Abstract
Evolocumab is a fully human monoclonal IgG2 antibody that inhibits proprotein convertase subtilisin/kexin type 9, a protein that targets LDL receptors for degradation and thereby reduces the liver’s ability to remove LDL-C from the blood. In Phase II and III trials in more than 6000 subjects with primary hypercholesterolemia, evolocumab reduced LDL-C by 50–75% compared with placebo and by 35–45% compared with ezetimibe. Evolocumab reduced the proatherogenic lipid profile, including Lp(a), and modestly increased HDL-C and ApoA1. In subjects with homozygous familial hypercholesterolemia, evolocumab reduced LDL-C by 30%. Safety and tolerability of evolocumab was similar to that of placebo and ezetimibe. The ongoing FOURIER trial, anticipated to report in 2017, will provide definitive evidence on cardiovascular endpoints and additional long-term safety.
The term primary hypercholesterolemia denotes a heterogeneous group of conditions with elevated LDL-C levels. It comprises both autosomal-dominant familial hypercholesterolemia (ADH) with an estimated frequency of 1 per 200–500, and the more common polygenic non-familial hypercholesterolemia, often in combination with adverse environmental factors, such as a hypercholesterolemic diet and obesity. Epidemiological studies have shown a graded relation between the risk of coronary heart disease (CHD) and cholesterol level Citation[1,2], and clinical trials have demonstrated that LDL-C reduction decreases cardiovascular morbidity and mortality Citation[3–6]. International treatment guidelines therefore recommend lowering LDL-C in hypercholesterolemic patients, depending on the individual’s estimated risk for cardiovascular disease, also taking into account other risk factors Citation[7,8].
When added to diet and lifestyle, statins are the current drug of choice for the treatment of hypercholesterolemia. European guidelines from 2011 recommend that LDL-C levels should be below 1.8 mmol/l in high- and very high-risk patients and below 2.5 mmol/l in moderate-risk patients Citation[7]. The American Heart Association/American College of Cardiology guidelines from 2013 recommend treating high-risk patients with high-intensity statins with the aim of lowering LDL-C by at least 50%, and to treat moderate-risk patients with moderate-intensity statins with the aim of lowering LDL-C by 30–50% Citation[8]. Achieving recommended treatment targets can be difficult with current treatment options, especially in familial hypercholesterolemia Citation[9]. In addition, it is estimated that 5–10% of patients are unable to tolerate any statin or an effective dose of a statin mainly because of statin-associated muscle symptoms Citation[10–13].
Serum levels of LDL-C are determined by several factors, including dietary saturated fats, which upregulate cholesterol synthesis in the liver, and uptake of LDL particles in the liver cells by LDL receptors on the surface of hepatocytes. The LDL receptors are recirculated in the hepatocytes, but are eventually degraded. In 2003, the glycoprotein proprotein convertase subtilisin/kexin type 9 (PCSK9) was discovered Citation[14], and shortly after it was reported that ‘gain of function’ mutations in the PCSK9 gene could cause familial hypercholesterolemia Citation[15]. PCSK9, which is synthesized and secreted by the liver, was determined to be involved in the degradation of the LDL receptor, binding to the receptor in serum and targeting it for degradation intracellularly in the lysosome Citation[16,17]. This discovery led to the development of therapeutic monoclonal antibodies that specifically bind to circulating PCSK9, neutralizing the protein and thereby inhibiting degradation of the LDL receptor Citation[18]. Currently, three monoclonal antibodies targeting PCSK9 are in Phase III development, evolocumab, alirocumab and bococizumab. Evolocumab is a fully human IgG2 monoclonal antibody, alirocumab is a fully human IgG1 monoclonal antibody and bococizumab is a humanized monoclonal antibody against PCKS9. In Phase I and Phase II studies, treatment with anti-PCSK9 antibodies were highly effective in reducing LDL-C levels, with few side effects Citation[19–29]. In addition, other approaches to inhibiting PCSK9 are in pre-clinical and Phase I development, such as PCSK9-binding peptides and small proteins (adnectins) as well as antisense and small interfering RNA (siRNA), interfering with PCSK9 mRNA and inhibiting translation Citation[30–33].
