Abstract
Insulin resistance (IR) associated with obesity represents a well-known risk factor for chronic disease. IR development may occur to hinder stressful conditions to provide an appropriate energetic supply to non-insulin-sensitive tissues. However, conditions of stress turn out to be ‘maladaptive’ in the long term, leading to chronic diseases. Paradoxically, insulin hypersensitivity and/or hypersecretion causing post-prandial hypoglycemia resulting in increased food intake and weight gain, can represent an event preceding obesity and IR. By performing an OGTT in obese or obese-prone individuals we observed that tardive post-prandial hypoglycemia (3h from glucose load) is not a rare event (32%); in 12% of cases it paralleled with low insulin levels, resulting in the ‘true insulin hypersensitivity’. By using Matsuda-method, we confirmed the presence of insulin hypersensitivity in this group. Therefore the early recognition of this phenomenon could be useful as a predictive biomarker to identify patients prone to develop obesity and obesity related-disorders.
Alice: “This is impossible...”
The Mad Hatter: “Only if you believe it is.”
–“Alice in Wonderland”
Obesity incidence is increasing worldwide, reaching an epidemic rate. Obesity is a well-recognized risk factor for the development of cardiovascular diseases, such as coronary artery disease and heart failure, and also for Type 2 diabetes mellitus and cancer, driving the obesity-attributable death rate to around 3 million per year Citation[1].
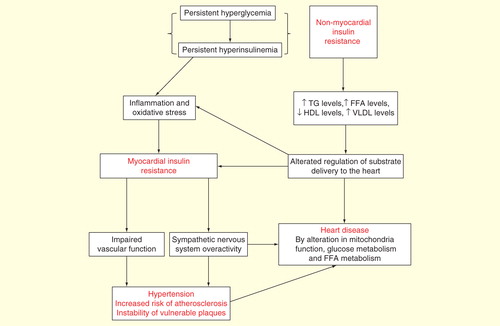
The development of insulin resistance (IR) associated with obesity, together with an inflammatory state, has been considered the pivotal phenomenon that can trigger a series of physiopathological events that may culminate in increased cardiovascular morbidity and mortality Citation[2]. Physiologically, after a meal, insulin is released from pancreatic β cells, which constitutes the main anabolic hormone that regulates substrate utilization and storage in multiple tissues, including the liver, skeletal muscle, adipose tissue and the heart Citation[3]. During fasting, when insulin levels are low, the substrates stored become available to maintain normal plasma glucose concentrations, for example, to prevent hypoglycemic events.
IR: systemic, hepatic & cardiac
When inefficient glucose uptake by muscle and adipose tissue occurs, a compensatory insulin hyperproduction is necessary to compensate for high plasma glucose concentrations. Moreover, during chronic hyperinsulinemia, secondary to IR to the glucose pathway, the relative normal insulin sensitivity to the lipid pathway could lead to increased fat storage at the peripheral level (adipose tissue and skeletal muscle) and in the liver.
Hepatic IR appears to be a selective process Citation[4]: in this condition, insulin fails to suppress gluconeogenesis but continues to promote lipogenesis of free fatty acids and their esterification in triglycerides, leading to hepatic increased lipid storage (hepatic steatosis). This condition is associated to an excessive release of triglycerides into the bloodstream. Thus, the detrimental combination at the systemic level of hyperglycemia and hypertriglyceridemia heralds the state of IR.
The heart is not immune to the deleterious effects of IR. A central point in the evaluation of myocardial IR is to be able to distinguish between the primary metabolic effects of peripheral IR (e.g., hyperinsulinemia, hyperglycemia and hyperlipidemia) from the secondary changes of insulin signaling, independent of the humoral milieu, that could be intrinsically altered at the myocardial level, for example, the true myocardial IR . It is not easy to discern in vivo between the primary and the secondary myocardial IR. IR-associated dyslipidemia promotes elevated rates of myocardial fatty acid uptake and oxidation, while glucose oxidation rate is depressed with the consequent decrease in cardiac efficiency Citation[5]. Elevated levels of fatty acids may cause an excessive accumulation of lipid intermediates, such as diacylglycerols and ceramide, which could induce myocardial IR and myocyte apoptosis Citation[6].
Other negative effects of IR/hyperinsulinemia on cardiovascular disease include the potential role of insulin on essential hypertension. Noteworthily, high insulin levels can contribute to deterioration of cardiac and renal function by antinatriuretic effect Citation[7] and have stimulating effects on sympathetic nervous system activity Citation[8].
Is IR an adaptive or maladaptive mechanism? can IR operate as a protective factor?
