Abstract
The WHO recommends the administration of sulfadoxine-pyrimethamine (SP) to all pregnant women living in areas of moderate (stable) to high malaria transmission during scheduled antenatal visits, beginning in the second trimester and continuing to delivery. Malaria parasites have lost sensitivity to SP in many endemic areas, prompting the investigation of alternatives that include azithromycin-based combination (ABC) therapies. Use of ABC therapies may also confer protection against curable sexually transmitted infections and reproductive tract infections (STIs/RTIs). The magnitude of protection at the population level would depend on the efficacy of the azithromycin-based regimen used and the underlying prevalence of curable STIs/RTIs among pregnant women who receive preventive treatment. This systematic review summarizes the efficacy data of azithromycin against curable STIs/RTIs.
The WHO recommends the administration of sulfadoxine-pyrimethamine (SP) to all pregnant women who live in areas of moderate (stable) to high malaria transmission during scheduled antenatal care (ANC) visits, beginning in the second trimester and continuing to delivery Citation[1]. This intervention, known as intermittent preventive treatment of malaria in pregnancy (IPTp), is national policy in 36 countries worldwide, 35 of which are in sub-Saharan Africa Citation[2]. The objective of IPTp-SP is to reduce the incidence of low birthweight and maternal anemia attributable to malaria. In recent years, however, malaria parasites have developed resistance to SP such that IPTp no longer reduces the incidence of low birthweight in some epidemiological settings, particularly in East Africa Citation[3]. Evidence suggests that in areas where parasites express the 581G dhps mutation that is associated with SP resistance, the administration of IPTp-SP may even harm fetal growth Citation[4–6]. Thus, the urgency to replace SP has never been greater and azithromycin-based combination (ABC) therapies are among leading candidates to do so.
Azithromycin is a slow-acting analog of erythromycin in the macrolide (azalide) class of drugs, which targets the ribosomal subunit of susceptible microorganisms and causes cellular death by inhibiting protein synthesis Citation[7]. It has in vitro and in vivo antimalarial properties Citation[8] and can be safely administered during pregnancy Citation[9]. Two human challenge studies have published results of azithromycin monotherapy treatment against Plasmodium falciparum infection. The first study reported a protective effect of 40% (n = 10; 95% CI: 12–74%) among immunologically naïve patients who received 250 mg azithromycin daily for 2 weeks prior to inoculation and for 1 week more following exposure Citation[10]. When the same regimen was used for one additional week post-inoculation, treatment efficacy was 100% (n = 10) Citation[11]. Despite this finding, comparable results have not been replicated in endemic settings where patients often have mixed and multiple infections. However, in vitro evidence suggests that azithromycin may be combined with antimalarial partner drugs to prevent or to cure P. falciparum infection Citation[12], the malaria species most prevalent in sub-Saharan Africa and which uniquely adhere to the placenta of pregnant women. In addition to reducing the burden of malaria infection, ABC therapies may also protect against adverse birth outcomes attributable to curable sexually transmitted and reproductive tract infections (STIs/RTIs). This could offer considerable public health impact. A recent meta-analysis suggests that curable STIs/RTIs are as prevalent as malaria parasitemia, if not more so, among pregnant women who attend ANC facilities in sub-Saharan Africa Citation[13]. Five curable STIs/RTIs – Treponema pallidum, Neisseria gonorrheae, Chlamydia trachomatis, Trichomonas vaginalis and bacterial vaginosis – are associated with adverse birth outcomes that include spontaneous abortion Citation[14–18], stillbirth Citation[19–21], intrauterine growth retardation Citation[20,22,23], premature rupture of membranes Citation[24–26], preterm birth Citation[17,22,23,26–33] and low birthweight Citation[20,23,24,28,29,33–35]. This paper summarizes azithromycin efficacy and sensitivity against these curable STIs/RTIs and highlights important issues for policymakers to consider while determining the potential use of ABC therapies in IPTp.
Table 1. Effect of curable STIs/RTIs on pregnancy outcomes.
Methodology
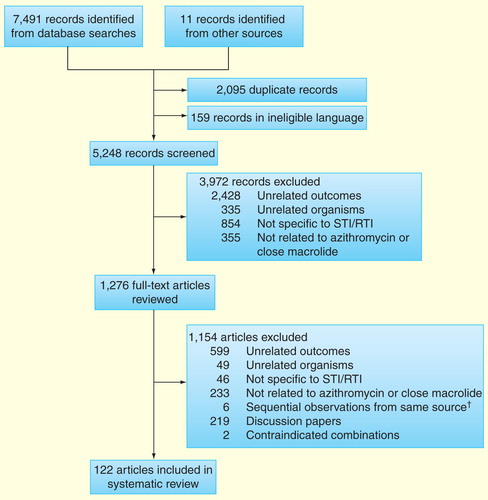
Between April and May 2013, PubMed, MEDLINE and EMBASE were searched using Medical Subject Headings and free-text terms for publications specific to the curable STIs/RTIs noted above. With each query, the infection and causal organism were used together, for example, ‘Syphilis’ AND ‘Treponema pallidum’, and then combined with search terms ‘azithromycin’ OR ‘macrolide’. Because the evidence base is limited with respect to azithromycin and some curable STIs/RTIs, both ‘azithromycin’ and ‘macrolide’ were used as filters. We had particular interest in randomized clinical trials (RCTs) that compared azithromycin against the current first-line treatments for curable STIs/RTIs in pregnancy, noting that azithromycin is the WHO-recommended treatment for pregnant women infected with C. trachomatis. Searches were limited to the English language and strict inclusion and exclusion criteria were applied so as to narrow the number of papers retained. Reference lists were also reviewed for additional documents. Excluded records and full-text articles were in seven categories:
‘Unrelated outcomes’ were studies that reported nonclinical aspects of azithromycin use such as cost-effective analysis, noncommunicable diseases such as heart disease or pharmacological outcomes involving a route of administration that is not applicable to this review (e.g., intravenous);
‘Unrelated organisms’ were papers dedicated to microbes that are not the focus of this review;
‘Not specific to STI/RTI’ were articles on the subject of same genus of interest, for example, Chlamydia, but were not specific to the genital tract, for example, Chlamydia pneumoniae;
‘Not related to azithromycin or close macrolide family’ were papers that did not contain macrolides in their analysis or outcomes, but focused on different antimicrobials against the organisms in question;
‘Sequential observations from same source’ involved surveillance reports from which most recent data set was used;
‘General discussion papers’ contained information pertinent to the search, but failed to provide specific data for STIs/RTIs.