Introduction to evolocumab
Evolocumab is a fully human monoclonal IgG2 antibody with high binding affinity for PCSK9 (KD = 16 pM). As a human IgG2 antibody, the clearance of evolocumab is mediated by both nonlinear and linear pathways. The nonlinear pathway is because of specific binding and complex formation with its target ligand, PCSK9, and therefore predominates at low evolocumab serum concentrations when there is insufficient evolocumab to completely bind all circulating PCSK9. When serum-unbound evolocumab is in excess, the nonlinear pathway is saturated and the majority of clearance is mediated by the typical IgG clearance processes in the reticuloendothelial system. This process results in linear elimination. In both instances, evolocumab is eventually degraded into small peptides and amino acids via these catabolic pathways.
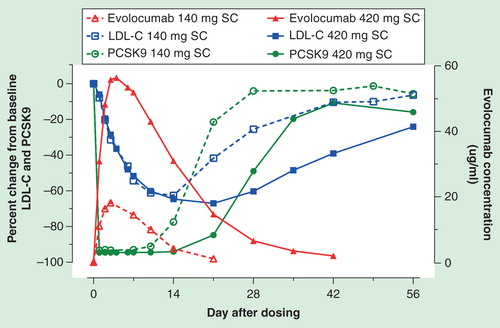
The relationship between unbound evolocumab, unbound PCSK9 and LDL-C levels in the serum following a single subcutaneous (SC) dose is illustrated in . Absorption from a SC dose of evolocumab gives rise to peak serum concentrations in 3–4 days after administration. Within 4 h of SC administration of evolocumab, there is rapid and maximal suppression of circulating unbound PCSK9 to undetectable levels. This suppression is followed by a dose-dependent reduction in LDL-C that reaches a maximum lowering effect with evolocumab doses ≥140 mg; higher doses extend the duration of lowering. The mean nadir in LDL-C response occurs between 7 and 21 days. PCSK9 continues to be produced and secreted by the liver, contributing to the elimination of evolocumab. The amount of unbound evolocumab eventually declines and unbound PCSK9 reappears in the circulation followed by a return in LDL-C toward baseline because of the PCSK9 role in LDL receptor degradation.
Figure 1. Relationship between unbound evolocumab (AMG 145), unbound PCSK9 and LDL-C following a 140 or 420 mg subcutaneous dose of evolocumab. Reproduced with permission from Amgen.
In clinical studies with repeated SC dosing of evolocumab over 12 weeks, dose-proportional increases in exposure were observed with dose regimens of ≥140 mg. No time-dependent changes were observed in unbound evolocumab concentrations over a period of 52 weeks and changes in unbound PCSK9 and serum lipoproteins were reversible upon discontinuation of evolocumab. No increase in unbound PCSK9 or LDL-C above baseline was observed after cessation of evolocumab, suggesting that compensatory mechanisms to increase production of PCSK9 and LDL-C do not occur during treatment. Population pharmacokinetic analyses suggest that no dose adjustments are necessary for age (18–79 years), gender, race/ethnicity, statin therapy, baseline PCSK9 and body weight.
Evolocumab dose regimens in clinical efficacy studies deliver approximately 80% of the theoretical maximum LDL-C lowering from PCSK9 inhibition by this mechanism. Unlike therapies that are given on a daily basis, convenient biweekly or monthly SC dosing schedules for hypercholesterolemic patients on current anti-PCSK9 monoclonal antibody therapies under investigation, like evolocumab, give rise to u-shaped LDL-C lowering over the dosing interval with deeper u-shapes and more variability observed with lower doses. The magnitude of these changes is dependent on the dose, dose interval and specific monoclonal antibody binding characteristics toward PCSK9. Thus, the average LDL-C lowering over the dosing interval is an important measure of efficacy given the pharmacodynamic profile of anti-PCSK9 antibodies.
