Abstract
Heart failure is a major cause of morbidity and mortality in chronic kidney disease (CKD). Rather than merely secondary to traditional vascular factors, CKD is also an independent risk factor for heart failure, termed uremic cardiomyopathy (UCM). Echocardiography commonly reveals structural left ventricular hypertrophy in CKD, without clarifying whether it is adaptive or maladaptive. Corresponding functional assessments have been mostly conducted at rest. To unravel the extents and mechanisms UCM, a next step involves the adoption of direct measurements of CKD-induced cardiac pumping incapacity at peak exercise. This could potentially lead to future novel interventions to ameliorate or reverse UCM.
Chronic kidney disease (CKD) carries a high burden of morbidity and mortality. Recent registry data from the US Renal Data System show that >40% of end-stage renal disease (ESRD) deaths were due to cardiovascular disease (CVD) Citation[1]. Indeed, 50% of ESRD patients suffer from heart failure (HF), thus forming the predominant cardiac abnormality observed in CKD Citation[1]. It is a common perception that HF in CKD is secondary to vascular comorbid conditions such as ischemic heart disease (IHD), hypertension and diabetes mellitus, but it is becoming apparent that primary CKD is an independent risk factor for incident HF even in nondiabetic and normotensive patients Citation[2]. It is also becoming clearer that CVD in CKD is more than just accelerated atherosclerosis Citation[3]. Thus, nontraditional risk factors such as uremic toxins, anemia, calcium phosphate imbalance, renin–angiotensin–aldosterone system (RAAS) activation, sympathetic activation, inflammation and oxidative stress are gaining recognition for their roles in the pathogenesis of cardiac disease in CKD Citation[4]. A recent Kidney Disease: Improving Global Outcome report emphasized the need for improving our understanding of cardiac dysfunction in CKD and highlighted the need for research that is capable of evaluating asymptomatic left ventricular (LV) dysfunction Citation[5]. Of the five subtypes of cardiorenal syndromes so far classified Citation[6], it is the fourth subtype, CKD directly leading to cardiac dysfunction, which will form the focus of this brief editorial, and for simplicity, we shall ascribe the term uremic cardiomyopathy (UCM) to this type of dysfunction.
Structural & ultrastructural abnormalities of UCM
By far the commonest, easily accessible method of studying the structural abnormalities of UCM is echocardiography, with which it is observed that a predominant feature in CKD is LV hypertrophy (LVH). A recent large observational study has demonstrated a graded relationship between the severity of CKD and the prevalence and severity of LVH Citation[7]. The prevalence increased from 32 to 75% as one moved from patient groups with estimated glomerular filtration rate (eGFR) >60 to <30 ml/min. The corresponding mean left ventricular mass increased from 46.1 to 57.8 g/m2.7. Perhaps, because CKD is closely associated with hypertension and LVH is a recognized consequence of chronic hypertension, no evidence has so far been proffered to establish whether LVH is a direct outcome of primary CKD rather than due solely to associated hypertension. A study using cardiac MRI with gadolinium contrast showed evidence of diffuse myocardial fibrosis in uremic patients, different in distribution to the subendocardial fibrosis observed in IHD Citation[8]. Histopathological examination of postmortem cardiac tissue samples in hemodialysis patients showed increased cardiomyocyte diameter, reduced capillary length density and increased interstitial volume Citation[9]. Although the above studies offer insight into the structural abnormalities of UCM, information on the functional consequences of such changes is still lacking. In order to understand whether these hypertrophic changes are adaptive or maladaptive, it is essential to study their functional correlates.
Pathophysiological consequences of UCM
Studies reporting functional abnormalities in UCM have mostly employed resting echocardiography. Parameters such as left ventricular ejection fraction, left ventricular fractional shortening and midwall fractional shortening have been used to assess systolic dysfunction, while transmitral flow velocity has been used to assess diastolic dysfunction. In a recent large observational study evaluating cardiac structure and function in CKD, despite a clear association between LVH and renal dysfunction, no association between systolic or diastolic cardiac function and renal function was demonstrated Citation[7]. In contrast, it is not uncommon to find normal or enhanced echocardiographic indices of resting cardiac function in the presence of LVH Citation[10]. It is, however, now well established that merely assessing the resting, unstressed heart can provide unrepresentative or misleading information about true cardiac function, unless in cardiogenic shock Citation[11]. Stress echocardiography (via exercise or pharmacological challenge) and cardiopulmonary exercise tests (CPXs) are some of the tools available to evaluate cardiac dysfunction. Unfortunately, because recommendations on ‘appropriateness criteria for stress echocardiography’ excluded patients with renal diseases Citation[12], very limited information is so far available on stress echocardiography in CKD patients.