‘Contraindicated in pregnancy’ were papers that reported outcomes of azithromycin combined with antimicrobial compounds that are considered unsafe in pregnancy.
A total of 122 articles met our primary inclusion criteria .
Results
Treponema pallidum
In vivo evidence
The WHO recommends treating pregnant women with syphilis infection using 2.4 million units of benzanthine penicillin G (BPG) administered by intramuscular injection Citation[36]. Thus, we summarize the results of the six clinical trials that reported outcomes among nonpregnant adults following treatment with BPG, azithromycin or a combination of BPG and azithromycin . The oldest data are from a trial in the USA (1993–1997) in which individuals who discovered they had been exposed to infectious stage syphilis through sexual intercourse in the preceding 30 days were given either 1 g azithromycin (n = 40) or BPG (n = 23). Three months post-treatment, rapid plasma reagin (RPR) and fluorescent treponemal antibody absorption tests (FTA-ABS) were negative for all participants in both treatment groups Citation[37]. Another trial in the USA during the same time period was designed to measure treatment outcomes in a population at high risk of contracting STIs/RTIs. Although diagnostic methods were not reported, the trial was suspended after two of the first 12 patients were provided 1 g azithromycin failed their test of cure while all 13 participants were cured using BPG (p = 0.18) Citation[38]. A three-arm trial of early syphilis in the USA then compared treatment outcomes among patients given BPG, or 2 g azithromycin once or 2 g azithromycin two-times with 1 week in between doses. RPR and FTA-ABS testing showed that cure was achieved in 85.7% (n = 14; 95% CI: 60.0–95.7%) of patients given BPG, 94.1% (n = 17; 95% CI: 72.7–98.6%) among recipients of 2 g azithromycin once and 82.8% (n = 29; 95% CI: 65.3–92.3%) in participants who twice received 2 g azithromycin Citation[39].
Table 2. Randomized clinical trials of azithromycin versus benzathine penicillin G for the treatment of Treponema pallidum
In sub-Saharan Africa, three trials have investigated BPG versus azithromycin, the first being a community-randomized trial in Uganda (1994–1998) among nonpregnant adults with serological syphilis. Diagnosis and test of cure were based on toluidine red unheated serum tests (TRUSTs) and Treponema pallidum hemagglutination assays. Treatment efficacy varied across regimens depending on TRUST titers at enrolment. Among patients with initial titers <1:2, BPG cured 71.0% (n = 93; 95% CI: 61.0–79.2%) of cases compared with 58.5% (n = 94; 95% CI: 48.4–68.0%) among recipients of 1 g azithromycin and 70.6% (n = 313; 95% CI: 65.3–75.4%) of participants given 1 g azithromycin plus BPG. If titers at enrolment were >1:4, the efficacy of BPG was reduced to 41.3% (n = 75; 95% CI: 30.9–52.7%). Treatment efficacy was also lower among groups given azithromycin but higher than BPG alone. Recipients of 1 g azithromycin alone had a cure rate of 53.3% (n = 71; 95% CI: 42.0–64.7%), whereas 1 g azithromycin plus BPG cured 54.7% of cases (n = 309; 95% CI: 49.1–60.2%) Citation[40].
These results were followed by a trial carried out in Tanzania (2000–2003) among patients who were recruited by screening high-risk populations. All 328 subjects had a titer of at least 1:8 on RPR test; 106 had baseline titers of >1:64, levels indicative of active syphilitic lesions. Confirmed by RPR test and T. pallidum particle agglutination assay, serological cure was observed in 97.5% (n = 163; 95% CI: 93.9–99.0%) of participants given 2 g azithromycin versus 95.2% (n = 165; 95% CI: 90.7–97.5%) in the BPG group Citation[41].
The most recent study comparing the efficacy of azithromycin versus BPG is a multicenter trial (2000–2007) in Madagascar (n = 421) and North America (n = 94) among HIV-negative patients with early syphilis. Based on RPR testing, serological cure was reported in 77.6% of subjects given 2 g azithromycin (n = 232; 95% CI: 71.8–82.5%) and 78.5% (n = 237; 95% CI: 72.8–83.3%) in the BPG group. Nonserious adverse events were reported by 61.5% (n = 174; 95% CI: 55.7–67.0%) of individuals treated with 2 g azithromycin, most of whom had self-limiting gastrointestinal discomfort, whereas 46.1% (95% CI: 40.6–52.1%) of BPG recipients reported nonserious adverse events Citation[42].
In vitro evidence
Fourteen in vitro studies met our inclusion criteria, seven with isolates from low-risk populations and seven from high-risk or mixed-risk groups . A report from San Francisco in 2001 was the first to associate azithromycin treatment failure with A→G mutations at the 2,058 position of the 23S rRNA gene of T. pallidum Citation[43]. Retrospective analysis of samples revealed that 4.0% (n = 25; 95% CI: 0.9–19.6%) of isolates had A→G mutations between 1999 and 2002. In 2003, the proportion of isolates with A→G mutations increased to 36.7% (n = 30; 95% CI: 21.9–54.6%) Citation[44]; by 2004, 56.1% (n = 66; 95% CI: 44.0–67.3%) had selected for resistance Citation[43]. However, in Dublin, 88.2% (n = 17; 95% CI: 65.3–96.4%) of isolates already had A→G mutations by 2002 Citation[44].
Table 3. Sensitivity testing of T. pallidum isolates to azithromycin and other macrolides.
Table 4. Sensitivity testing of T. pallidum isolates to azithromycin and other macrolides.
Macrolide resistance is strongly associated with use by an individual in the previous year. Isolates from Seattle (2001–2005) were two-times more likely to be resistant if patients had been treated with macrolides in the past 12 months (RR: 2.2; 95% CI: 1.1–4.4; p = 0.02) Citation[45]. This relationship persisted over the decade. A2058G and A2059G mutations, which are associated with clinical failures of azithromycin, were found in 88.9% (n = 36; 95% CI: 74.6–95.5%) of isolates from 2001 to 2010 among patients exposed to macrolides in the preceding 12 months, whereas 61.2% (n = 98; 95% CI: 51.3–70.4%) of isolates from patients who had not received prior macrolide treatment contained the same mutations Citation[46]. Similar mutations were found among strains of T. pallidum in eight cities across China (2008–2011). A2058G was present in 97.0% individuals who had taken macrolides in the previous 12 months versus 62.5% of patients who had not (n = 211; OR: 19.65; 95% CI: 5.8–66.9) Citation[47]. The opposite was found in Taiwan (2009–2011) where no single A2058G or A2059G mutation was seen among 211 isolates tested from a population where only one person had been given macrolide therapy in the previous year Citation[48]. Similarly, there was no evidence of resistance among 141 amplified samples from HIV-negative heterosexual patients in Madagascar Citation[49]. Although use of macrolides in the previous year was not reported, the Essential Drugs List of the Malagasy Ministry of Health does not include macrolides Citation[301].