Clinical efficacy
Phase II trials
An extensive Phase II program evaluating the safety and efficacy of evolocumab in primary hypercholesterolemia (familial and non-familial) was completed Citation[22–26,28,34]. The studies assessed dosing regimens ranging from 70 to 140 mg SC every 2 weeks (Q2W) and/or 280 to 420 mg SC monthly (QM) in combination with statins with or without ezetimibe and as an adjunct to diet alone in hypercholesterolemic subjects with cardiovascular disease or at cardiovascular risk. In particular, the safety and efficacy of evolocumab was studied in combination with statins in patients with heterozygous familial hypercholesterolemia (HeFH), homozygous familial hypercholesterolemia (HoFH), non-familial hypercholesterolemia and in high-risk Japanese subjects, in statin-intolerance, and as an adjunct to diet (i.e., monotherapy). The 12-week duration Phase II studies were all double-blind, randomized, placebo- and/or ezetimibe-controlled trials except for TESLA Part A, which was a single arm, open-label pilot study to determine whether evolocumab would lower LDL-C in HoFH subjects. The long-term, open-label study, OSLER, allowed patients completing LAPLACE-TIMI 57, RUTHERFORD, GAUSS, MENDEL or YUKAWA to be randomized 2:1 to evolocumab plus standard-of-care (SoC) or SoC alone for 1 year and then receive evolocumab plus SoC for up to four additional years. The open-label extension trial, TAUSSIG Citation[35], facilitated collection of long-term safety and efficacy data in HoFH subjects completing TESLA Part A as well as de novo HoFH and HeFH subjects, including those on apheresis.
Table 1. Phase II trial summaries.
In nearly 1700 Phase II subjects with primary non-familial and heterozygous familial hypercholesterolemia, evolocumab 140 mg Q2W and 420 mg QM resulted in significant reductions in LDL-C of approximately 50–70% compared with placebo and approximately 35–40% compared with ezetimibe Citation[22–26,28]. Significant reductions in proatherogenic lipid parameters, including Lp(a), and increases in anti-atherogenic lipid parameters were observed. Safety and tolerability for all dosing regimens evaluated were similar and comparable with placebo or ezetimibe among all of the populations studied. No clinically meaningful differences in efficacy or safety were observed for the 140 mg Q2W and 420 mg QM SC dosing regimens. It was concluded that these two dosing regimens were clinically equivalent and were chosen to be further studied in Phase III. Finally, the effects on LDL-C and other lipid parameters observed at week 12 in OSLER were maintained with long-term evolocumab administration and were reversible upon cessation of treatment with no evidence of rebound Citation[24].
In eight subjects with HoFH in TESLA Part A, a significant reduction in LDL-C of 17% compared with baseline was observed with evolocumab; however, no reduction in LDL-C was observed in two receptor-negative subjects Citation[34]. The safety and tolerability profile of evolocumab in these eight subjects was consistent with that seen in the primary non-familial and heterozygous familial hypercholesterolemia population. Given this efficacy and safety profile in HoFH, 420 mg QM was studied in the double-blind, randomized, placebo-controlled Phase III TESLA Part B trial Citation[36].
Phase III trials
The completed Phase III program along with the ongoing open-label extension study OSLER-2 Citation[37] is outlined in Citation[36,38–42]. These studies enrolled over 4000 subjects with primary non-familial and heterozygous familial hypercholesterolemia as well as approximately 50 HoFH subjects. The 12- and 52-week duration randomized, double-blind, placebo- and/or ezetimibe-controlled studies evaluated the safety and efficacy of evolocumab on high- or moderate-intensity statins, other lipid-lowering therapies, and diet alone. Subjects included those with HeFH, HoFH, statin-intolerance and non-familial hypercholesterolemia. Subjects completing the primary non-familial and heterozygous familial hypercholesterolemia Phase III trials were eligible to enroll in the long-term extension study, OSLER-2, where the first year included a SoC control arm. Patients with HoFH completing TESLA Part B were eligible to participate in the one-arm open-label extension study TAUSSIG.