Assessment of myocardial contractile reserve in pediatric CKD patients, using exercise echocardiography, has shown that contractile reserve is impaired even when resting parameters are normal Citation[13]. In another study of pediatric CKD patients, peak oxygen consumption (VO2max), a measure of physical functional reserve and a surrogate for peak cardiac performance, is shown to be impaired compared with healthy controls Citation[14]. Since these pediatric populations are assumed to have minimal atherosclerosis, the findings suggest that CKD per se may have direct deleterious effects upon cardiac function. However, results from pediatric patients may not translate fully to adults. Therefore, studies specifically designed to evaluate cardiac reserve in adult CKD patients without comorbid atherosclerosis substrates are needed to obtain useful insights into UCM.
A CPX study in ESRD patients has shown impaired physical functional reserve and a negative survival impact Citation[15]. However, this study did not exclude patients with IHD, diabetes mellitus or pre-existing HF, and therefore, it is difficult to ascertain whether the observations were due to primary CKD or secondary to CVDs. Moreover, other comorbid factors including anemia, peripheral vascular disease, hypertension and skeletal myopathy in CKD patients would contribute variably to the VO2max and anaerobic threshold values obtained Citation[16]. These considerations highlight the importance of patient selection and the choice of optimum parameters to measure cardiac dysfunction arising directly from primary CKD.
Cardiac functional reserve
When a heart begins to fail, compensatory mechanisms are activated that maintain the resting cardiac performance within as normal a range as possible. Peak performance, however, becomes compromised and diminished. It is this diminution of cardiac reserve that needs to be measured Citation[11]. This loss of cardiac reserve leads to a reduced ability to cope with stress such as exercise, septicemia and surgery. Diminution of cardiac reserve occurs with any functional or quantitative cardiomyocyte insult. Conditions such as myocardial infarction, myocarditis and cardiotoxic insults through chemotherapy, sympathetic and renin–angiotensin–aldosterone neurohumoral overactivity and cytokines can cause myocyte loss through necrosis, apoptosis and autophagy Citation[17]. Cardiac functional reserve can be best represented by quantification of cardiac power output (CPO, calculated as the product of cardiac output and mean arterial pressure) at peak stimulation Citation[11]. Measured invasively or noninvasively, peak cardiac power (CPOmax) has been shown to be a direct indicator of cardiac dysfunction and the best predictor of mortality in patients with HF Citation[18,19].
CKD & cardiac functional reserve
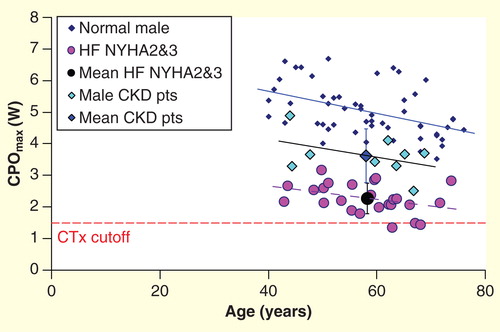
The uremic milieu, with its components of sympathetic activation Citation[20], RAAS activation and inflammation and oxidative stress Citation[21] has the potential to inflict cardiomyocyte cell injury. Some uremic toxins have been shown to cause cardiac damage including fibrosis Citation[22]. Studies performed on isolated cardiomyocytes have shown abnormal calcium handling and impaired myocyte relaxation in uremia Citation[23]. The uremic environment also appears to impair cardiomyocyte energetics Citation[24]. Although the above considerations suggest that cardiac functional reserve is likely to be impaired in CKD, empirical evidence is still lacking. A proof-of-concept study has demonstrated that CPO at peak exercise (CPOmax) is impaired in asymptomatic patients with advanced CKD even in the absence of CVD and diabetes mellitus, as shown in Citation[25]. In this study, cardiac functional reserve was measured noninvasively using CPX. Since the CKD patients in this study were asymptomatic from the cardiac point of view, and the key difference with healthy controls was late-stage CKD, the possible factor accounting for the cardiac functional impairment was uremia and its knock-on effects. Since CPOmax has been shown to be a powerful predictor of mortality in HF patients Citation[18,19], it might be hypothesized that further compromise of cardiac function by uremic processes in CKD could exacerbate poor prognosis.
