Abstract
Paravalvular leak is a common complication occurring after transcatheter aortic valve implantation and is associated with at least a 2-fold increase in 30-day and 1-year mortality risk. In high-risk, inoperable patients with severe aortic stenosis, paravalvular leak may even negate the survival benefit of transcatheter aortic valve implantation. This editorial reviews the anatomy, pathophysiology and predictors of paravalvular leak and discusses preventative and therapeutic strategies to optimally treat this previously underappreciated complication.
Transcatheter aortic valve implantation (TAVI) offers significant short- and mid-term survival benefits compared to medical therapy alone in inoperable patients with severe aortic stenosis and results in early outcomes similar to conventional surgical aortic valve replacement (SAVR) in very high-risk, elderly surgical patients Citation[1]. Although TAVI has demonstrated excellent reduction in transvalvular pressure gradients, aortic insufficiency (AI) remains a common problem after TAVI Citation[1]. Early in the TAVI experience, various degrees of paravalvular leak (PVL) were reluctantly ‘accepted’, as AI was thought to be a better tolerated and more slowly progressive lesion than aortic stenosis. PVL severity is commonly believed to be worst at the time of implantation and to be relatively stable over time. More recent evidence suggests that any PVL portrays significant patient risk and may even potentially negate the survival benefits of TAVI when moderate or severe PVL occurs Citation[2,3].
We discuss the incidence, clinical implications, prevention and management of PVL after TAVI.
PVL is common after TAVI
Some degree of PVL is thought to occur after most TAVI procedures Citation[1,3]; however, wide variation in the reported incidence of PVL exists, likely related to various imaging modalities, lack of reporting standards, different time points for evaluation and reporting bias. In observational studies, moderate-to-severe PVL has been reported in 10–25% of patients Citation[3]. Pooled estimates of 45 studies with 12,926 patients demonstrated moderate or severe AI in 11.7% (CI: 9.6–14.1), with a higher incidence in self-expanding prosthesis than balloon-expandable prosthesis (16.0 vs 9.1%; p = 0.005) Citation[2]. Evidence from randomized trials confirm the presence of moderate or severe PVL in 12% of patients post-procedurally, with nearly 25% of patients experiencing worsening PVL over time Citation[1].
PVL is associated with early & late mortality
The incidence of greater than trace residual PVL after SAVR ranges from 0 to 4.8% Citation[4,5] and is associated with an increased risk of early and late mortality Citation[5]. Similarly, a recent meta-analysis demonstrated that moderate or severe AI post-TAVI was associated with significant hazard ratios for 30-day and 1-year mortality of 2.95 (1.73, 5.02; p = 0.001) and 2.27 (1.84, 2.81; p = 0.001), respectively Citation[2]. Even mild AI after TAVI was associated with an increased hazard ratio for death of 1.829 (1.005, 3.329; p = 0.048). The 2-year PARTNER trial results confirm the significant mortality impact of any PVL with mild-to-severe PVL conferring an increased hazard ratio for death of 2.11 (1.43, 3.10; p = 0.0001) Citation[1]. Additionally, these patients also experienced significant residual dyspnea and reduced functional status. The long-term mortality outcomes associated with PVL have also been shown to be poor. After 535 ± 333 days of follow-up, in patients with at least moderate PVL, an increased hazard ratio for death of 4.38 (2.78, 8.56; p < 0.001) has been demonstrated Citation[6]. This has been further validated with 5-year outcome data, demonstrating a hazard ratio for death of 2.98 (1.44, 6.17; p < 0.01) in patients with at least moderate PVL Citation[7]. In summary, the presence of any PVL greater than trace AI doubles the expected mortality rate at 1 year, negating the survival benefit of TAVI in high-risk patients with severe aortic stenosis.
