Dengue is a growing global problem that urgently needs to be addressed [Citation1]. Similarly to yellow fever, dengue is caused by viruses of the flaviviridae family and transmitted by Aedes mosquitoes, but unlike yellow fever for which we have had a vaccine for more than 80 years, the first dengue vaccine is only available now. Research and Development funding for dengue vaccine development has more than tripled in the past decades, much of which is driven by vaccine manufacturers [Citation2]. We have a robust pipeline with several promising dengue vaccine candidates, all using different approaches [Citation3]. The only vaccine to have completed Phase 3 trials is the CYD-TDV (a live attenuated recombinant chimeric tetravalent dengue vaccine developed by Sanofi Pasteur) sponsored by Sanofi Pasteur. Interestingly, the relatedness to other flaviviruses played a role in the development of CYD-TDV in which the 17D yellow fever virus backbone is used for chimerization with dengue viruses (DENVs). CYD-TDV is a formulation of four chimeras, each one engineered to express the envelope and pre-membrane proteins from one of the four serotypes of DENV [Citation4]. Dengvaxia (CYD-TDV) was first licensed in Mexico in December 2015, and is meanwhile licensed in three other countries.
Pooled efficacy data of CYD-TDV from phase 3 trials conducted in over 30,000 children aged 2–16 years in 10 countries (5 in Asia, and 5 in Latin America) have shown this vaccine to have mixed performance: efficacy varied widely depending on serotype, baseline flavivirus priming status (i.e. prior DENV infection status) and age [Citation5]. Protection against serotypes 3 and 4 was good, but marginal against serotype 1 and poor against serotype 2. Absence of prior DENV infection was associated with low efficacy. Higher age had an overall beneficial impact on efficacy: among individuals aged 9–16 years, efficacy against virologically confirmed DENV infection regardless of severity and serotype was higher (66%) than in those below 9 years (45%). Efficacy against severe dengue and hospitalization was also higher (93% and 81%, respectively) in individuals aged 9 years or older, compared with 45% and 56% in children younger than 9 years [Citation6]. Eliciting the reasons for the imbalanced efficacy results is now a subject of intense research. Did the CYD-TDV act as a monovalent or bivalent vaccine mainly against serotype 3 and 4 only despite high antibodies against all serotypes as measured by the plaque reduction neutralization assay [Citation7]? Are the high geometric mean titre titers for antibodies against all four serotypes after administering three doses of the vaccines perhaps more a reflection of the short-lived heterotypic immunity seen after natural infection with one serotype rather than long-lasting homotypic immunity? Did viral interference among the vaccine strains impact this imbalance in efficacy? Were the target neutralizing antibody titers during vaccine development too low for serotype 2? Alternatively, is there something unique about serotype 2 with unclear implications for different vaccine design strategies? As the CYD-TDV vaccine does not contain the nonstructural proteins of DENV, one area of research has included the role of nonstructural proteins in dengue pathogenesis. For example, the potentially important role of NS1, one of the nonstructural proteins, in the pathway leading to increased vascular permeability was recently demonstrated in a mouse model. Immunization of mice with NS1 from DENV-1 to DENV-4 provided protection against lethal DENV-2 challenge [Citation8]. Furthermore, since natural DENV infection elicits both neutralizing antibodies and broad T-cell responses, efficient and long-lasting immunity induced by vaccination may require elements of both humoral and cell-mediated immunity [Citation9]. Natural infection induces dengue-specific CD8(+) T lymphocytes that are highly activated and exhibit antiviral effector functions [Citation10]. A vigorous response by multifunctional CD8(+) T cells has been associated with protection from dengue disease [Citation11]. Lastly, still little is known about the role of innate immune response in pathogenesis which would warrant further research [Citation8].
Data from three years of follow-up of the CYD-TDV multi-country phase 3 trials combined with four years of follow-up of the single-country phase 2b trial in Thailand allow more insights into the safety of CYD-TDV. It should be noted that after the second year, only passive (hospital-based) surveillance was conducted and, hence, only data on hospitalized dengue cases (but not overall number of virologically confirmed dengue cases) are available. This follow-up data revealed an increased number of hospitalized dengue cases in vaccinees compared to controls during the third year of the Asian Phase 3 trial in those younger than 9 years, which was much more pronounced in the youngest children (i.e. 2–5 years of age) who had an almost eight-fold increased risk of hospitalizations due to dengue. This increased risk was not observed in this age group during the first two years of the Asian phase 3 trial, nor in the fourth year of the phase 2b trial in Thailand. The reasons for the safety observation in the young age group are unknown at present. The possibility of immune enhancement in the context of waning or incomplete immunization in flavivirus-naïve and primed individuals is being investigated. In response to the reversed risk/benefit ratio in younger age groups, Sanofi Pasteur reinstituted active surveillance in many of the Phase 3 trial sites for the remainder of the trial period.