Potential applications of evaluating cardiac reserve in CKD
Interdisciplinary collaborative research is being intensified to unravel the complexities of cardiorenal syndromes Citation[6]. Unlike in nephrology where measurement of organ dysfunction (e.g., by monitoring eGFR) is integral to management, there is so far no counterpart in cardiological practice or research, such that cardiologists cannot readily quantify the impact of therapeutic interventions upon cardiac function and tailor management accordingly. A major reason is that hitherto, there has been no quantitative measure of cardiac function, analogous to eGFR, in cardiological practice or research. The solution may be found in the clinically applicable measurement of cardiac functional reserve such as CPOmax Citation[11]. Such a tool would help early detection of cardiac dysfunction from primary CKD even at presymptomatic phases. It can also help to evaluate the efficacy of conventional cardioprotective strategies and develop novel therapies to prevent progression of and/or reverse UCM. It would also help to evaluate the relative merits of conventional therapeutic strategies such as maintenance of fluid and electrolyte balance, anemia treatment, CVD risk modification, β-blockade and RAAS blockade. It provides a cardiac outcome measure that can help evaluate novel therapies such as management of inflammation and oxidative stress, removal of uremic toxins with special dialysis techniques (e.g., plasma separation and adsorption) and strategies to minimize generation of uremic toxins through dietary modification and/or gastrointestinal binders of uremic toxins.
Acknowledgement
This work is supported by funding from Yorkshire Kidney Research Fund and Sheffield Kidney Research Foundation.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Annual Data Report 2013. US Renal Data System. Available from: www.usrds.org/atlas.aspx [Last accessed December 2013]
- Dhingra R, Gaziano JM, Djousse L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail 2011;4(2):138-44
- Remppis A, Ritz E. Cardiac problems in the dialysis patient: beyond coronary disease. Semin Dial 2008;21(4):319-25
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382(9889):339-52
- Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int 2011;80(6):572-86
- Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010;31(6):703-11
- Park M, Hsu CY, Li Y, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012;23(10):1725-34
- Mark PB, Johnston N, Groenning BA, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 2006;69(10):1839-45
- Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 1998;9(6):1018-22
- Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol 1995;26(1):195-202
- Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol 2003;18(3):215-22
- Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol 2008;51(11). 1127-47
- Mitsnefes MM, Kimball TR, Witt SA, et al. Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation 2003;107(6):864-8
- Weaver DJ Jr, Kimball TR, Knilans T, et al. Decreased maximal aerobic capacity in pediatric chronic kidney disease. J Am Soc Nephrol 2008;19(3):624-30
- Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 2004;65(2):719-24
- Sietsema KE, Hiatt WR, Esler A, et al. Clinical and demographic predictors of exercise capacity in end-stage renal disease. Am J Kidney Dis 2002;39(1):76-85
- Goldspink DF, Burniston JG, Tan LB. Cardiomyocyte death and the ageing and failing heart. Exp Physiol 2003;88(3):447-58
- Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet 1986;2(8520):1360-3
- Williams SG, Cooke GA, Wright DJ, et al. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur Heart J 2001;22(16):1496-503
- Schlaich MP, Socratous F, Hennebry S, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 2009;20(5):933-9
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002;62(5):1524-38
- Lekawanvijit S, Adrahtas A, Kelly DJ, et al. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010;31(14):1771-9
- Periyasamy SM, Chen J, Cooney D, et al. Effects of uremic serum on isolated cardiac myocyte calcium cycling and contractile function. Kidney Int 2001;60(6):2367-76
- Raine AE, Seymour AM, Roberts AF, et al. Impairment of cardiac function and energetics in experimental renal failure. J Clin Invest 1993;92(6):2934-40
- Chinnappa S, Mooney A, Lewis NT, et al. New evidence of cardiac dysfunction associated with renal impairment. Int J Cardiol 2011;152(3):411-13