The anatomy & pathophysiology of PVL
PVL occurs when there is incomplete TAVI stent apposition with the native aortic annulus, often related to a heavily calcified, eccentric aortic annulus Citation[3]. The fracture pattern of the calcified native aortic valve during balloon valvuloplasty and calcium shift during valve implantation is unpredictable. As a result, triangular gaps remaining between the implanted TAVI prosthesis and the calcified annulus can result in PVL . Current intraprocedural imaging modalities, fluoroscopy and transesophageal echocardiography do not have the spatial resolution to allow visualization of the calcium shift and these residual gaps Citation[8].
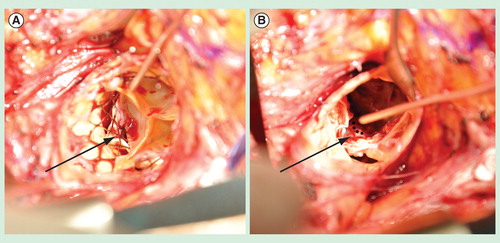
Figure 2. The anatomy of a typical posterior paravalvular leak after transcatheter aortic valve implantation, as viewed intraoperatively during valve explantation. (A) Intraoperative photograph demonstrating an aortic annular triangular gap (arrow) between two calcified cusps of the native aortic valve and the implanted transcatheter aortic valve implantation (TAVI) prosthesis. This patient had developed symptomatic, moderate-to-severe paravalvular leak 18 months after implantation of the TAVI prosthesis, necessitating re-operative aortic valve replacement. (B) Intraoperative photograph taken after surgical removal of the TAVI prosthesis, demonstrating the bulky calcium at the base of the left coronary cusp and non-coronary cusp, leaving a triangular gap at the commissure preventing apposition of the TAVI prosthesis (dashed line) against the native aortic annulus.
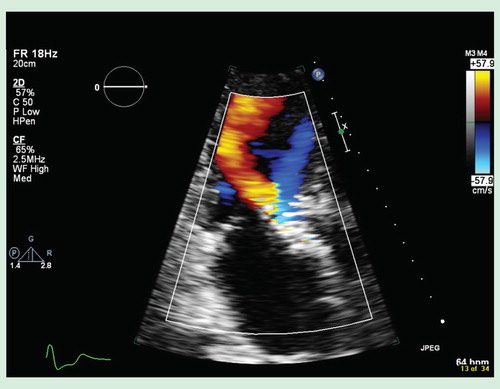
The pathophysiology of AI in PVL is quite different from that of chronic AI. In chronic AI, the lesion is slowly progressive and generally well tolerated, while most PVL represent acute AI or acutely worsened AI. As a result, the left ventricular end diastolic pressure rises within a non-compliant, hypertrophied left ventricle, and pulmonary congestion ensues. Without time for left ventricular adaptation or accommodation, these patients present acutely with intolerable symptoms of dyspnea, requiring early intervention. After TAVI implantation, the patients that tend to struggle the most clinically with PVL are usually those patients who experience more AI following the procedure than before (i.e., no AI before, mild-to-moderate AI after TAVI) Citation[9].
Predictors of PVL after TAVI
Identified risk factors for PVL include severe, bulky, eccentric aortic annular calcification, too low valve implantation and undersized valve selection Citation[3,8]. Inadequate valve sizing by transesophageal echocardiography, defined as a low area cover index (the ratio of 100 × [prosthesis diameter – native annulus diameter]/prosthesis diameter ≤8%), has recently been recognized as a major risk factor for PVL Citation[6,10]. All available TAVI prostheses are at risk for PVL, although there may be some differences between self-expanding and balloon-expandable prosthesis Citation[2]. However, the incidence of PVL was not affected by the delivery route used in a study that compared to transfemoral to transapical TAVI Citation[11].
