Abstract
Robotic percutaneous interventional systems represent the future of interventional cardiology. Robotic technology significantly reduces operator exposure to ionizing radiation, and improves ergonomics in the cardiac catheterization laboratory, thereby preventing orthopedic injuries. It may enable more accurate stent implantation and reduce the incidence of contrast-induced nephropathy. Robotics has the potential of converting the current ‘high-risk’ catheterization laboratory into a safe, physician- and patient-friendly environment.
Andreas Gruntzig pioneered percutaneous coronary intervention (PCI) almost four decades ago, describing his findings in a landmark article published in the New England Journal of Medicine in 1979 Citation[1]. Since then, catheter-based interventions have revolutionized the management of cardiovascular medicine. Technical innovations have led to increasingly complex coronary interventions, new approaches to treatment of peripheral vascular and structural heart disease, and numerous other procedures performed under fluoroscopy by interventional cardiologists, radiologists and vascular surgeons. Clinical outcomes continue to improve, leading to application of the percutaneous interventions to an ever-growing number of patients. Despite all these advances, the basic approach of performing the procedure has not changed much. The interaction of the operator with the patient and the basic workflow of the procedure are very similar to how it was performed almost four decades ago.
So, why consider changing the basic design and ergonomics of the procedure? The answer lies in the impact of these procedures on the first generation of interventional cardiologists, who have been exposed to the effects of performing these procedures for the last 25–30 years. It has become clear that the cardiac catheterization laboratory is a ‘high-risk’ environment for the operators. Day after day, the operator is exposed to three major health risks: ionizing radiation, orthopedic trauma and infectious hazards. As the volume of PCI grows, operators are spending more time in the catheterization laboratory. In addition, increasing complexity of these procedures means that the average procedure time has also significantly increased over the years, exposing the operators to increased risk on a daily basis Citation[2].
The Society for Cardiovascular Angiography and Interventions, in conjunction with other professional societies, has made great efforts to publicize the occupational hazards to the interventionalist and has made recommendations to minimize the risk to operators Citation[3]. Despite application of these strategies, the interventional operator continues to be exposed to a high-risk environment. Thus, it has become apparent that it is time to consider a fundamental change to the way these procedures are performed.
Occupational hazards to the interventional cardiologist
Ionizing radiation
One of the largest and most serious concerns is the exposure of personnel in the catheterization laboratory to ionizing radiation Citation[4]. Although radiation dosage is closely monitored with radiation badges, there is no established minimum safety threshold Citation[5]. Radiation-induced malignancies remain the most feared long-term complication to the interventional cardiologist, and there is mounting evidence to suggest that there is significant impact on the molecular level to physicians exposed to radiation in the catheterization laboratory. Interventional cardiologists develop DNA damage and chromosomal abnormalities at a significantly higher rate than non-invasive cardiologists Citation[6]. The degree of DNA damage correlates with the number of years spent in the catheterization laboratory. Epidemiological studies of occupational radiation exposure from the 1950s identified an increased risk of death from leukemia, skin cancer and breast cancer Citation[7]. Lead aprons are now required to be worn in the catheterization laboratory; despite this shielding, the brain remains one of the most exposed organs during interventional procedures. Prior exposure to ionizing radiation is an established risk factor for brain tumors. Recently, there have been case reports of interventionalists with left hemisphere brain malignancies Citation[8]. This has led to significant safety concerns, as operators typically receive higher doses of radiation to the left side of the body.
Radiation-induced cataracts are another major concern. Multiple studies have documented increased rate of cataracts in interventional cardiologists compared with controls not exposed to fluoroscopy Citation[9]. Physicians who do not wear eye protection are at particular risk and can develop cataracts with only a few years of clinical practice.
