Bayas JM, Vilella A, et al. Immunogenicity and reactogenicity of a combined hepatitis A and B vaccine in young adults. Vaccine. 1996;14(15):1407–1411.
- Czeschinski PA, Binding N, Witting U. Hepatitis A and hepatitis B vaccinations: immunogenicity of combined vaccine and of simultaneously or separately applied single vaccines. Vaccine. 2000;18(11–12):1074–1080.
- De Schryver A, Verstrepen K, Vandersmissen L, et al Comparative immunogenicity of two vaccination schedules of a combined hepatitis A and B vaccine in healthy volunteers. J Viral Hepat. 2011;18(4):e5–10.
- Höhler T, Groeger-Bicanic G, Hoet B, et al. Antibody persistence and immune memory elicited by combined hepatitis A and B vaccination in older adults. Vaccine. 2007;25(8):1503–1508.
- Joines RW, Blatter M, Abraham B, et al. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine. 2001;19(32): 4710–4719.
- Kallinowski B, Bock HL, Clemens R, et al. Immunogenicity and reactogenicity of a combined hepatitis A/B candidate vaccine: first results. Liver. 1996;16(4):271–273.
- Knöll A, Hottenträger B, Kainz J, et al. Immunogenicity of a combined hepatitis A and B vaccine in healthy young adults. Vaccine. 2000;18(19):2029–2032.
- Rendi-Wagner P, Kundi M, Stemberger H, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19(15–16):2055–2060.
- Rieger MA, Hofmann F, Michaelis M. Simultaneous vaccination against hepatitis A and B: results of an open, randomized study from the occupational health point of view. Int J Occup Med Environ Health. 2004;17(3):379–391.
- Van Der Wielen M, Van Damme P, Chlibek R, et al. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24(26):5509–5515.
- Chlibek R, Von Sonnenburg F, Van Damme P, et al. Antibody persistence and immune memory 4 years post-vaccination with combined hepatitis A and B vaccine in adults aged over 40 years. J Travel Med. 2011;18(2):145–148.
- Van Herck K, Leroux-Roels G, Van Damme P, et al. Ten-year antibody persistence induced by hepatitis A and B vaccine (Twinrix) in adults. Travel Med Infect Dis. 2007;5(3):171–175.
- Williams RE, Sena AC, Moorman AC, et al. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine. 2012;30(21):3147–3150.
- Wolters B, Muller T, Ross RS, et al. Comparative evaluation of the immunogenicity of combined hepatitis A and B vaccine by a prospective and retrospective trial. Hum Vaccin. 2009;5(4):248–253.
- Wolters B, Junge U, Dziuba S, et al. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21(25–26):3623–3628.
- Thompson SC, Norris M. Immunogenicity and reactogenicity of a combined hepatitis A-hepatitis B vaccine in adolescents. Int J Infect Dis. 1998;2(4):193–196.
- Connor BA, Blatter MM, Beran J, et al. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14(1):9–15.
- Frey S, Dagan R, Ashur Y, et al. Interference of antibody production to hepatitis B surface antigen in a combination hepatitis A/hepatitis B vaccine. J Infect Dis. 1999;180(6):2018–2022.
- Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20(7–8):1157–1162.
- Levie K, Beran J, Collard F, et al. Long term (24 months) follow-up of a hepatitis A and B vaccine, comparing a two and three dose schedule in adolescents aged 12-15 years. Vaccine. 2002;20(19–20):2579–2584.
- Ostergaard L, Silfverdal SA, Berglund J, et al. A tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when co-administered with Twinrix((R)) in subjects aged 11-17 years: an open, randomised, controlled trial. Vaccine. 2012;30(4):774–783.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health. 2012;50(1):38–46.
- Roberton D, Marshall H, Nolan TM, et al. Reactogenicity and immunogenicity profile of a two-dose combined hepatitis A and B vaccine in 1-11-year-old children. Vaccine. 2005;23(43):5099–5105.
- Van Damme P, Leroux-Roels G, Law B, et al. Long-term persistence of antibodies induced by vaccination and safety follow-up, with the first combined vaccine against hepatitis A and B in children and adults. J Med Virol. 2001;65(1):6–13.
- Diaz-Mitoma F, Law B, Subramanya A, et al. Long-term antibody persistence induced by a combined hepatitis A and B vaccine in children and adolescents. Vaccine. 2008;26(14):1759–1763.
- Duval B, Gilca V, Boulianne N, et al. Immunogenicity of two paediatric doses of monovalent hepatitis B or combined hepatitis A and B vaccine in 8-10-year-old children. Vaccine. 2005;23(31):4082–4087.