Neisseria gonorrhoeae
In vivo evidence
The WHO recommends treating pregnant women with Neisseria gonorrhoeae infection using 400 mg cefixime as a single dose or 125 mg ceftriaxone by intramuscular injection Citation[50]. However, azithromycin has been used for the treatment of gonorrhea among nonpregnant adults during the past two decades. Eleven trials were identified through our review . Nine trials conducted between the late 1980s and 1999 investigated the use of 1 g azithromycin among individuals attending sexually transmitted infection (STI) clinics. Of these, three were open label without comparators Citation[51–53] and six were two-arm trials that compared azithromycin to ciprofloxacin and/or doxycycline Citation[54–59]. The pooled efficacy of azithromycin against N. gonorrhoea, estimated using random effects models Citation[60], was 97.0% (n = 539; 95% CI: 95.5–98.5%). This is slightly higher than 96.5% (n = 539; 95% CI: 94.3%–97.6%) reported in a 2010 review Citation[61] that added numerators and divided the sum of denominators among the same nine trials. Such an approach does not account for heterogeneity across study populations and gives equal weight to all trials regardless of their precision. Regardless of pooling methods, it is unlikely that the same efficacy would be observed today using 1 g azithromycin in high-income countries following 25 years of cumulative drug pressure. However, the epidemiological context in sub-Saharan Africa is likely different where azithromycin use has been almost exclusively limited to trachoma eradication campaigns Citation[62].
Table 5. Randomized clinical trials of azithromycin for the treatment of Neisseria gonorrhoeae
We identified two RCTs that investigated the use of 2 g azithromycin among patients at STI clinics. The first was a multicenter trial in the USA (1991–1992) in which 98.9% (n = 374; 95% CI: 97.3–99.6%) of patients were cured Citation[63]. A similar RCT in New Delhi (2005–2006) involved 42 participants; loss to follow-up was high, 52.4%, but all 22 subjects who returned for a test of cure had their N. gonorrhoeae infections cured Citation[64].
In vitro evidence
Over the past decade, in vitro studies have documented the loss of N. gonorrhoeae sensitivity to azithromycin. There are no standard breakpoints of minimum inhibitory concentrations (MICs) used to categorize N. gonorrhoeae resistance to azithromycin, but >1 µg/ml Citation[65] and >2 µg/ml Citation[66] have both been used. In this section, we summarize the key regional observations from 36 in vitro studies .
The Public Health Agency of Canada reported that 0.17% (n = 40,875; 95% CI: 0.001–0.002%) of N. gonorrhoeae samples were resistant to azithromycin between 2000 and 2009, although the modal value of the MIC shifted from 0.25 µg/ml in 2001 to 0.5 µg/ml between 2007 and 2009 Citation[67]. During the same 10-year period, the Centers for Disease Control and Prevention in the USA reported that 0.04% (n = 87.566; 95% CI: 0.03–0.06%) of N. gonorrhoeae isolates tested had MICs ≥8 µg/ml (including 25 with 8 µg/ml and 14 with 16 µg/ml) Citation[68]. This did not include five cases of azithromycin-resistant N. gonorrhoeae found between August and October 2009 among men who have sex with men; three had MICs of 8 µg/ml and two had 16 µg/ml Citation[69]. Resistance may have appeared in Europe slightly before North America. Analysis of isolates from 17 European countries found that 3.2% (n = 836; 95% CI: 0.02–0.05%) of gonococcal isolates were resistant to azithromycin in 2006. By 2007, 6.8% (n = 973; 95% CI: 0.05–0.09) of samples were resistant. The overall proportion of resistant isolates declined in 2008 to 1.8% (n = 940; 95% CI: 0.01–0.03%), although only 5.2% (95% CI: 4.1–6.8%) of strains tested in the same year were fully susceptible to azithromycin and ciprofloxacin. Four isolates from Scotland and one from Ireland exhibited MICs >256 mg/l Citation[68].
Gonococcal isolates examined from South America and Cuba exhibited high but stable levels of resistance between 2000 and 2009 in most settings Citation[70]. Collectively, azithromycin resistance was 13.0% (n = 8,373; 95% CI: 12.3–13.7%) based on data from six countries including Chile, an outlier. Averaged over the decade, 26.7% (n = 3,116; 95% CI 25.2–28.3%) of samples from Chile were resistant, rising to 45.6% (n = 463; 95% CI: 41.1–50.1%) according to the most recent data from 2009. Removing Chile from the regional summary, 4.4% (n = 5,257; 95% CI: 3.9–5.1%) of isolates were resistant over the decade.
All 60 gonococcal isolates from India between 2004 and 2005 were susceptible to azithromycin Citation[71]. Pooled analysis of samples collected from India, Pakistan and Bhutan between 2007 and 2011 found that 76.9% (n = 65; 95% CI: 65.3–85.5%) were susceptible. Results were not stratified by country and, therefore, it is not known whether the sensitivity of isolates from India had changed Citation[72]. Applying the more conservative breakpoint of >1 µg/ml to the in vitro studies identified in this review, 35% (7 of 20) of the in vitro studies reported upper range MICs that included gonococcal isolates resistant to azithromycin. This percentage does not include 16 studies we identified and included in that did not report MICs.
Table 6. Sensitivity testing of Neisseria gonorrhoeae isolates to azithromycin.
Table 7. Sensitivity testing of N. gonorrhoeae isolates to azithromycin.