Prevention of PVL
More accurate estimation of the native aortic annular dimensions by multidetector computed tomography for valve sizing remains one of the few prophylactic maneuvers to reduce PVL Citation[12]. In a recent randomized trial, TAVI valve sizing by a multidetector computed tomography algorithm versus control demonstrated a statistically significantly reduced incidence of significant PVL from 13 to 5% (p = 0.032) Citation[13]. Oversizing of the valve prosthesis by 10–20% and higher valve implantation within the native aortic annulus may help to reduce the incidence of PVL as well Citation[2,12]. The most significant problem with preventing PVL is the unpredictable nature of calcific fracture patterns of the native aortic valve and the inability to seat the valve prosthesis circumferentially flush against the aortic annulus . Additionally, current intraoperative imaging guidance is insufficient to accurately delineate the shift of calcium during stent deployment in a meaningful, 3D manner to guide intraprocedural changes to improve suboptimal stent apposition Citation[2,8].
Treatment of PVL
Several strategies may help to reduce PVL after valve implantation. Post-implantation balloon valvuloplasty may help to reduce the severity of PVL , especially in an underdeployed prosthesis; however, it is associated with increased risks of annular rupture and stroke Citation[8]. Valve-in-valve deployment can extend the valve cuff higher or lower in the aortic annulus to cover any residual gaps to reduce PVL Citation[14]. However, the success of this maneuver can be variable as it is not always clear where the PVL is originating from. Furthermore, a recent retrospective analysis of the PARTNER trial showed that valve-in-valve implantation for AI was associated with an increased hazard ratio for cardiovascular mortality of 1.86 (1.03, 3.38; p = 0.041) Citation[15]. Repositioning of the implanted prosthesis by complicated snaring techniques has been described, but not widely validated Citation[6,16]. Lastly, but importantly, if significant PVL remains, conversion to conventional SAVR should be considered because of the high mortality associated with leaving a patient with moderate or severe PVL Citation[2,8].
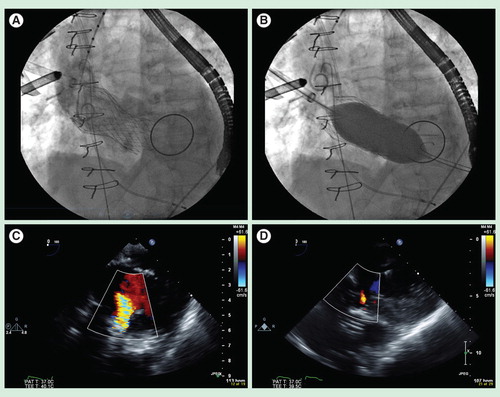
Figure 3. Post-implant balloon valvuloplasty represents a commonly performed first-line maneuver to treat severe paravalvular leak. (A) Fluoroscopic image of a 31 mm Medtronic CoreValve Transcatheter aortic valve implantation prosthesis (Minneapolis, MN, USA), implanted via direct aortic access, in a high-risk elderly patient with severe aortic stenosis and a large aortic annulus who had a previous bileaflet mechanical mitral valve prosthesis. (B) Fluoroscopic image demonstrating post-implant balloon valvuloplasty under rapid ventricular pacing with a 28-mm balloon. (C) Intraoperative transesophageal echocardiography demonstrating a deep transgastric view of the CoreValve Transcatheter aortic valve implantation prosthesis, with color Doppler showing severe paravalvular leak post-implantation. (D) Intraoperative transesophageal echocardiography (deep transgastric view) demonstrating resolution of severe paravalvular leak to trace residual aortic insufficiency after post-implant balloon aortic valvuloplasty with a 28-mm balloon.
Future directions
The incidence of significant PVL will decrease as multi-slice computed tomography becomes the gold standard for TAVI valve sizing, and experience in optimal valve positioning is developed. However, these important preventative measures are unlikely to eliminate PVL in its entirety. Advancements in valve prosthesis design including repositioning/redeployment and more robust annular cuffs will be critical to reduce PVL. Recently, two newer generation transapical prostheses have demonstrated that thicker annular cuffs can systematically eliminate PVL in almost all patients (no patients with moderate or severe AI and <5% with any PVL) Citation[17,18]. Currently, intraoperative imaging modalities such as transesophageal echocardiography and aortography only allows for qualification of PVL. A recent study by Vasa-Nicotera et al. validated the ‘aortic regurgitation index’ in a small cohort of patients Citation[19]. The AR index allows for quantification of PVL by the following calculation 100 × (diastolic blood pressure – left ventricular end diastolic pressure)/systolic blood pressure. An index less than 25 was associated with increased 1-year mortality. Further evaluation of the AR index in large multicentred trials to further generalizability of this tool is warranted. Future image guidance systems that allow real-time, 3D visualization of the aortic annulus, aortic root, valve prosthesis and calcium motion may be equally important to solving the problem of PVL as well.