Orthopedic trauma
Long procedure times and heavy lead aprons take a significant toll on the interventional cardiologist. Musculoskeletal back pain and spinal disc disease are so common among these physicians that these conditions are commonly referred to as ‘interventionalists’ disc disease’ Citation[10]. Multiple recent studies have documented an alarming prevalence of orthopedic problems among physicians working in the catheterization laboratory. Protective lead can weigh up to 10 lbs and lead aprons can generate intervertebral disc pressures as high as 300 pounds/square inch Citation[11]. The commonest orthopedic problems are lumbosacral and cervical disc disease, and hip, knee and ankle arthritis. Physicians with more than 20 years of experience have a very high rate of back pain with almost 60% reporting spinal disc disease. More than one-third of physicians with spinal problems miss work due to their symptoms. Despite recognition of all these orthopedic risks, conditions in the cardiac catheterization laboratory continue to cause significant musculoskeletal injuries.
Infectious hazards
Despite increased awareness, needle stick and sharps injuries are widely prevalent in the catheterization laboratory. This is an often overlooked risk to the catheterization laboratory staff. Accidental exposure can transmit blood-borne pathogens to the operator, including hepatitis B virus, hepatitis C virus and HIV. Percutaneous procedures are associated with a needle stick injury rate of 0.6% and a glove perforation rate of 1% Citation[12].
Robotic PCI
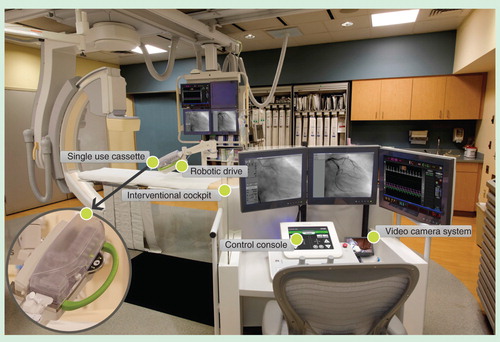
Robotic catheter-based systems that enable remote-control interventions have the potential to mitigate some of the hazards of practicing interventional cardiology. Robotic systems also have the potential to improve stability, precision and accuracy of coronary and vascular interventions. Medical robotic technologies were first developed for use in surgical procedures, and are currently successfully utilized in robotic-assisted mitral valve repair and robotic coronary artery bypass grafting. The first application of robotics in cardiology was in the electrophysiology laboratory. The system utilized external magnetic fields to perform electrophysiological mapping and radiofrequency ablation Citation[13]. Subsequently a system was developed which worked without magnetic navigation (SenseiRobotic Catheter System, Hansen Medical, Mountain View, CA, USA). Successful application of robotics in electrophysiology was quickly followed by using robotic technologies for remote coronary interventions. The first-generation device was developed by Beyar et al., and consisted of a joystick-controlled operator module connected to a tabletop-mounted motorized drive used to navigate intravascular devices. The first-in-man clinical study of robotic PCI utilizing this device was reported by Beyar et al. in 2006 Citation[14]. After refinements and multiple modifications, this robotic system has been further developed and redesigned as the CorPath Vascular Robotic system (. This system allows interventional cardiologists to perform remote-controlled coronary interventions while being seated in an ergonomic and radiation shielded cockpit, which can be positioned either in the catheterization laboratory or in the control room. The physician sits in a radiation-free environment, without the need for heavy lead aprons. The operator interface consists of a touchscreen console, joysticks to control movements of intravascular devices and a remote-control contrast delivery system. Fluoroscopic images and hemodynamic parameters are relayed to monitors within the cockpit. An articulated arm mounted to the bedside contains a robotic drive with a sterile single-use cassette. The cassette can control standard coronary wires as well as angioplasty balloons and stents.
The system has two major components: cockpit and bedside unit.
The interventional cockpit is a radiation-shielded workstation that is mobile and can be positioned anywhere in the catheterization laboratory. It allows the interventional cardiologist to perform the PCI procedure remotely from the control console while sitting in the cockpit unit. This is an open architecture system and is compatible with 0.014 inch guide wires, rapid exchange catheter systems and other standard catheterization laboratory hardware. The system allows manipulation of the guide wire, balloon and stents with one hand, while operating the automatic contrast injector with the other hand.