Table 2. Evolocumab Phase III trial summaries.
Similar to the Phase II results, the Phase III data demonstrate that evolocumab 140 mg Q2W and 420 mg QM resulted in significant reductions in LDL-C of approximately 55–75% compared with placebo Citation[36,38–41] and approximately 35–45% compared with ezetimibe . This consistent response was observed regardless of baseline characteristics such as age, race, gender, body mass index, cardiovascular risk, PCSK9 level and statin dose/intensity Citation[40–42]. No clinically meaningful differences between evolocumab doses of 140 mg Q2W and 420 mg QM were observed with respect to reducing LDL-C, total cholesterol, ApoB, non-HDL-C, VLDL-C, triglycerides, Lp(a), total cholesterol/HDL-C and ApoB/ApoA1, and increasing HDL-C and ApoA1 . As observed in OSLER, the effect of evolocumab on LDL-C reduction and improvements in other lipid parameters were maintained with long-term evolocumab administration Citation[24,39].
Table 3. Effect of evolocumab compared with placebo on lipid parameters in the Phase III trials.
Table 4. Effect of evolocumab compared with ezetimibe on lipid parameters in the Phase III trials.
In 49 subjects with HoFH, a Phase III double-blind, placebo-controlled study (TESLA Part B) demonstrated statistically significant reductions in LDL-C of approximately 30% compared with placebo Citation[36]. Improvements in other lipid parameters (total cholesterol, ApoB and non-HDL-C) were also observed. Early data in the long-term extension study, TAUSSIG, suggest that these changes in lipid parameters were maintained with long-term treatment in HoFH subjects Citation[43]. Furthermore, it appears that in HoFH subjects, who typically have higher baseline PCSK9 levels, a dose of 420 mg Q2W provides additional LDL-C reduction compared with 420 mg QM. This observation will require further validation in the ongoing TAUSSIG study.
Safety & tolerability
The safety and tolerability of evolocumab in the nearly 1700 hyperlipidemic subjects from the seven Phase II trials described in were published previously Citation[22–26,28,34,44]. In six Phase III trials that enrolled over 4000 non-familial and familial hyperlipidemic subjects, the safety and tolerability of evolocumab is similar to that of the comparator (placebo and/or ezetimibe) and with that observed in the Phase II program Citation[36,38–42]. The overall incidence of treatment-emergent adverse events (TEAEs) in patients receiving evolocumab was comparable with those receiving control (placebo and/or ezetimibe). Among the 12-week duration studies, the incidence of TEAEs was modestly higher, but balanced, in the statin-intolerance study, GAUSS-2 (ezetimibe 73%; evolocumab 66%) compared with the other 12-week studies (placebo 39, 44 and 49%; ezetimibe 40 and 45%; evolocumab 36, 44 and 56%). In the 52-week DESCARTES trial, the incidence of TEAEs was balanced between evolocumab (75%) and placebo (74%). The incidence of serious AEs was low and balanced ranging from 1–3% on evolocumab to 1–5% on placebo or ezetimibe in the 12-week duration studies. In the 52-week DESCARTES study, rates of serious AEs were 6 and 4% in the evolocumab and placebo arms, respectively. TEAE that led to discontinuation of investigational product were low (≤2%) in the evolocumab and comparator (≤4%) arms except in the statin-intolerance study where the rate was 8% in the evolocumab arm and 13% in the ezetimibe arm.
Evaluation of the most common TEAEs (≥6%) across the trials did not identify safety issues Citation[36,38–42]. TEAEs were generally balanced between evolocumab and comparator. Of note, the incidence of myalgia was balanced and low (<4%) in the 12- and 52-week duration trials except in the double-blind, double-dummy statin-intolerant study GAUSS-2, where the incidence of myalgia on evolocumab was 8% compared with 18% on ezetimibe. A standardized Medical Dictionary for Regulatory Activities (MedDRA) search strategy for TEAEs that could be muscle-related AEs revealed a pattern similar to what was observed with the adverse event of myalgia alone. Although evolocumab is administered as a SC injection, a standardized MedDRA search strategy was performed for possible injection site reactions. Using this search strategy, the incidence of TEAEs that could be injection site reactions ranged from 1 to 8% on control and 1 to 6% on evolocumab. No clinically meaningful difference was observed between control and evolocumab. Given concerns around statins, low LDL-C and neurocognitive dysfunction, a standardized MedDRA search strategy for TEAEs that could be related to neurocognitive dysfunction was performed. These events were rare; no clinically meaningful difference was noted between control and evolocumab.