Chlamydia trachomatis
In vivo evidence
The WHO recommends treating pregnant women with Chlamydia trachomatis infection using 1 g azithromycin as a single oral dose Citation[50]. We found eight RCTs in the literature that reported the treatment efficacy of 1 g azithromycin among pregnant women Citation[73–80]. Using random effect models, we estimate the pooled treatment efficacy to be 92.1% (n = 268; 95% CI: 88.4–95.7%). The estimated efficacy would be higher if we excluded two trials that were conducted in the USA. The first trial (1995–1997) reported a 3-week test of cure rate to be 88.1% (n = 42; 95% CI: 74.9–94.7%) Citation[76], whereas the second trial (1998–2000) was terminated early due to poor efficacy, 63.3% (n = 55; 95% CI: 50.4–75.1%), based on test of cure ≥4 weeks post-treatment Citation[74]. These results need to be interpreted with caution because no distinction was made between treatment failures and new infections, sex partners were not treated by trial staff, but were referred to a treatment center, and only 35% of women were seen within 7 days of the scheduled test of cure.
Table 8. Treatment efficacy studies of 1 g azithromycin for the treatment of Chlamydia trachomatis in pregnant women.
Studies investigating sexual activity following treatment offer some perspectives on post-treatment infections and the extent to which they may be failures or de novo infections. A trial in Seattle (1998–2003) found that persistent or recurrent chlamydial or gonorrheal infection occurred in 7.6% (n = 289; 95% CI: 5.1–4.9%) of female patients who reported no sexual intercourse after treatment Citation[81]. Another study reported that 19.0% (n = 79; 95% CI 11.9–29.0%) of women were positive for C. trachomatis 3 months after treatment using 1 g azithromycin. Of these women, 13.3% (n = 15; 95% CI: 4.0–38.3%) reported being sexually inactive during the post-treatment period Citation[82]. These findings may be attributable to false reporting of sexual contact, or treatment failure or may lend credence to the hypothesis that C. trachomatis enters a latent asymptomatic state that is undetectable by culture or, possibly, Nucleic Acid Amplification Tests, but can later reactivate Citation[83].
In vitro evidence
Thresholds for antimicrobial susceptibility and resistance of C. trachomatis are not universally standardized, although MICs >4 μg/ml are often used to characterize therapeutic failure Citation[84–87]. The lowest concentration of antimicrobial compound needed to inhibit chlamydial formation is between 0.03 and 0.125 μg/ml, whereas the minimum bactericidal concentration (MBC; also referred to as the minimum chlamydicidal concentration, or MCC) is between 0.06 and 0.5 μg/ml Citation[88,89]. The published in vitro studies of azithromycin collectively suggest the persistence of high and widespread treatment efficacy . One noted exception is the study of six isolates from three patients who experienced treatment failure in Russia (2000–2002); four isolates were resistant to azithromycin, doxycycline and ofloxacin at MICs and MBCs >5.12 μg/ml Citation[90]. Not surprisingly, in vitro resistance appears to be more common in individuals with greater severity of disease or recurrent disease. A study in the USA during the early 1990s described decreased susceptibility and emerging resistance to azithromycin and doxycycline in isolates from women with mucopurulent cervicitis but not in isolates from women with asymptomatic infections Citation[91]. Similar observations were reported in 2010 from India; six of eight isolates with modified susceptibility had been obtained from recurrently infected individuals, whereas the remaining two were from nonrecurrently infected patients. MICs and MBCs for azithromycin were 8 µg/ml for two of the patients from which the modified susceptibility isolates were taken. One individual had chronic cervicitis and the other had pelvic inflammatory disease Citation[92].
Table 9. Sensitivity testing of Chlamydia trachomatis isolates to azithromycin.
Trichomonas vaginalis
In vivo evidence
Trichomonas vaginalis is a protozoal infection which causes cervicitis and nongonococcal urethritis. The WHO recommends treating pregnant women with T. vaginalis infection after the first trimester using 2 g metronidazole orally as a single dose, or 400–500 mg twice daily for 7 days or 300 mg clindamycin orally twice a day for 7 days Citation[36]. If treatment is imperative during the first trimester of pregnancy, the single-dose regimen of 2 g metronidazole orally is recommended Citation[36]. Azithromycin has not been used directly for prevention or treatment purposes because T. vaginalis is anaerobic. Nevertheless, azithromycin has demonstrated protection against T. vaginalis in studies of mass STI/RTI treatment .
Table 10. Trials using azithromycin alone and in combination with other drugs reporting protection curable STIs/RTIs among pregnant women.
Table 11. Trials using azithromycin alone and in combination with other antimicrobial therapies not contraindicated in pregnancy and reporting protection curable STIs/RTIs among commercial sex workers.
In Kenya (1998–2002), 1 g azithromycin or placebo was given once per month to 466 HIV-negative female sex workers Citation[93]. At the end of the trial, HIV incidence was the same across treatment groups, the primary endpoint, but the incidence of T. vaginalis was reduced significantly among those given azithromycin versus placebo (RR: 0.56; 96% CI: 0.40–0.78; p < 0.001). A similar observation was made in a three-arm IPTp trial in Malawi (2003–2006) Citation[94]. Pregnant women received standard IPTp-SP, or monthly IPTp-SP or monthly IPTp-SP plus 1 g azithromycin during two antenatal visits; the prevalence of T. vaginalis at delivery was 16.7% (n = 411; 95% CI: 13.5–20.7%), 15.1% (n = 411; 95% CI: 12.0–18.9%) and 11.0% (n = 419; 95% CI: 8.3–14.3%), respectively. Thus, women who received azithromycin had 35% (RR: 0.65; 95% CI: 0.46–0.93; p = 0.02) fewer T. vaginalis infections at delivery compared with monthly recipients of IPTp-SP.
A cluster randomized trial in Uganda (1994–1998) compared the incidence of HIV infections among nonpregnant adults who received 1 g azithromycin, 250 mg ciprofloxacin and 2 g metronidazole versus multivitamins plus antihelminthics Citation[95]. Although the trial was terminated early for lack of protection against the primary endpoint, the incidence of several curable STIs/RTIs was lower in the control group, most notably T. vaginalis. The cumulative incidence of newly diagnosed T. vaginalis infection was 4.8/100 person-years (116/2,397 person-years) in the intervention group compared with 9.1/100 person-years (182/1,993 person-years) in the control group (RR: 0.52; 95% CI: 0.35–0.79).
The same combination of antimicrobials was provided to female sex workers in rural Zimbabwe as a one-time treatment followed by 3 monthly check-ups Citation[96]. The prevalence of T. vaginalis was just under 20% at baseline, decreased to approximately 5% at visit 2, rose to nearly 13% at visit 3 and lowered again to just over 10%, that is, one-half of the pretreatment levels.