It has been demonstrated that IR is directly associated with obesity, diabetes and cardiovascular disease, entailing an increased risk of mortality. Nevertheless, from an evolutionary point of view, the development of IR may have a protective role during physiological or pathological states associated with negative energy balance, such as starvation, infection or trauma, to assure sufficient glucose provision toward the energy-demanding brain Citation[9]. In this setting, the development of IR that occurs during physiological states, such as pregnancy, could be considered a protective phenomenon able to maintain a proper energetic supply to the fetus during body growth Citation[10]. Interestingly, it has been suggested than even in some pathological conditions, such as myocardial infarction, obesity (and probably, by extension IR) could be advantageous in terms of size of myocardial infarct Citation[11] and mortality Citation[12,13].
IR: from adaptive mechanism to disease state
Which events can transform an adaptive, protective, physiological response to stressful conditions into a pathological element capable of producing a chronic devastating disease?
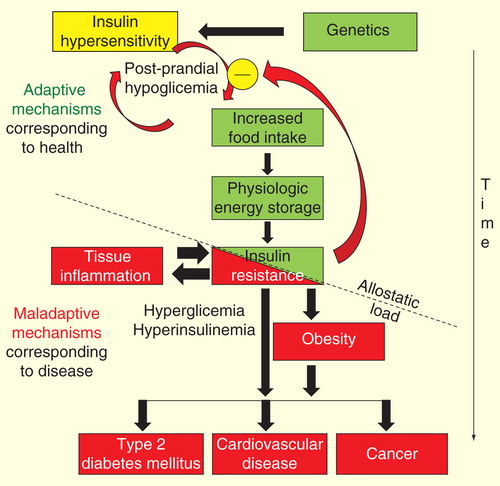
As shown in , it can be hypothesized that the allostatic load (i.e., the ‘point of rupture’) of the system could be reached by repetitive or prolonged exposure to stressful variables, such as multiple and frequent exposure to high-calorie diets succeeding infections or aging. The relative irreversibility of the process is suggested by the enormous difficulty to return to the ‘healthy status,’ observed in several conditions associated with IR, such as obesity, diabetes mellitus, essential hypertension and cardiovascular disease. These key mediators of obesity-induced metabolic disease have been recently defined as ‘evolutionarily conserved adaptive traits with maladaptive effects in the modern obesogenic environment’ Citation[14]. The above concept calls for our maximal attention to improve our diagnostic skills to be used at a point where the process could be yet ‘reversible.’ As observed in , a potential target for the prevention of IR obesity would necessarily be a correct identification of early predictors of IR-associated diseases, such as insulin hypersensitivity.
Figure 2. Representation of the hypothetic chain of events that lead from insulin hypersensitivity to insulin resistance-associated diseases. On the basis of genetic predisposition, the early events are initially displayed by insulin hypersensitivity and postprandial hypoglycemia. Postprandial hypoglycemia leads to increased caloric consumption and, therefore, to body weight increase. A new steady state of insulin sensitivity is reached to maintain stable body weight. With iterative exposure to the deleterious stimulus (frequency factor) and/or the duration of exposure is extended (time-exposure factor), an allostatic load is reached and insulin resistance is transformed from an adaptive to a maladaptive mechanism.
Insulin hypersensitivity, a potential early biomarker of obesity development
Needless to say, in any definition that applies a criterion of ‘normality’ or ‘resistance’ to the effect of any hormone, a subgroup of ‘hypersensitive’ individuals to the putative hormonal effects must exist. The physiological adaptations and pathological states that could result from changes in target tissue sensitivity to the action of hormones are of extreme importance in normal and pathological states. Hypersensitivity syndromes due to prereceptor, receptor and postreceptor abnormalities have been described in endocrine physiology. In these settings, the presence of a hypersensitive state to the hormonal effects of thyroid Citation[15], sexual Citation[16] and glucocorticoid hormones Citation[17] has already been reported.
Hypersensitivity to the physiological action of insulin had been well described in the 1940s by the prominent physiologist, the Nobel Prize winner Bernardo Houssay Citation[18]. It is astonishing that compared with IR research, insulin hypersensitivity has received very poor scientific attention. By applying the keywords ‘insulin’ and ‘hypersensitivity’ in Medline Scholar Google and after excluding animal studies, we have found less than 50 published articles in the past 25 years compared with the nearly 763,000 references found during the same period when the keyword ‘insulin resistance’ was used.