Table 5. Subject incidence of safety and tolerability of evolocumab in Phase III trials.
Laboratory analyses did not identify safety issues. Elevation of creatine kinase (CK) and increases in transaminases were infrequent and balanced between evolocumab and control. In the Phase III evolocumab program, there were no cases of anti-evolocumab neutralizing antibodies and the development of anti-evolocumab binding antibodies was rare. Among the 2480 subjects receiving evolocumab in Phase III, only one subject tested positive for the development of new anti-evolocumab binding antibodies. The absence of neutralizing antibodies to evolocumab and the rare (<1%) observation of binding antibodies is consistent with what was observed in the Phase I and II evolocumab trials.
Cardiovascular events, composed of death by any cause, cardiovascular death, myocardial infarction, hospitalization for unstable angina, coronary revascularization, stroke, hospitalization for heart failure or transient ischemic attack were adjudicated. These events were infrequent. There was no significant imbalance in events between control and evolocumab Citation[45].
Conclusion
The accumulated data with evolocumab presented here in nearly 6000 subjects with primary non-familial and heterozygous familial hypercholesterolemia demonstrates that evolocumab consistently reduces LDL-C by 50–75% compared with placebo and by 35–45% compared with ezetimibe. This result is consistent across the populations studied which ranged from subjects with atherosclerotic cardiovascular disease on high-intensity statin therapy with ezetimibe to subjects with cardiovascular risk being managed by diet alone. Evolocumab reduced the proatherogenic lipid profile, including a 20–33% reduction in Lp(a), and resulted in modest increases in HDL-C and ApoA1. In receptor-defective HoFH subjects, evolocumab reduced LDL-C by approximately 30% compared with placebo; favorable, clinically meaningful changes were noted in the lipid profile in HoFH subjects, although the magnitude of these changes was less marked than in non-HoFH subjects. One patient with two receptor negative mutations and one patient with autosomal recessive HoFH did not respond to evolocumab treatment. This observation appears to be consistent with the mechanism of action, which requires some degree of LDL-receptor functionality.
Based on the accumulated safety data in the Phase II and Phase III program, the safety profile of evolocumab appears similar to comparator. TEAE were balanced between evolocumab and control with the overall incidence of TEAE leading to discontinuation of investigational product and serious AEs being low and balanced. A careful evaluation of muscle-related AEs, abnormalities in CK and transaminases and anti-evolocumab antibodies was unremarkable. Although the number of cardiovascular events was modest, there does not appear to be evidence of harm and the first examination of long-term data in OSLER was encouraging Citation[24]. Definitive evidence will be provided from the ongoing FOURIER trial Citation[46].
Expert commentary
PCSK9-inhibition with monoclonal antibodies is a new, powerful tool to reduce LDL-C in high-risk patients. The changes in the lipid profile compare favorably to what is achieved with high-intensity statin therapy with the exception of PCSK9-mediated reduction of Lp(a). Importantly, the impact of LDL-C reduction by PCSK9 inhibition on cardiovascular outcomes is unknown and currently under investigation in large clinical trials.