- Gilca V, Dionne M, Boulianne N, et al. Long-term immunogenicity of two pediatric doses of combined hepatitis A and B or monovalent hepatitis B vaccine in 8 to 10-year-old children and the effect of a challenge dose given seven years later. Pediatr Infect Dis J. 2009;28(10):916–918.
- Belderok SM, Sonder GJ, Van Rossum M, et al. Evaluation of immune responses to combined hepatitis A and B vaccine in HIV-infected children and children on immunosuppressive medication. Vaccine. 2013;31(38):4156–4163.
- Burgess MA, McIntyre PB, Hellard M, et al. Antibody persistence six years after two doses of combined hepatitis A and B vaccine. Vaccine. 2010;28(10):2222–2226.
- Burgess MA, Rodger AJ, Waite SA, et al. Comparative immunogenicity and safety of two dosing schedules of a combined hepatitis A and B vaccine in healthy adolescent volunteers: an open, randomised study. Vaccine. 2001;19(32):4835–4841.
- Cunningham CK, Rudy BJ, Xu J, et al. Randomized trial to determine safety and immunogenicity of two strategies for hepatitis B vaccination in healthy urban adolescents in the United States. Pediatr Infect Dis J. 2010;29(6):530–534.
- Lee SD, Chan CY, Yu MI, et al. A two dose combined hepatitis A and B vaccine in Chinese youngsters. J Med Virol. 1999;59(1):1–4.
- Ramonet M, Da Silveira TR, Lisker-Melman M, et al. A two-dose combined vaccine against hepatitis A and hepatitis B in healthy children and adolescents compared to the corresponding monovalent vaccines. Arch Med Res. 2002;33(1):67–73.
- Usonis V, Meriste S, Bakasenas V, et al. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12-18 months. Vaccine. 2005;23(20):2602–2606.
- Van Damme P, Kafeja F, Van Der Wielen M, et al. Long-term immunogenicity and immune memory after two doses of the adult formulation of a combined hepatitis A and B vaccine in children 1 to 11 years of age. Pediatr Infect Dis J. 2011;30(8):703–705.
- Van Der Wielen M, Van Damme P, Collard F. A two dose schedule for combined hepatitis A and hepatitis B vaccination in children ages one to eleven years. Pediatr Infect Dis J. 2000;19(9):848–853.
- Domínguez A, Oviedo M, Carmona G, et al. Epidemiology of hepatitis A before and after the introduction of a universal vaccination programme in Catalonia, Spain. J Viral Hepat. 2008;15(Suppl 2):51–56.
- Vilella A, Dal-Ré R, Simó D, et al. Reactogenicity profile of a combined hepatitis A and B vaccine in clinical practice: a naturalistic study in adult travellers. Vaccine. 2005;23(19):2465–2469.
- Woo EJ, Miller NB, Ball R. Adverse events after hepatitis A B combination vaccine. Vaccine. 2006;24(14):2685–2691.
- Moro PL, Museru OI, Niu M, et al. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210(6):561.e1–561.e6.
- Thudi K, Yadav D, Sweeney K, et al. Physicians infrequently adhere to hepatitis vaccination guidelines for chronic liver disease. PLoS One. 2013;8(7):e71124.
- Van Damme P, Chlibek R, Keeffe E. Hepatitis A and B vaccination in elderly travellers. European Gastroenterology & Hepatology Review. 2011;7(2):84–92.
- Guess HA. Combination vaccines: issues in evaluation of effectiveness and safety. Epidemiologic Reviews. 1999;21(1):89–95.
- Levine H, Kopel E, Anis E, et al. The impact of a national routine immunisation programme initiated in 1999 on hepatitis A incidence in Israel, 1993 to 2012. Euro Surveill. 2015;20(7):3–10.
- Chiang CJ, Yang YW, You SL, et al. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310(9):974–976.
Appendix 1. Search string
‘twinrix’ [Supplementary Concept] OR Twinrix[tiab] OR Ambirix[tiab] OR combined hepatitis A and B vaccin*[tiab] OR hepatitis A/B vaccin*[tiab] OR hepatitis A/hepatitis B vaccin*[tiab] OR (((‘Hepatitis A’[Mesh] OR hepatitis A[tiab]) AND (‘Hepatitis B’[Mesh] OR hepatitis B[tiab])) AND (‘Vaccination’[Mesh] OR vaccin*[tiab])) OR ((‘Hepatitis A’[Mesh] OR hepatitis A[tiab]) AND (‘Hepatitis B’[Mesh] OR hepatitis B[tiab]) AND (‘Immunization’[Mesh] OR immun*[tiab]))
Limit: Publication date from January 1990 to present (October 13th, 2015)
Appendix 2. Study characteristics
Table A1. Study characteristics.