Bacterial vaginosis
The WHO recommends treating bacterial vaginosis in pregnant women, preferably after the first trimester, with 200 or 250 mg metronidazole three-times per day for 7 days, or 5 g metronidazole gel (0.75%) applied intravaginally twice a day for 5 days or 300 mg clindamycin 300 mg orally twice a day for 7 days Citation[36]. As with T. vaginalis, if treatment is imperative during the first trimester of pregnancy, 2 g metronidazole orally is recommended Citation[36]. Bacterial vaginosis has no single causative agent, but is thought to result from destabilization of Lactobacillus species (spp.) with secondary colonization of anaerobic organisms that include Gardnerella vaginalis, Bacteroides spp, Mobiluncus spp. and Mycoplasma hominis alongside an increase in vaginal pH Citation[97,98].
In vivo evidence
Our review identified no trials that have attempted to measure the treatment efficacy of azithromycin alone against bacterial vaginosis. Only one study in the USA (2002–2005) investigated the use of azithromycin as a partner drug with metronidazole for the treatment of symptomatic bacterial vaginosis. Nonpregnant women received one of four treatments: 750 mg metronidazole once per day for 7 days, or metronidazole once per day for 7 days plus 1 g azithromycin on days 1 and 3, or metronidazole for 14 days or metronidazole for 14 days plus azithromycin on days 1 and 3 Citation[99]. No additional benefit of cure was observed among women who received metronidazole plus azithromycin compared with metronidazole alone.
Antibiotic treatment for bacterial vaginosis is challenging, in part, because it is a syndrome that involves multiple microorganisms rather than a single etiological agent. Comparable data from other macrolides suggest potential therapeutic value for azithromycin against bacterial vaginosis . Analysis of azithromycin against the anaerobic and carboxyphilic bacteria that replace the normal vaginal flora may provide a better understanding as to the potential role of azithromycin against bacterial vaginosis.
Table 12. Sensitivity of macrolides and structurally related agents against key causative organisms in bacterial vaginosis
Discussion
Azithromycin has been used against curable STIs/RTIs for 25 years. It has been an attractive option for preventive and curative treatment because it is efficacious as a single dose and offers reasonable tolerability against T. pallidum, N. gonorrhoeae and C. trachomatis. During the 1990s, and prior to the advent of antiretroviral therapies for HIV, the management of curable STIs/RTIs received renewed importance, particularly as trials showed that treatment of N. gonorrhoeae, C. trachomatis and T. vaginalis reduced the genital viral load of HIV among men and women Citation[100–103]. Groups at high risk for transmitting HIV have since been targeted by treatment campaigns using 1 g azithromycin. Thus, it is not a surprise that changes in azithromycin sensitivity within high-income settings have often been observed first among members of high-risk groups. Pregnant women attending ANC facilities in sub-Saharan Africa do not have the same risk profile. Thus, on this basis alone, it is less likely that the use of ABC therapies in IPTp would be a catalyst for the rapid emergence of azithromycin resistance, although its emergence cannot be ruled out. The potential benefits of ABC therapies may be best viewed through prior experience with mass drug administration among pregnant women. In the context of the AIDS epidemic and before the age of antiretroviral therapies, researchers attempted to prevent maternal-to-child transmission (MTCT) of HIV by providing pregnant women in Uganda 1 g azithromycin, in combination with 250 mg ciprofloxacin and 2 g metronidazole Citation[104]. The data safety monitoring board suspended the trial early for reasons of futility, despite having cut neonatal deaths by 17% (RR: 0.83; 95% CI: 0.71–0.97), decreased the incidence of low birthweight by 32% (RR: 0.68; 95% CI: 0.53–0.86), and reduced the incidence of preterm delivery by 23% (RR: 0.77; 95% CI: 0.56–1.05). These impressive results were achieved at a time when neither IPTp-SP nor insecticide treated bed nets for the control of malaria in pregnancy had been deployed.
If ABC therapies are used in IPTp, then there are several key factors to consider that are pathogen specific. Regarding syphilis, 1 g azithromycin should be used alongside 2.4 mu BPG for three reasons: combination therapy has been shown to achieve higher rates of cure than either therapy alone Citation[105]; use of ABC therapy with BPG would likely reduce selection of the A→G mutation associated with azithromycin and preserve T. pallidum sensitivity; and only BPG can be expected to cure congenital infection if the placenta has been invaded by spirochetes Citation[106]. As for N. gonorrhoeae, 1 g azithromycin may be just above the MIC of fully susceptible strains. Thus, ABC therapies containing >1 g azithromycin may be preferable from the standpoint of reducing selection pressure. However, a single 2 g dose may not be well tolerated as 6 in 10 patients reported self-limiting gastrointestinal discomfort when treated for syphilis infection with such a regimen Citation[42]. The dose could be split over 2 days to improve tolerability. ABC therapies that contain 2 g azithromycin, either a single- or split-dose, would be protective against C. trachomatis. Although the data are limited and the mechanism of action is not understood, ABC therapies that have 1 g azithromycin may protect against T. vaginalis based on reports from Malawi among pregnant women Citation[107] and commercial sex workers in Kenya Citation[93]. It is curious, however, that T. vaginalis infection during pregnancy is associated with adverse birth outcomes, but the first-line treatment of 2 g metronidazole does not always improve birth outcomes. A trial in Uganda reported that pregnant women treated for T. vaginalis infection were 2.5-times more likely to deliver a low birthweight infant than untreated women (RR: 2.49; 95% CI: 1.12–5.50) Citation[108]. The authors suggest that this may be attributable to metronidazole exposure. Another trial in the USA reported an increase in the risk of preterm delivery among pregnant women exposed to metronidazole for the treatment of asymptomatic trichomoniasis compared with those who were not treated (RR: 1.8; 95% CI: 1.2–2.7) Citation[109]. In contrast to these findings from high-income settings, data from a multicenter trial in sub-Saharan Africa indicate that treatment of T. vaginalis infection using metronidazole does not increase the chances of preterm birth Citation[110]. Apart from bacterial vaginosis, which is not transmitted through sexual contact, re-infection will remain a risk for pregnant women and, therefore, providers should continue to offer education and screening as appropriate.