Current data supports the cardiovascular benefits of marked LDL-C reduction. There are observational data that proatherogenic non-LDL-C lipids may contribute to the pathogenesis of atherosclerosis and clinical manifestations of cardiovascular disease. There has been discussion of the impact of remnant particles as well as Lp(a) on cardiovascular outcomes. With regards to remnant particles, current data with evolocumab indicate that remnant particles will be reduced as well. There are some genetic data that suggest that other proatherogenic lipids, particularly Lp(a), may contribute to manifestations of atherosclerosis, particularly as it relates to aortic stenosis. The impact of PCSK9 is most marked on LDL-C reduction and it is clear that LDL-C is a major modifiable cardiovascular risk factor. Thus, it will be challenging to dissect the proven cardiovascular benefits of marked LDL-C reduction from the other potentially beneficial effects on lipids, such as the reduction in Lp(a) of approximately 20–30% and the reduction in triglycerides of up to approximately 20%. Studies specifically recruiting hypertriglyceridemic patients have not been performed, but the drug could possibly also become a treatment option in difficult-to-treat hypertriglyceridemic patients.
To date, the safety and tolerability in the trials show adverse event profiles comparable between evolocumab and comparators and low rates of discontinuation and serious AEs. Careful evaluation of the safety database has not revealed evidence of muscle-related side effects, neurocognitive or glycemic impairment, issues with anti-evolocumab antibodies or laboratory evidence of muscle or liver injury. Nevertheless, evolocumab and other anti-PCSK9 antibodies allow for achievement of LDL-C levels not previously attainable. Although there are data beyond 1 year, the long-term effects of low and very low levels of LDL-C are unknown. PCSK9 is also expressed in the nervous system, intestines and pancreas and long-term PCSK9 inhibition could possibly affect these organ systems.
Concerns emerged related to an increased risk of diabetes and neurocognitive dysfunction associated with statin therapy. Thus, theoretical safety issues for long-term PCSK9 inhibition relating to cellular membrane integrity, levels of fat-soluble vitamins, steroid hormones, diabetes and possible neurological side effects need to be addressed. In the 52-week results from the OSLER open-label study, overall TEAEs, serious AEs and elevations of CK and aminotransferases were not appreciably greater in those who achieved low (<1.3 mmol/l) and very low (<0.65 mmol/l) LDL-C levels, compared with those with LDL-C ≥1.3 mmol/l Citation[24]. Analyses of OSLER and DESCARTES have not identified safety issues with fat-soluble vitamins, steroid hormone biosynthesis, glycemic parameters or neurocognitive function. These observations regarding vitamin and steroid hormones are not unexpected because intracellular cholesterol originates primarily via intracellular synthesis and is not reliant on circulating LDL-C. Heterozygous PCSK9 loss-of-function mutations, observed in approximately 1–4% of the population, have been associated with a reduced risk of CHD, whereas increased morbidity for other conditions, such as cognitive performance, has not been observed Citation[47,48]. Furthermore, a woman with loss-of-function PCSK9 mutations in both genes (compound heterozygote), and LDL-C of approximately 0.4 mmol/l is healthy, fertile and without evidence of adverse consequences of life-long very low LDL-C Citation[49]. While these observations are encouraging, larger and long exposure to PCSK9 inhibition and achievement of very low LDL-C levels will enhance our confidence in clinical and observational data.
Anti-PCSK9 antibodies are injectable protein therapeutics and as such carry the risk of anti-drug antibody development, allergic reactions and injection site reactions. The incidence of anti-evolocumab binding antibodies is rare and there were no neutralizing antibodies. The rare occurrence of anti-evolocumab binding antibodies was not associated with allergic reactions or impaired pharmacokinetics or pharmacodynamics in the clinical program. Clinical trial data show that the incidence of injection site reactions was low and similar to placebo. The majority of injection site reactions were mild and did not impact adherence to therapy.