The bedside unit includes a bedrail-mounted articulated arm supporting the robotic drive with an attached single-use cassette. The robotic drive is connected to the cockpit via a communication cable.
The procedure is started by obtaining vascular access manually utilizing standard technique. A standard guiding catheter is manually introduced, and the target coronary artery is selectively cannulated using standard manual technique by the operator. The guide catheter is manually connected to the Y-connector, which is placed manually into the Y-connector holder of the cassette. The guide catheter between the Y-connector and the skin entry site is supported by an adjustable robotic extension arm. The coronary guide wire is manually introduced through the Y-connector into the guiding catheter and the proximal end is loaded into the cassette. From here onward, the operator, via the control console, is capable of controlling the guide wire robotically. This offers linear and rotational movement; so the device can be advanced, retracted and rotated. After coronary guide wire introduction using the robotic system, the operator or assistant manually loads a rapid exchange coronary angioplasty balloon into the system. The balloon can then be advanced to the coronary lesion with robotic precision in order to perform dilatation of the target lesion by standard technique. Subsequently, the balloon is retracted with the robotic system and exchanged for a rapid exchange stent. The procedure for stent insertion, deployment and retrieval is performed in a similar fashion. Angiography is then performed from the cockpit to assess the final angiographic result.
The system allows the operator to switch to a standard manual approach if required. In case of an emergency, complication or failure of the robotic system, the operator has the ability to switch to a traditional manual intervention seamlessly and without significant delay. It is important to emphasize that the system is not designed to replace traditional interventional training, but is engineered to be complimentary to it. Training for the system is provided on site by the manufacturer. It is estimated that it takes three to five cases to adapt to the cardiac catheterization laboratory workflow and get proficient with device manipulation. It takes a minimum of 15–20 cases to gain proficiency to perform complex cases. It is important to note that the robotic system is likely more valuable in long and complex cases where there is prolonged procedural time and significant radiation exposure to the interventionalist.
The safety, feasibility and technical performance of robotic PCI was evaluated in the PRECISE trial Citation[15]. This was a prospective, single-arm, multi-center registry that demonstrated the safety and feasibility of the CorPath system. A total of 164 patients with coronary artery disease and clinical indications for percutaneous intervention were enrolled at nine sites. The primary endpoints were clinical procedural success, defined as <30% residual stenosis at the completion of the robotic-assisted procedure without major adverse cardiovascular events within 30 days, and device technical success, defined as the successful manipulation of intracoronary devices using the robotic system only. Major inclusion criteria included a de novo stenosis of at least 50% by visual estimate, with maximal length of 24 mm and reference diameter of 2.5–4.0 mm, which could be completely covered by a single stent. Major exclusion criteria included planned PCI or coronary artery bypass graft surgery, required treatment of more than one coronary artery, previous stent implantation within 5 mm of the target lesion, planned treatment with directional or rotational atherectomy, intraluminal thrombus, severe tortuosity or calcification, total occlusion, postal location, involvement of a bifurcation, or unprotected left main coronary artery. PCI was completed successfully without conversion to manual operation, and device technical success was achieved in 162 of 164 patients (98.8%). There were no device-related complications. Clinical procedural success was achieved in 160 of 164 patients (97.6%), whereas 4 (2.4%) had periprocedural non–Q-wave myocardial infarctions. No deaths, strokes, Q-wave myocardial infarctions or revascularization occurred in the 30 days after the procedure. The robotic remote-control procedure met the expected technical and clinical performance with significantly (95%) lower radiation exposure to the operator.
Another trial evaluating the feasibility of robotic systems in endovascular interventions is currently underway.