None of the studies identified in this review indicate that azithromycin offers preventive or curative effect against bacterial vaginosis, the most prevalent of curable STIs/RTIs. Antibiotic therapy has only been shown to reduce the risk of preterm delivery by one-half (RR: 0.53; 95% CI: 0.34–0.84) among pregnant women with bacterial vaginosis (Nugent scores 7–10) or intermediate flora (Nugent scores 4–6) Citation[111]. A Nugent score of 0–3 is considered normal Citation[112] for which no protection against adverse birth outcomes has been observed.
Conclusions
ABC therapies are among leading candidates to replace SP for use in IPTp and may offer important public health benefits by also reducing the burden of curable STIs/RTIs in pregnancy. Evidence from nonpregnant adults suggests that ABC therapies containing 1 g azithromycin may cure maternal T. pallidum infection. BPG should still be administered with azithromycin because the combination has been shown to be more efficacious in nonpregnant adults than either treatment alone. Moreover, evidence from pregnant women indicates that eradication of congenital syphilis may require BPG treatment. Neisseria gonorrhoeae infection among pregnant women in sub-Saharan Africa, where strains are likely to be fully sensitive to azithromycin, is likely to be cured by ABC therapies containing 1 g azithromycin. However, 2 g may be needed to reduce persistent and/or recurrent infection, and opportunities for the emergence of drug resistance. ABC therapy containing 1 g azithromycin would be curative of C. trachomatis infection, whereas some protection against T. vaginalis infection could be expected with the same dose. It remains unknown whether ABC therapies could offer protection against bacterial vaginosis if administered during the first half of pregnancy. ABC therapies merit investigation for the use in IPTp given their potential to reduce the dual burden of malaria and curable STIs/RTIs in pregnancy and improve maternal, fetal and neonatal health.
Expert commentary
Current strategies for addressing the dual-burden of malaria and curable STIs/RTIs in pregnancy are suboptimal. In West Africa, IPTp-SP continues to provide protection against the effects of malaria infection but, as previously noted, malaria parasites in East Africa have developed resistance so that IPTp-SP no longer protects against the malaria attributable fraction of low birthweight Citation[3]. Some evidence suggests that IPTp-SP may even be harmful in areas where parasites express the 581G dhps mutation Citation[4–6]. ABC therapies are likely to be more efficacious against malaria parasites in these settings. However, there is an urgent need for trials of ABC therapies to be conducted by independent researchers for policymakers to review alongside the results of trials produced and reported by industry.
In the case of curable STIs/RTIs, the focused ANC package recommended by the WHO includes screening for syphilis and the provision of BPG to women who test positive Citation[50]. Screening would need to continue even if ABC therapies were used in IPTp. The WHO currently recommends the use of rapid point of care tests for syphilis in the ANC setting Citation[113]. Using such tests will expedite case finding and treatment with BPG because results are available during the same consultation. As for the four other curable STIs/RTIs of this review, health care providers are limited to the use of a syndrome-based management algorithm to diagnose and to treat suspected infections. However, 80% of gonococcal and 70–75% chlamydial infections in women are asymptomatic Citation[114] and, therefore, rarely recognized using the syndromic approach. As a result, the diagnostic algorithm has a low sensitivity (30–80%) and specificity (40–80%) for N. gonorrhoeae and C. trachomatis among pregnant women Citation[115–117]. The sensitivity for T. vaginalis (54–83%) and bacterial vaginosis (51–69%) is slightly higher, with moderate specificity for T. vaginalis (40–54%) and bacterial vaginosis (40–58%) Citation[118]. Given the evidence of this review, ABC therapies used in IPTp could be expected to mitigate a considerable proportion of this unattended burden of curable STIs/RTIs.
Much is debated about the utility of a syndrome-based approach to diagnosing and treating many STIs/RTIs. Its shortcomings, as described above, illuminate a much-needed area for research. Specifically, more refined definitions of diagnosis need to be used when characterizing adverse birth outcomes. This is particularly so with T. vaginalis for which successful treatment does not necessarily reduce the risk of adverse birth outcomes. Similarly with bacterial vaginosis, treatment of women who have Nugent scores of 1–3 has not reduced the incidence of preterm birth. With both of these infections, is the recommended regimen of metronidazole inadequate for radical cure? Is it administered too late in pregnancy to alter the course of events? Or are asymptomatic infections simply much less virulent and, therefore, treatment has a marginal effect on selected downstream measures of the adverse birth outcome? Studies of descriptive epidemiology are needed to understand better the extent to which symptomatic versus asymptomatic curable STIs/RTIs contribute to adverse birth outcomes. Such descriptive epidemiology, however, would be incomplete if the prevalence of co-infections were not also considered. The apparent failure to reduce the incidence of adverse birth outcomes following treatment for one infection may be masked by the presence of co-infection(s) that will only be mitigated with the use of combination therapies and consideration of downstream outcomes. The trial in Uganda that failed to reduce the incidence of MTCT of HIV is illustrative. HIV transmission was not interrupted for providing combination treatment against curable STIs/RTIs, but significant reductions were observed in the incidence of neonatal deaths by 17% (RR: 0.83; 95% CI: 0.71–0.97) and low birthweight by 32% (RR: 0.68; 95% CI: 0.53–0.86) Citation[104].
Five-year view
Discussion of the future of IPTp needs to be placed in the context of broader malaria elimination efforts. IPTp-SP has long been considered a malaria control intervention that can be expected to protect less against the fraction of low birthweight attributable to malaria infection as malaria transmission decreases. Recent evidence suggests that IPTp-SP continues to protect against low birthweight among multigravidae in areas where the prevalence of malaria parasitemia measured in children is between 7 and 8%, whereas protection is conferred by IPTp-SP among paucigravidae until very low levels of transmission Citation[119]. Unpublished results from a recently completed multicenter trial in West Africa, where there remain malaria parasites sensitive to SP, indicate that an approach of intermittent screening and treatment (IST) of malaria in pregnancy is noninferior to IPTp-SP (Manuscript under review/personal communication with D. Chandramohan). Thus, there is an urgent need for clinical trials in an area of high SP resistance in East Africa, designed to compare ABC therapies versus IST versus IPTp-SP. ABC therapies, given their action against malaria and curable STIs/RTIs, would be superior to IST and IST would be superior to IPTp-SP, potentially paving the way for adoption of an integrated malaria and curable STI/RTI control package that employs the use of combination treatment.