By definition, insulin hypersensitivity must be suspected in the presence of combined hypoglycemia associated with normal or low plasma insulin levels. Therefore, the key diagnostic element of insulin hypersensitivity is heralded by the presence of hypoglycemia, usually postprandial. This condition is elicited by the ingestion of carbohydrates and/or can be diagnosed using an oral glucose tolerance test. The usual threshold for the diagnosis of postprandial hypoglycemia is 70 mg/dl (or 3.9 mmol/l) Citation[19]. Hypoglycemia may occur earlier, that is, in the first 2 h after oral glucose load (and it is usually secondary to some well-known disorders such as hyperthyroidism or gastric surgery), and tardive, that is, 3–5 h after glucose load or meal stimulus. In more than 50% of the cases, tardive postprandial hypoglycemia could be secondary to insulin hypersensitivity Citation[19].
Many authorities have denied the pathological role of postprandial hypoglycemia, at least when this alteration arises in the absence of symptoms. This assumption is, however, in sharp contrast with other well-accepted clinical entities that similarly occur in the absence of symptoms, such as hypoglycemia secondary to the use of insulin or antidiabetic drugs, asymptomatic hyperglycemia and hypercalcemia. When symptomatic postprandial hypoglycemia occurs, it presents with manifestations of increased sympathetic activity, such as anxiety, tremor, dizziness, tachycardia, weakness, increased perspiration and hunger. The symptoms subside with carbohydrate ingestion, but it may be oligosymptomatic or completely asymptomatic.
The mechanism behind tardive hypoglycemia has been predominantly attributed to increased first phase of insulin secretion secondary to IR. As defined above, this condition is not ‘true’ insulin hypersensitivity, for example, a hypoglycemia episode that occurs in the presence of normal or low plasma insulin levels.
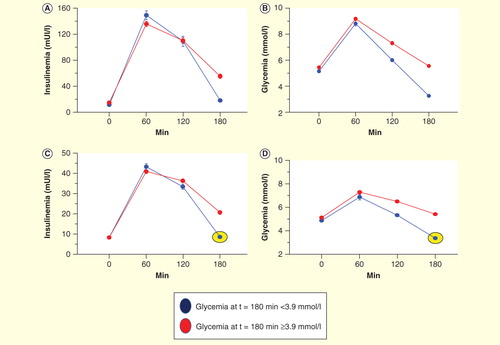
By evaluating insulin and glucose responses during an oral glucose test tolerance (OGTT) performed in our research hospital in a large group of obese and obesity-prone individuals (lean or overweight subjects with a strong family history of obesity) (n = 941) we were able to identify two different types of tardive postprandial hypoglycemia. All patients were not taking any medications potentially affecting insulin sensitivity . The first group showed elevated insulin levels during OGTT (that we have termed ‘hypoglycemia secondary to hyperinsulinemia and IR, , blue line) and the second group exhibited normal or low levels of plasma insulin (that we have termed ‘true’ insulin hypersensitivity group’, , blue lines and circles). Overall, nearly more than a quarter (31%) of the whole population showed tardive hypoglycemia, while 10% of the entire examined population had true insulin hypersensitivity. Interestingly, we observed this late condition predominantly in lean patients prone to the development of obesity.
Figure 3. Graphical results of 941 oral glucose test tolerance (OGTT) studies, according to glucose and insulin response to oral glucose load. Insulin-resistant OGTTs: (A) Insulin curves of normal and insulin-resistant subjects. Blue line indicates insulinemic response of subjects with hypoglycemia (<3.9 mmol/l). (B) Glucose curves of subjects with high insulin responses during OGTT. Red lines indicate normal glucose responses. Insulin-sensitive OGTTs. (C) Insulin curves of subjects with normal and insulin hypersensitivity. Blue lines and circles indicate insulinemic responses of patients with hypoglycemia and low plasma insulin levels (<70 mU/ml at 1 h after glucose load). (D) Glucose curves of subjects with low insulin responses during OGTT. Red lines indicate normal glucose responses.
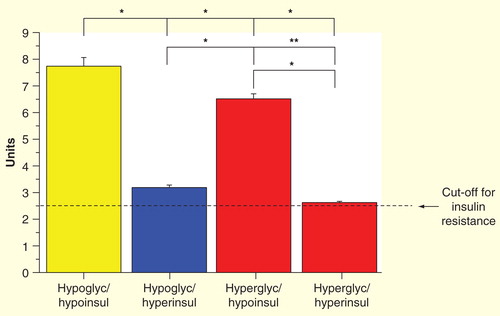
Notably, the insulin sensitivity index, as assessed using the Matsuda method Citation[20], was threefolds higher in low-insulin hypoglycemic patients than the cut-off for IR and it allows to discriminate between IR and insulin hypersensitivity in patients with postprandial hypoglycemia .
Figure 4. Insulin sensitivity index by the Matsuda method: ‘true’ insulin hypersensitive group (yellow bar); hypoglycemic–hyperinsulinemic group (blue bar); and hyperglycemic groups (red bars).
