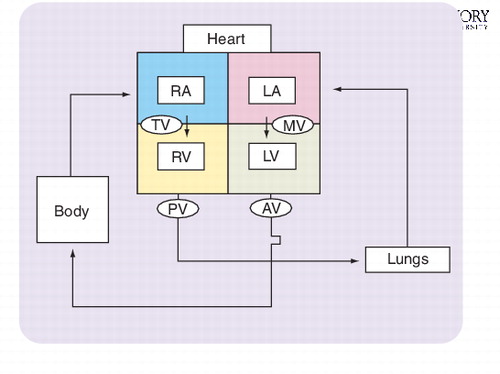
RA: Right atrium; RV: Right ventricle.; TV: Tricuspid valve.
The first attempt to dilate a stenotic heart valve occurred nearly a century ago in 1912. Although this first patient improved, subsequent attempts were often unsuccessful. Development in interventional valve therapy continued slowly and sporadically until the early 1960s. Finally, invention of the first artificial heart valve and the ability to support the circulation using the heart–lung machine allowed the surgeon to replace diseased valves safely. Today, 90,000 valve operations are performed annually in the USA alone (300,000 procedures worldwide) for various valvular heart conditions.
Systematic and timely flow of blood through the heart largely explains the essential function of the four heart valves. Schematically depicted in , oxygen-depleted blood returns to the right filling chamber of the heart (right atrium), crossing the tricuspid valve into the right pumping chamber (right ventricle) and on through the pulmonary valve to the lungs. The blood then becomes oxygenated and drains from the lungs into the left atrium, passing through the mitral valve and into the left ventricle. The left ventricle then pumps blood through the aortic valve to begin its journey to all parts of the body. In general, damaged heart valves are stenotic (narrowed), insufficient (leaky) or a combination of the two. If allowed to progress unabated, the natural history of diseased valves will often result in irreversible damage to vital organs and premature death.
In the adult population, aortic and mitral valve abnormalities are the most common. In the USA, aortic stenosis is the most common adult valvular heart disease, responsible for approximately 60% of all valve operations. In the case of aortic stenosis, valve replacement is often superior to repair. The environment of the aortic root is characterized by high blood pressure, often making repair less durable, and the typical patient with severe aortic stenosis has a very calcified, distorted valve, bearing little resemblance to normal.
In contrast to aortic valve surgery, the goal of mitral valve surgery is predominantly one of valve repair. As the mitral valve serves a vital role in the shape and function of the left ventricle, surgeons strive to retain as much of the native mitral valve as possible, ensuring both a short- and long-term survival benefit. Additionally, mitral regurgitation, the second most common heart valve abnormality in the adult US population, is not as often associated with widespread native valve damage, as in aortic stenosis. In many cases, vital components of the native mitral valve may be salvaged with important clinical benefit.
Justification
The development of percutaneous heart valve technology (PHVT) is still in its infancy, and ultimately the question of whether it is necessary must be addressed. Surgical valve repair and replacement therapy have a long, successful and durable history. The risk-adjusted mortality for isolated aortic and mitral valve replacement now stands at 4 and 6%, respectively [101]. Even in elderly and high-risk patients, the track record is excellent, further reinforcing surgery as the gold standard. So why would a patient select an unproven device over a well established open surgical valve procedure? Is it even ethical to give the patient a choice of PHVT in its current form? With the aging population, there are patients who possess enough comorbid conditions to render them a high risk for surgery, but this is a minority. Clearly, proponents of PHVT must start by acknowledging a high performance standard in searching for its justification.
In its current state, PHVT can be broadly categorized into two categories: devices that repair diseased mitral and tricuspid valves and expandable valves to replace diseased aortic or pulmonary valves. Percutaneous mitral valve repair approaches currently include reshaping the annulus through the coronary sinus (Edwards Lifesciences, Mitralsolutions, Viacor, Cardiac Dimensions), reshaping the annulus through the ventricle (Mitralign, Myocor, QuantumCor, Ample Medical) or connecting the middle scallops of the anterior and posterior leaflets (Edwards Lifesciences, Evalve). Percutaneous pulmonary and aortic valve replacement (Aortx, Cook, Corazon, CoreValve, Direct Flow Medical, Edwards Lifesciences, Heart Leaflet Technologies, Medtronic, Palmaz/Baley’s, Sadra Medical, Shelhigh, 3F Therapeutics, ValveXchange) presents greater technological challenges. A stent-mounted valve must be crimped into a guiding catheter and then expanded into a precise position, presumably with enough radial force to prevent paravalvular leak and valve migration. While percutaneous pulmonary valve replacement continues to evolve rapidly, percutaneous aortic valve replacement has been characterized by paravalvular regurgitation and early mortality, primarily due to the greater complexity of systemic circulatory pathology and pathophysiology. Further, the durability of these valves remains unknown, particularly when accounting for the potential detrimental effects of valve crimping and re-expansion before implantation.
Percutaneous therapy for aortic stenosis will lag behind the rest. No greater are the technological hurdles than in patients with aortic stenosis, where the pathology is often advanced and the procedural risks are greater. The often heavily calcified aortic valve needs to be excised, decalcified or pushed aside for the new collapsible valve to be implanted. Further, this has to be accomplished without embolization (resulting in stroke), paraprosthetic regurgitation, device migration and compromise of coronary blood flow, all of which are potentially life threatening.