Key issues
Use of azithromycin-based combination (ABC) therapies may have a transformative effect on maternal, fetal and newborn heath by mitigating the dual-burden of malaria and curable sexually transmitted infections and reproductive tract infections sexually transmitted infections and reproductive tract infections (STIs/RTIs) in pregnancy.
ABC therapies containing two or more grams of azithromycin may be less likely to select for resistance when exposed to Treponema pallidum, Neisseria gonorrhoeae and potentially, Chlamydia trachomatis.
In the absence of evidence that azithromycin is curative of congenital syphilis, not simply maternal infection, benzanthine penicillin G (BPG) will still need to be administered to pregnant women who have a syphilis infection; however, the combination of azithromycin plus BPG is more efficacious that BPG alone.
ABC therapies may be preventive of Treponema vaginalis infection during pregnancy, although its impact on birth outcomes needs to be investigated.
The most prevalent of all STIs/RTIs, bacterial vaginosis, may or may not be mitigated by the use of ABC therapies.
Studies of descriptive epidemiology are needed to understand better the extent to which symptomatic versus asymptomatic curable STIs/RTIs contribute to adverse birth outcomes. There is an urgent need for clinical trials in an area of high sulfadoxine-pyrimethamine resistance in East Africa, designed to compare ABC therapies versus IPTp-SP versus providing IST for malaria in pregnancy during ANC visits.
Financial & competing interests disclosure
MJ Newport is Director of the Wellcome Trust-Brighton and Sussex Centre for Global Health Research (grant number 100715). RM Chico receives funding from Medicines for Malaria Venture, a nonprofit foundation based in Geneva, Switzerland. E Ngulube is a Commonwealth Scholar. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- WHO. WHO Policy Recommendation: Intermittent Preventive Treatment of Malaria in Pregnancy using Sulfadoxine-Pyrimethamine (IPTp-SP). 11 April 2013 (2013).
- World Health Organization. World malaria report: 2012. World Health Organziation, Geneva (2012).
- Chico RM and Chandramohan D. Intermittent preventive treatment of malaria in pregnancy: at the crossroads of public health policy. Trop. Med. Int. Health 16(7), 774–785 (2011).
- Harrington WE, Mutabingwa TK, Muehlenbachs A et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc. Natl Acad. Sci. USA 106(22), 9027–9032 (2009).
- Harrington WE, Mutabingwa TK, Kabyemela E, Fried M and Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin. Infect. Dis. 53(3), 224–230 (2011).
- Harrington WE, Morrison R, Fried M and Duffy PE. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring. PLoS ONE, 8(2), e56183 (2013).
- Whitman MS and Tunkel AR. Azithromycin and clarithromycin: overview and comparison with erythromycin. Infect. Control Hosp. Epidemiol. 13(6), 357–368 (1992).
- Chico RM, Pittrof R, Greenwood B and Chandramohan D. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar. J. 7(1), 255 (2008).
- Sarkar M, Woodland C, Koren G and Einarson AR. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 6, 18 (2006).
- Kuschner R, Heppner D, Andersen S et al. Azithromycin prophylaxis against a chloroquine resistant strain of Plasmodium falciparum. Lancet 343(8910), 1396–1397 (1994).
- Anderson S, Berman J, Kuschner R et al. Prophylaxis of Plasmodium falciparum malaria with azithromycin administered to volunteers. Ann. Intern. Med. 123, 771–773 (1995).
- Ohrt C, Willingmyre G, Lee P, Knirsch C and Milhous W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 46, 2518–2524 (2002).
- Chico R M, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-saharan Africa. JAMA 307(19), 2079–2086 (2012).
- Williams JW. A textbook of obstetrics. D Appleton & Co, New York, USA 1923.
- Ratnam AV, Din SN, Hira SK et al. Syphilis in pregnant women in Zambia. Br. J. Vener Dis. 58(6), 355–358 (1982).
- Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C and Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 308(6924), 295–298 (1994).
- Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 189(1), 139–147 (2003).
- Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Redzko S, Przepiesc J, Zdrodowski M. The influence of Chlamydia trachomatis infection on spontaneous abortions. Adv. Med. Sci. 54(1), 86–90 (2009).
- McDermott J, Steketee R, Larsen S and Wirima J. Syphilis-associated perinatal and infant mortality in rural Malawi. Bull. World Health Organ. 71(6), 773–780 (1993).
- Temmerman M, Gichangi P, Fonck K et al. Effect of a syphilis control programme on pregnancy outcome in Nairobi, Kenya. Sex. Transm. Infect. 76(2), 117–121 (2000).
- Watson-Jones D CJ, Balthazar G, Weiss H et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J. Infect. Dis. 186, 940–947 (2002).
- Association of Chlamydia trachomatis and Mycoplasma hominis with intrauterine growth retardation and preterm delivery. The John Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Am. J. Epidemiol. 129(6), 1247–1257 (1989).
- Watson-Jones D, Changalucha J, Gumodoka B et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J. Infect. Dis. 186(7), 940–947 (2002).
- Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA and Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 256(14), 1899–1903 (1986).
- McGregor JA, French JI, Parker R et al. Prevention of premature birth by screening and treatment or common genital tract infections: results of a prospective controlled evaluation. Am. J. Obstet. Gynecol. 173(1), 157–167 (1995).
- Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: A population-based cohort study in Washington State. Sex. Transm. Infect. 83(4), 314–318 (2007).
- Elliott B, Brunham RC, Laga M et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J. Infect. Dis. 161(3), 531–536 (1990).
- Cotch MF, Pastorek JG 2nd, Nugent RP et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex. Transm. Dis. 24(6), 353–360 (1997).
- Svare JA, Schmidt H, Hansen BB, Lose G. Bacterial vaginosis in a cohort of Danish pregnant women: prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG 113(12), 1419–1425 (2006).
- Odendaal HJ, Schoeman J, Grove D et al. The association between Chlamydia trachomatis genital infection and spontaneous preterm labour. S. Afr. J. Obstet. Gynaecol. 12(3), 146–149 (2006).
- Watson-Jones D, Weiss HA, Changalucha JM et al. Adverse birth outcomes in United Republic of Tanzania--impact and prevention of maternal risk factors. Bull. World Health Organ. 85(1), 9–18 (2007).
- Rours GI, Duijts L, Moll HA et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur. J. Epidemiol. 26(6), 493–502 (2011).
- Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex. Transm. Dis. 38(3), 167–171 (2011).