Five-year view
Large cardiovascular outcomes trials with PCSK9 inhibitors are being conducted and results are anticipated as early as 2017. These studies will be instrumental in defining the place for PCSK9 inhibitors in the treatment of hypercholesterolemia. Until then, if approved and available as anticipated in 2015–2016, these drugs will probably be reserved for use in difficult-to-treat patients, that is, the substantial number of ADH patients not responding sufficiently to treatment with statins and ezetimibe Citation[50–53]. Patients with ADH and CHD should be treated to LDL-C levels below 1.8 mmol/l (70 mg/dl), which is extremely difficult to achieve in these high-cholesterol patients. If approved, the drug will probably also be used in secondary prevention in other difficult-to-treat primary hypercholesterolemia patients, such as those with recurrent cardiovascular events, as well as in the 5–10% of patients who are unable to tolerate statins. While much has been made of the challenge of defining statin-intolerant patients, the approach employed in clinical trials to date has been pragmatic based on what clinicians do when encountering patients with statin-associated muscle symptoms. Physicians switch to another statin and usually try several statins before endeavoring to find a low or atypical dose regimen that a patient can tolerate. To date, these trials enrolled subjects with a mean LDL-C of approximately 4.9 mmol/l (190 mg/dl) and significant cardiovascular risk Citation[42,54]. PCSK9 inhibitors may represent an impactful solution for these patients.
Evolocumab and similar PCSK9 inhibitors open the possibility to lower LDL-C to levels well beyond current treatment targets. The ongoing endpoint studies should provide data to address questions about the level of low LDL-C associated with clinical benefit and the long-term safety of low LDL-C. If these trials show a substantial benefit in cardiovascular disease reduction and there are no major long-term safety issues, these drugs could turn out to be the next lipid-lowering drug of choice after statins.
Cost of the PCSK9-inhibitors have not been determined, but could be anticipated to be on par with recombinant monoclonal antibodies for use in other therapeutic areas. Competition between pharmaceutical companies and focus on cost–effectiveness will hopefully reduce the price. Widespread use is not medically warranted until results from the large outcomes studies become available. If substantial benefits in CVD reduction are achieved, treatment with PCSK9 inhibitors in a wider group of patients could prove to be cost-effective.
Given the excitement around the PCSK9 target and its biology, the alternative mechanisms to PCSK9 inhibition will continue to be explored.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a circulating protein secreted by the liver that regulates LDL-C uptake by binding to the LDL-C receptor, targeting the receptor for degradation in the lysosomes.
Evolocumab is a fully human monoclonal IgG2 antibody with high binding affinity for PCSK9 that binds PCSK9 and prevents lysosomal degradation of the LDL-C receptor.
Evolocumab reduced LDL-C by 50–75% compared with placebo and by 35–45% compared with ezetimibe in Phase II and III studies.
Safety and tolerability of evolocumab in trials of up to 52 weeks duration were similar to that of placebo and ezetimibe.
Results from FOURIER, a large cardiovascular endpoint trial, are anticipated no later than 2017.
If cardiovascular endpoints are favorable and no long-term safety and tolerance issues emerge, anti-PCSK9 antibodies could revolutionize lipid-lowering treatment in difficult-to-treat hypercholesterolemic patients, allowing patients to attain LDL-C levels not achievable with conventional treatment.
Financial & competing interests disclosure
G Langslet has received moderate advisory board fees from Amgen, Sanofi and Janssen Pharmaceuticals. M Emery is an employee of Amgen and thus receives salary, stock and benefits from Amgen. S Wasserman is an employee of Amgen and thus receives salary, stock and benefits from Amgen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Meera Kodukulla was involved in the editorial and formatting of this document. Mary Elliott, Thomas Liu and Jingyuan Yang were involved in the statistical quality control of this document.