In subjects above the age of 9 years, hospitalized dengue cases remained consistently higher in the control group throughout the three years of the phase 3 trials. Given the consistently higher efficacy and better benefit/risk profile for individuals aged 9 years and above, Sanofi Pasteur has sought licensure with an indication in individuals from 9 years of age and above [Citation6].
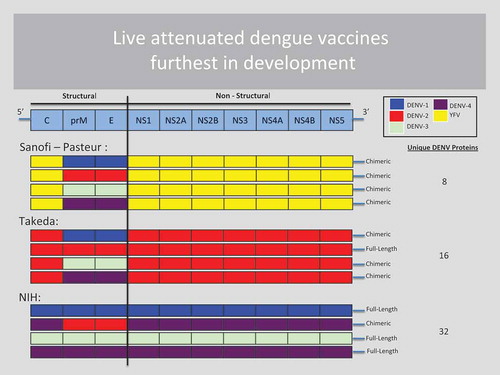
Two other live attenuated vaccine candidates are in active development in human trials. Unlike CYD-TDV, they incorporate DENV nonstructural proteins: Takeda’s TDV vaccine contains whole attenuated DENV-2 including the nonstructural proteins, but only the premembrane and envelope proteins for serotypes 1, 3 and 4 [Citation12], while the U.S. NIH-developed TV003/TV005 vaccine has whole attenuated virus for three of four serotypes but only premembrane and envelope proteins for DENV-2 [Citation13]. illustrates the amount of nonstructural proteins that each of the leading live attenuated dengue vaccines contains. The Takeda and NIH vaccines will soon be assessed for their clinical efficacy in Phase 3 trials. We will need to wait for those results before we know whether these vaccines will be able to overcome some of the shortcomings that were encountered with the CYD-TDV vaccine.
Although the three most advanced dengue vaccine candidates have used live attenuated strategies, other approaches have been proposed to improve the immunogenicity of non-live candidates, including the use of recent FDA-approved adjuvants, direct coupling with immune-potentiators, and ligand-mediated immune cell targeting [Citation14]. Furthermore, conformational epitopes, such as the previously unknown envelope dimer epitope, which is broadly cross-reactive and elicits highly neutralizing antibodies against all four serotypes, may provide new targets for vaccine design and monitoring [Citation15]. The future success of dengue vaccines depends, at least in part, on our ability to understand the complexity of DENV antigens and the human immune response to them [Citation16], and on the ongoing pursuit of potential immune correlates of protection and risk [Citation17]. Without a better understanding of immune correlates, large resource-intensive efficacy trials will continue to be necessary to evaluate new dengue vaccine candidates. Improved preclinical models, and also controlled human infection studies (challenge studies) will have increasingly important roles to play in down-selecting vaccine candidates before entering Phase 3 efficacy trials.
Any introduction of dengue vaccine will require extensive preparation. Lessons learned from past vaccine introductions have shown that the usual long delays between licensure and roll-out are counterproductive [Citation18]. The Dengue Vaccine Initiative (DVI) (www.denguevaccines.org), mainly funded through the Bill & Melinda Gates Foundation, has worked toward the goal of accelerating dengue vaccine development and introduction. DVI partners have coordinated efforts to generate more data on the burden of dengue, conduct economic evaluations and strategic demand forecasting for dengue vaccines, elaborate on the necessary immunization systems to be in place for vaccine introduction, and provide assistance on advocacy and communications [Citation19–Citation21]. Such work has set the foundation for potential dengue vaccine introduction. As a thorough review by Tozan highlights, country-level analytical expertise in economic analyses needs to be strengthened to facilitate evidence-based decision-making on dengue vaccine introduction in endemic countries [Citation22].