Five-year view
At this early developmental stage of PHVT, industry has focused on establishing feasibility, reserving issues of durability for later investigation. The US FDA circulatory device approval process largely dictates both preclinical and clinical development with the initial requirement of gathering safety and feasibility data. Following appropriate bench and animal studies, the technology should be studied closely in a small group of highly selected patients considered poor candidates for standard surgical therapy. In this situation, good surgical candidates are not subjected to an increased risk. Conversely, these early feasibility patients are very ill and have a high risk for a multitude of complications, regardless of the procedure.
After establishing a safety and feasibility record, the more daunting challenge of efficacy looms ahead. So while a percutaneous device may be able to safely repair the mitral valve in the short term, we can only speculate on how the device will hold up with time. It is understood that current surgical techniques can completely correct mitral regurgitation and result in improved long-term survival and quality of life for the patient. There is, however, an early risk of mortality and morbidity that the patient ‘pays’ up front for that long-term benefit. A more evolved percutaneous technique may be safer from a procedural standpoint, but impart less long-term benefits to the patient as a result of inferior durability. Such a relationship already exists between coronary artery bypass surgery and percutaneous coronary intervention. Bypass surgery provides unequalled durability at higher levels of procedural risk and invasiveness. Stenting, on the other hand, carries less procedural risk and invasiveness, but is less durable. Most patients prefer stenting to bypass surgery partly because they may lack an appreciation of the durability of their choices and are sometimes led to believe that stent failures do not increase their risk for eventual surgery. A similar phenomenon could easily develop for percutaneous valve therapy. Seemingly safer, less invasive approaches, without adequate long-term durability data, will likely delay surgical therapy until a patient’s overall health status has deteriorated. In addition, the surgical procedure at that later time will be more complex and, in the case of the mitral valve, result in the need for replacement rather than a repair.
Information resources
| • | The Cardiothoracic Surgery Network. www.ctsnet.org/ | ||||
| • | Stephensen L. In: State of the Heart: The Practical Guide to Your Heart and Heart Surgery. Write Stuff Syndicate, Inc, FL, USA (2001). | ||||
| • | Edmunds L, Cohn L. In: Cardiac Surgery in the Adult. McGraw-Hill Professional, OH, USA (2003). | ||||
| • | At this point in the evolution of percutaneous heart valve technology (PHVT), there has been more commercial and professional ‘hype’ than solid data. Overall, heart valve technology has grown to a billion dollar market and PHVT is likely to cause the market to grow further still. There are several key issues to note as we move into a potential renaissance period of heart valve intervention. | ||||
| • | Will PHVT develop in the same manner as percutaneous coronary artery intervention? The primary drivers in growth for percutaneous coronary intervention were the tremendous appeal of less invasion and faster recovery. Despite a preponderance of long-term data demonstrating the superiority of bypass surgery, patients continue to prefer an easier quick fix. If PHVT is to develop in a more responsible way, primary care physicians and general internists should initially screen patients with an opportunity for both the surgeon and the interventional cardiologist to evaluate the patient on an equal footing. The decision to perform a procedure should be the product of a multidisciplinary discussion. Such a proposal may be too idealistic and the reality is that these technologies will develop based on many individual self-interests. | ||||
| • | PHVT devices should be tested first in the controlled, fully capable environment of the cardiac operating room. Safety and efficacy data obtained from implanting the device in an open incision can then be applied to the percutaneous deployment in the cardiac catheterization lab. However, many interventional cardiologists would oppose this view, believing PHVT is fundamentally different from surgical therapies. As such, most of the PHVT companies have elected not to use the operating room as an incubator or proving ground for their devices. Rather, the norm has been to go from preclinical testing directly to the catheterization lab for clinical feasibility testing. In most of these cases, the cardiac surgeon has been relegated to the familiar role of surgical ‘back-up’ in the event of a procedural catastrophe. | ||||
| • | With the eventual clinical introduction of new, less-invasive valve procedures, we will need new guidelines for these less invasive procedures to encompass patients with minimal or no symptoms and less severe valve pathology. Revising practice guidelines regarding the indications and timing of valvular heart surgery is likely to generate vigorous debate. For these reasons, professional societies, industry and government need to continue to work together. Collaborative conferences, such as the one held in April 2004 Citation[1], should be organized with the intent of developing consensus statements for PHVT. | ||||
References
- Vassiliades TA Jr, Block PC, Cohn LH et al. The clinical development of percutaneous heart valve technology: a position statement of the Society of Thoracic Surgeons (STS), the American Association for Throacic Surgery (AATS), and the Society for Cardiovascular Angiography and Interventions (SCAI) J. Thorac. Cardiovasc. Surg. 129(5), 970–976. J. Am. Coll. Cardiol. 45, 1554–1560. Ann. Thorac. Surg. 79, 1812–1818. Catheter. Cardiovasc. Interv. 65, 73–79
Website
- Society of Thoracic Surgeons National Database www.sts.org/sections/stsnationaldatabase/ (Accessed December 2005)