We believe that postprandial hypoglycemia is a ‘true’, underestimated and in many cases, neglected disorder, that can lead to increased chronic caloric consumption and then to obesity. In this regard, it is intriguing to note that in some classic longitudinal studies analyzing risk factors for the development of obesity, increased insulin sensitivity (as measured by the glucose clamp technique) emerges as a potent predictor of obesity Citation[21]. Accordingly, in the EGIR study, a large number of obese individuals exhibited high or normal insulin sensitivity Citation[22]. Also, in a recent article, it has been reported that obesity-prone women were more insulin sensitive compared with a group of lean, non-obesity-prone subjects Citation[23]. In line with these findings, increased insulin sensitivity was found in a group of normal-weight, healthy men with a strong familial history of obesity, Citation[24], suggesting that increased insulin sensitivity could be genetically determined and could be an early predictor of weight gain.
As observed in , insulin hypersensitivity could be seen predominantly after 2 h of oral glucose stimulus. This finding implies that it could be useful to extend the OGTT study to at least 3 h to diagnose a potential hypoglycemic event. We also suggest that insulin level determinations must be added to the usual, simplistic approach of determining plasma glucose concentrations only.
In conclusion, our findings obtained from a large dataset of OGTTs show that postprandial hypoglycemia is not a rare event and could be found in both IR and insulin sensitivity states. Postprandial hypoglycemia could be clinically significant in terms of prediction value for the development of obesity. Early recognition of postprandial hypoglycemia could represent a useful biomarker to identify patients with predisposition to obesity and obesity-related disorders that can culminate in increased cardiovascular mortality.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Finnucane MM, Stevens GA, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557-67
- De Fronzo RA, Ferranini E. Insulin resistance a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173-94
- Dale Abel E, O’Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol 2012;32:2068-76
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 2008;7:95-6
- Rijzewijk LJ, van der Meer RW, Lamb HJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 2009;54:1524-32
- Drosatos K, Schulze PC. Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10:109-12
- Sarafidis PA, Bakris GL. The antinatriuretic effect of insulin: an unappreciated mechanism for hypertension associated with insulin resistance? Am J Nephrol 2007;27:44-54
- Dampney RA. Arcuate nucleus – a gateway for insulin’s action on sympathetic activity. J Physiol 2011;589:2109-10
- Chawla A, Nguyen KD, Sharon Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738-49
- Power ML, Schulkin J. Maternal obesity, metabolic disease, and allostatic load. Physiol Behav 2012;106:22-8
- Pingitore A, Di Bella G, Lombardi M, et al. The obesity paradox and myocardial infarct size. J Cardiovasc Med 2007;8:713-17
- Iozzo P, Rossi G, Michelassi C, et al. Interpretation of the “obesity paradox”: a 30-year study in patients with cardiovascular disease. Int J Cardiol 2013;168:112-16
- Lavie CJ, Milani RV, Ventura HO. Impact of obesity on outcomes in myocardial infarction combating the “obesity paradox”. J Am Coll Cardiol 2011;58:2651-3
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172-7
- Jaffiol C, Baldet L, Torresani J, et al. A case of hypersensitivity to thyroid hormones with normally functioning thyroid gland and increased nuclear triiodothyronine receptors. J Endorinol Invest 1990;13:839-45
- Santen R, Jeng MH, Wang JP, et al. Adaptive hypersensitivity to estradiol: potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Mol Biol 2001;79:115-25
- Malchoff CD, Malchoff DM. Glucocorticoid resistance and hypersensitivity. Endocrinol Metab Clin North Am 2005;34:315-26
- Houssay B. Advancement of knowledge of the role of the hypophysis in carbohydrate metabolism during the last twenty years. Endocrinology 1942;30:884-97
- Khan M, Kabadi UM. Postprandial hypoglycemia. In: Everlon R, editor. Diabetes - damages and treatments. 2011.InTech. Available from: www.intechopen.com/books/diabetes-damages-and-treatments/postprandial-hypoglycemia
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462-70
- Swinburn BA, Nyomba BL, Saad MF, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest 1991;88:168-73
- Ferrannini E, Natali A, Bell P, et al. Insulin Resistance and Hypersecretion in Obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 1997;100:1166-73
- Gower BA, Alvarez JA, Bush NC, et al. Insulin sensitivity affects propensity to obesity in an ethnic-specific manner: results from two controlled weight loss intervention studies. Nutr Metab 2013;10:3-11
- Giacco R, Clemente G, Busiello L, et al. Insulin sensitivity is increased and fat oxidation after a high-fat meal is reduced in normal-weight healthy men with strong familial predisposition to overweight. Int J Obes 2004;28:342-8