While research has intensified to address the shortcomings of CYD-TDV, there is an urgent need for policy-makers and the dengue scientific community to decide the best way forward for CYD-TDV in the absence of any other currently available dengue vaccine [Citation23]. Several dengue endemic countries are now considering licensing and introducing the CYD-TDV vaccine. A pressing question is how this vaccine will impact public health given that it will be limited to those aged 9 years and above in dengue endemic areas. As a stand-alone public health intervention, this vaccine will doubtlessly not be able to fully control dengue. Mathematical modeling efforts are under way to estimate the extent of the vaccine’s impact on public health and on virus transmission. But modeling will only be estimates based on assumptions. Demonstration projects are sorely needed. Vaccine effectiveness studies, vaccine probe studies, cost-effectiveness evaluations, pharmacovigilance and long-term safety monitoring, as well as studies to determine the need and timing of boosters should accompany any potential vaccine introduction, and indeed plans for such studies for CYD-TDV are now the subject of intense work. We have come very close to the first dengue vaccine, and how we approach the coming years will be critical in determining how quickly dengue vaccines continue to progress.
In light of recent developments, Expert Review of Vaccines is publishing a special issue on dengue vaccines, covering the immunology of dengue pertinent to dengue vaccine development, to a review of the current three leading dengue vaccine candidates (Sanofi Pasteur, Takeda, and NIH/NIAID) and potential new approaches such as sub-unit dengue vaccines, as well as tackling issues related to dengue vaccine introduction communication and advocacy, financing and economics, and various other points for consideration.
Financial and competing interests disclosure
A Wilder-Smith serves as Senior Advisor to the Dengue Vaccine Initiative. IK Yoon is Director of the Dengue Vaccine Initiative, which has received unrestricted grants from Sanofi Pasteur and Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Notes on contributors


Annelies Wilder-Smith


In-Kyu Yoon
References
- Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309.
- Horstick O, Tozan Y, Wilder-Smith A. Reviewing dengue: Still a neglected tropical disease? PLoS Negl Trop Dis. 2015;9(4):e0003632.
- Thomas SJ, Endy TP. Current issues in dengue vaccination. Curr Opin Infect Dis. 2013;26(5):429–434.
- Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Human Vaccines. 2010;6(9):696–705.
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–1206.
- Wilder-Smith A, Massad E. Age specific differences in efficacy and safety for the CYD-tetravalent dengue vaccine. Expert Rev Vaccines. 2016;15(4):437–441.
- Leo YS, Wilder-Smith A, Archuleta S, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother. 2012;8(9):1259–1271.
- Beatty PR, Puerta-Guardo H, Killingbeck SS, et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7(304):304ra141.
- Rivino L. T cell immunity during dengue virus infection and implications for vaccine design. Expert Rev Vaccines. 2016;15(4):443–453.
- Rivino L, Kumaran EA, Thein TL, et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci Transl Med. 2015;7(278):278ra35.
- Weiskopf D, Angelo MA, de Azeredo EL, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A. 2013;110(22):E2046–53.
- Osorio JE, Wallace D, Stinchcomb DT. A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone. Expert Rev Vaccines. 2016;15(4):497–508.
- Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Expert Rev Vaccines. 2016;15(4):509–517.
- Lam JH, Ong LC, Alonso S. Key concepts, strategies, and challenges in Dengue vaccine development: An opportunity for sub-unit candidates? Expert Rev Vaccines. 2016;15(4):483–495.
- Dejnirattisai W, Wongwiwat W, Supasa S, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16(2):170–177.
- Acosta EG, Bartenschlager R. Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development. Expert Rev Vaccines. 2016;15(4):467–482.
- Srikiatkhachorn A, Yoon IK. Immune correlates for dengue vaccine development. Expert Rev Vaccines. 2016;15(4):455–465.
- Hajjeh RA, Privor-Dumm L, Edmond K, et al. Supporting new vaccine introduction decisions: lessons learned from the Hib Initiative experience. Vaccine. 2010;28(43):7123–7129.
- Lim JK, Lee YS, Wilder-Smith A, et al. Points for consideration for dengue vaccine introduction – recommendations by the dengue vaccine initiative. Expert Rev Vaccines. 2016;15(4):529–538.
- Constenla D, Clark S. Financing dengue vaccine introduction in the Americas: challenges and opportunities. Expert Rev Vaccines. 2016;15(4):447–459.
- Carvalho A, Van Roy R, Andrus J. International Dengue Vaccine Communication and Advocacy: challenges and Way Forward. Expert Rev Vaccines. 2016;15(4):539–545.
- Tozan Y. Current issues in the economics of vaccination against dengue. Expert Rev Vaccines. 2016;15(4):519–528.
- Wilder-Smith A, Gubler DJ. PUBLIC HEALTH. Dengue vaccines at a crossroad. Science. 2015;350(6261):626–627.