Benefits to patients
While benefits to the interventional cardiologist are clear, there is also the potential for significant improvement in patient outcomes. Traditional PCI requires assessment of lesion length by visual assessment, which can often lead to inaccurate measurement. Quantitative coronary angiography can improve accuracy of lesion measurement, but is infrequently utilized in real-world practice and is still subject to measurement error. A recent trial highlighted the prevalence of stent misplacement and subsequent poor clinical outcomes Citation[16]. It is well known that geographic miss is associated with increased rates of target vessel revascularization and a significantly higher rate of myocardial infarction. Highly precise robotic systems with the ability of device manipulation with millimeter precision may prevent geographic miss, and also allow operators to accurately measure lesion length prior to stent selection. With accurate lesion length measurement, stent length can me minimized, thus favorably impacting outcomes Citation[17]. Decreased operator strain and fatigue during long procedures may improve technical precision and minimize fluoroscopy times. Increased simultaneous control over both coronary catheter positioning and contrast media injector may enable reductions in fluoroscopy and amount of contrast used per procedure. Lower volumes of contrast are associated with reduction in the incidence of contrast-induced nephropathy. Contrast-induced nephropathy has been clearly associated with increased morbidity and mortality Citation[18]. Minimizing radiation exposure to the patients has obvious benefits, as it may prevent radiation injuries such as radiation-induced skin burns, or iatrogenic malignancy, particularly in patients undergoing multiple staged procedures.
Future directions
While robotic PCI shows great promise, limitations and hurdles remain. The efficacy of robotic PCI in complex and high-risk coronary lesions remains to be established. However, a number of case studies have been published demonstrating the feasibility of the system in these lesion subsets. The precision of the system would be of significant benefit in treatment of ostial lesions, bifurcations and chronic total occlusions. As robotic coronary intervention is adopted, new applications of the technology are likely to emerge. Robotic systems could be easily applied to peripheral vascular procedures, which expose the interventionists and patients to large doses of radiation. Ultimately, robotic technologies will likely be seamlessly integrated into daily practice and may redefine the interventional cardiology standard of care.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med 1979;301:61-8
- Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory: time for a safer environment. Catheter Cardiovasc Interv 2009;73:432-8
- Dehmer GJ. Occupational hazards for interventional cardiologists. Catheter Cardiovasc Interv 2006;68:974-6
- Balter S. Radiation safety in the cardiac catheterization laboratory: operational radiation safety. Catheter Cardiovasc Interv 1999;47:347-53
- The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Annals of ICRP 2007;37:1-332
- Andreassi MG, Cioppa A, Botto N, et al. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J 2005;19:998-9
- Linet MS, Kim KP, Miller DL, et al. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat Res 2010;174:793-808
- Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 2013. [Epub ahead of print]
- Duran AD, Kleinman NK, Echeverri DE, et al. Retrospective evaluation of lens injuries and dose: RELID study. J Am Coll Cardiol 2011;57:E1951
- Ross AM, Segal J, Borenstein D, et al. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol 1997;79:68-70
- Khalil TM, Rosomoff HL. Ergonomics. Back pain: a guide to prevention and rehabilitation. Van Nostrand Reinhold; New York: 1993
- Leena RV, Shyamkumar MK. Glove perforations during interventional radiological procedures. Cardiovasc Intervent Radiol 2010;33:375-8
- Ernst S, Ouyang F, Linder C, et al. Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation 2004;109:1472-5
- Beyar R, Gruberg L, Deleanu D, et al. Remote-control percutaneous coronary interventions: concept, validation, and first in humans pilot clinical trial. J Am Coll Cardiol 2006;47:296-300
- Giora W, Metzger C, Caputo R, et al. Safety and feasibility of robotic percutaneous coronary intervention, (PRECISE study). J Am Coll Cardiol 2013;61:1596-600
- Costa MA, Angiolillo DJ, Tannenbaum M, et al. Impact of stent deployment procedural factors on long term effectiveness and safety of sirolimus eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol 2008;101:1704-11
- Gollapudi RR, Valencia R, Lee SS, et al. Utility of three dimensional reconstruction of coronary angiography to guide percutaneous coronary intervention. Catheter Cardiovasc Interv 2007;69:479-82
- Dangas G, Iakovou I, Nikolsky E, et al. Contrast induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005;95:13-19