Conclusion & future considerations
PVL is a common complication after TAVI that is associated with at least a twofold increase in early and late mortality. Preventative measures should be exercised and all therapeutic strategies should be employed to aggressively minimize PVL. Surgical AVR should be considered if patient risks are not considered truly prohibitive.
There is a much better understanding and appreciation of the significant implications of PVL following TAVI. Preventative measures such as optimal valve sizing by multi-slice computed tomography and 3D transesophageal echocardiography will decrease the prevalence of PVL significantly. Newer generation prostheses will need to further reduce PVL by being repositionable and having more robust transannular cuffs (perhaps at the cost of lower device profiles). Solving the problem of PVL after TAVI will undoubtedly improve overall patient safety and outcomes and represents a giant step forward in the rapidly evolving field of transcatheter valve therapy.
Financial & competing interests disclosure
MWA Chu has received grant support from the Canadian Institute of Health Research, Canadian Foundation for Innovation, Ontario Research Fund and AMOSO. The author has also received commercial research support from Neochordae, Inc. and Medtronic, Canada, and speaker's honorarium from Medtronic, Canada. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366(18):1686-95
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61(15):1585-95
- Genereux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new achilles' heel? A comprehensive review of the literature. J Am Coll Cardiol 2013;61(11):1125-36
- Conradi L, Seiffert M, Treede H, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a propensity score analysis in patients at high surgical risk. J Thorac Cardiovasc Surg 2012;143(1):64-71
- Sponga S, Perron J, Dagenais F, et al. Impact of residual regurgitation after aortic valve replacement. Eur J Cardiothorac Surg 2012;42(3):486-92
- Gotzmann M, Korten M, Bojara W, et al. Long-term outcome of patients with moderate and severe prosthetic aortic valve regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2012;110(10):1500-6
- Toggweiler S, Humphries KH, Lee M, et al. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol 2013;61(4):413-19
- Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation 2013;127(3):397-407
- Hayashida K, Lefevre T, Chevalier B, et al. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2012;5(12):1247-56
- Detaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv 2009;2(9):821-7
- Ewe SH, Delgado V, Ng ACT, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg 2011;92(4):1244-51
- Willson AB, Webb JG, LaBounty TM, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol 2012;59(14):1287-94
- Binder RK, Webb JG, Willson AB, et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol 2013;62(5):431-8
- Toggweiler S, Wood DA, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed balloon-expandable transcatheter aortic valves. JACC Cardiovasc Interv 2012;5(5):571-7
- Makkar RR, Jilaihawi H, Chakravarty T, et al. Determinants and outcomes of acute transcatheter valve-in-valve therapy or embolization: a study of multiple valve implants in the U.S. PARTNER trial (Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol 2013;62(5):418-30
- Latib A, Michev I, Laborde JC, et al. Post-implantation repositioning of the CoreValve percutaneous aortic valve. JACC Cardiovasc Interv 2010;3(1):119-21
- Holzhey D, Linke A, Treede H, et al. Intermediate follow-up results from the multicenter engager European pivotal trial. Ann Thorac Surg 2013;96(6):2095-100
- Kempfert J, Treede H, Rastan AJ, et al. Transapical aortic valve implantation using a new self-expandable bioprosthesis (ACURATE TA): 6-month outcomes. Eur J Cardiothorac Surg 2013;43(1):52-6. discussion 57
- Vasa-Nicotera M, Sinning JM, Chin D, et al. Impact of paravalvular leakage on outcome in patients after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2012;5(8):858-65