References
- Keys A. Seven countries. A multivariate analysis of death and coronary heart disease. Harvard University Press; Massachusetts: 1980
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78
- Buchwald H, Varco RL, Boen JR, et al. Effective lipid modification by partial ileal bypass reduced long-term coronary heart disease mortality and morbidity: five-year posttrial follow-up report from the POSCH. Program on the Surgical Control of the Hyperlipidemias. Arch Intern Med 1998;158:1253-61
- Cannon CP. Improve IT Investigators. IMPROVE-IT trial: a comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndromes. Presented at the American Heart Association Scientific Sessions, November 17, 2014. Chicago, IL; Abstract 2014
- Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011;124:2202-7
- European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (esc) and the European atherosclerosis society (EAS). Eur Heart J 2011;32:1769-818
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;129:S1-45
- Huijgen R, Kindt I, Verhoeven SB, et al. Two years after molecular diagnosis of familial hypercholesterolemia: majority on cholesterol-lowering treatment but a minority reaches treatment goal. PLoS One 2010;5:e9220
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients – the PRIMO study. Cardiovasc Drugs Ther 2005;19:403-14
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011;78:393-403
- Pasternak RC, Smith SCJr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567-72
- Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015. [Epub ahead of print]
- Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 2003;100:928-33
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154-6
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009;50(Suppl):S172-7
- Qian YW, Schmidt RJ, Zhang Y, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res 2007;48:1488-98
- Chan JC, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA 2009;106:9820-5
- Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891-900
- Ballantyne C, Neutel J, Cropp A, et al. Efficacy and safety of bococizumab (RN316/PF-04950615), a monoclonal antibody against proprotein convertase subtilisin/kexin type 9 in statin-treated hypercholesterolemic subjects: Results from a randomized, placebo-controlled, dose-ranging study (NCT 01592240). J Am Coll Cardiol 2014;63(Suppl):Abstr 1374
- Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol 2012;60:1888-98
- Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012;380:2007-17
- Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J 2014;78:1073-82
- Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 2014;129:234-43
- Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2012;380:1995-2006
- Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012;126:2408-17
- Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29-36
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012;308:2497-506
- McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344-53
- Zhang Y, Eigenbrot C, Zhou L, et al. Identification of a small peptide that inhibits PCSK9 protein binding to the low density lipoprotein receptor. J Biol Chem 2014;289:942-55
- Mitchell T, Chao G, Sitkoff D, et al. Pharmacologic profile of the Adnectin BMS-962476, a small protein biologic alternative to PCSK9 antibodies for low-density lipoprotein lowering. J Pharmacol Exp Ther 2014;350:412-24
- Borodovsky A, Querbes W, Yucius K, et al. Development of monthly to quarterly subcutaneous administration of RNAi therapeutics targeting the metabolic disease genes PCSK9, ApoC3 and ANGPTL3. Presented at the American Heart Association Scientific Sessions, November 17, 2014. Chicago, IL; Abstract 11936
- Stein E, Kasichayanula S, Turner T, et al. LDL cholesterol reduction with BMS-962476, an adnectin inhibitor of PCSK9: results of a single ascending dose study. J Am Coll Cardiol 2014;63(Supp):Abstr 1372
- Stein EA, Honarpour N, Wasserman SM, et al. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation 2013;128:2113-20
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). Available from: https://clinicaltrials.gov/ct2/show/NCT01624142
- Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:341-50
- Open label study of long term evaluation against LDL-C Trial-2 (OSLER-2). Available from: https://clinicaltrials.gov/ct2/show/NCT01854918
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331-40
- Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809-19
- Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2531-40
- Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014;311:1870-82
- Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541-8
- Harada-Shiba M, Kishimoto I, Makino H, et al. Efficacy of evolocumab (AMG 145) in patients with PCSK9 gain-of-function mutations. Atherosclerosis 235, e11. Abstract 2014
- Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J 2014;35:2249-59
- Blom DJ, Wasserman SM, Stein EA. Evolocumab in hyperlipidemia. N Engl J Med 2014;371:877-8
- Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER). Available from: https://clinicaltrials.gov/ct2/show/NCT01764633
- Cohen JC, Boerwinkle E, Mosley THJr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264-72
- Postmus I, Trompet S, de Craen AJ, et al. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res 2013;54:561-6
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 2006;79:514-23
- Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial hypercholesterolaemia of the European atherosclerosis society. Eur Heart J 2014;35:2146-57
- Watts GF, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Eur J Prev Cardiol 2014. [Epub ahead of print]
- Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478-90a
- Pijlman AH, Huijgen R, Verhagen SN, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis 2010;209:189-94
- Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody, alirocumab, versus ezetimibe, in patients with statin intolerance as defined by a placebo run-in and statin rechallenge arm. Presented at the American Heart Association Scientific Sessions, November 17, 2014. Chicago, IL; Abstract 20773