- Donders GG, Desmyter J, De Wet DH, Van Assche FA. The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourin. Med. 69(2), 98–101 (1993).
- Sutton MY, Sternberg M, Nsuami M, Behets F, Nelson AM, St Louis ME. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected congolese women: prevalence, risk factors, and association with low birth weight. Am J Obstet Gynecol, 181(3), 656-662 (1999).
- Organization WH. Guidelines for the Management of Sexually Transmitted Infections. Switzerland (2003).
- Hook EW 3rd, Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann. Intern. Med. 131(6), 434–437 (1999).
- Klausner JD, Kohn RP, Kent CK. Azithromycin versus penicillin for early syphilis. N. Engl. J. Med. 354(2), 203–205, author reply 203–205 (2006).
- Hook EW, 3rd, Martin DH, Stephens J, Smith BS and Smith K. A randomized, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis. Sex. Transm. Dis. 29(8), 486–490 (2002).
- Kiddugavu MG, Kiwanuka N, Wawer MJ et al. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex. Transm. Dis. 32(1), 1–6 (2005).
- Riedner G, Rusizoka M, Todd J et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N. Engl. J. Med. 353(12), 1236–1244 (2005).
- Hook EW, 3rd, Behets F, Van Damme K et al. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J. Infect. Dis. 201(11), 1729–1735 (2010).
- Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin. Infect. Dis. 42(3), 337–345 (2006).
- Lukehart SA, Godornes C, Molini BJ et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N. Engl. J. Med. 351(2), 154–158 (2004).
- Marra CM, Colina AP, Godornes C et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J. Infect. Dis. 194(12), 1771–1773 (2006).
- Grimes M, Sahi SK, Godornes BC et al. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex. Transm. Dis. 39(12), 954–958 (2012).
- Chen XS, Yin YP, Wei WH et al. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin. Microbiol. Infect. 8(10), 1469–0691 (2012).
- Wu H, Chang SY, Lee NY et al. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J. Clin. Microbiol. 50(7), 2299–2304 (2012).
- Van Damme K, Behets F, Ravelomanana N et al. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex. Transm. Dis. 36(12), 775–776 (2009).
- World Health Organization. Sexually transmitted and other reproductive tract infections: Guide to essential practice. Department of Reproductive Health and Research, World Health Organization, Geneva (2005).
- Odugbemi T, Oyewole F, Isichei CS, Onwukeme KE and Adeyemi-Doro FA. Single oral dose of azithromycin for therapy of susceptible sexually transmitted diseases: a multicenter open evaluation. West Afr. J. Med. 12(3), 136–140 (1993).
- Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J. Antimicrob. Chemother. 31( Suppl. E), 193–198 (1993).
- Swanston WH, Prabhakar P, Barrow L, Mahabir BS, Furlonge C. Single dose (direct observed) azithromycin therapy for Neisseria gonorrhoeae and Chlamydia trachomatis in STD clinic attenders with genital discharge in Trinidad and Tobago. West Indian Med. J. 50(3), 198–202 (2001).
- Lassus A. Comparative studies of azithromycin in skin and soft-tissue infections and sexually transmitted infections by Neisseria and Chlamydia species. J. Antimicrob. Chemother. 25( Suppl. A), 115–121 (1990).
- Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB and Tilton RC. Azithromycin in the treatment of sexually transmitted disease. J. Antimicrob. Chemother. 25( Suppl. A), 109–114 (1990).
- Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB and Tilton RC. Single dose azithromycin treatment of gonorrhea and infections caused by C. trachomatis and U. urealyticum in men. Sex. Transm. Dis. 21(1), 43–46 (1994).
- Gruber F, Grubisic-Greblo H, Jonjic A et al. Treatment of gonococcal and chlamydial urethritis with azithromycin or doxycycline. Chron. Derm. (Roma) 5, 213–218 (1995).
- Gruber F, Brajac I, Jonjic A, Grubisic-Greblo H, Lenkovic M, Stasic A. Comparative trial of azithromycin and ciprofloxacin in the treatment of gonorrhea. J. Chemother. 9(4), 263–266 (1997).
- Rustomjee R, Kharsany AB, Connolly CA, Karim SS. A randomized controlled trial of azithromycin versus doxycycline/ciprofloxacin for the syndromic management of sexually transmitted infections in a resource-poor setting. J. Antimicrob. Chemother. 49(5), 875–878 (2002).
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials 7(3), 177–188 (1986).
- Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex. Transm. Infect. 86(6), 422–426 (2010).
- World Health Organization, London School of Hygiene and Tropical Medicine and International Trachoma Initiative. Trachoma control: A guide for programme managers. World Health Organization, Switzerland (2006).
- Handsfield HH, Dalu ZA, Martin DH, Douglas JM Jr, McCarty JM, Schlossberg D. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex. Transm. Dis. 21(2), 107–111 (1994).
- Khaki P, Bhalla P, Sharma A, Kumar V. Correlation between In vitro susceptibility and treatment outcome with azithromycin in gonorrhoea: a prospective study. Indian J. Med. Microbiol. 25(4), 354–357 (2007).
- Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 21(5), 414–419 (2003).
- Martin I, Jayaraman G, Wong T, Liu G, Gilmour M, Canadian Public Health Laboratory N. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009. Sex. Transm. Dis. 38(10), 892–898 (2011).
- Martin I, Jayaraman G, Wong T, Liu G and Gilmour M. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009. Sex. Transm. Dis. 38(10), 892–898. (2011).
- Cole MJ, Chisholm SA, Hoffmann S et al. European surveillance of antimicrobial resistance in Neisseria gonorrhoeae. Sex. Transm. Infect. 86(6), 427–432 (2010).
- Centers for Disease Control and Prevention. Neisseria gonorrhoeae with reduced susceptibility to azithromycin --- San Diego County, California, 2009. MMWR Morb. Mortal. Wkly Rep. 60(18), 579–581 (2011).
- Starnino S, Galarza P, Carvallo ME et al. Retrospective analysis of antimicrobial susceptibility trends (2000-2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean shows evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone. Sex. Transm. Dis. 39(10), 813–821. (2012).
- Khaki P, Bhalla P, Sharma P, Chawla R, Bhalla K. Epidemilogical analysis of Neisseria gonorrhoeae isolates by antimicrobial susceptibility testing, auxotyping and serotyping. Indian J. Med. Microbiol. 25(3), 225–229 (2007).
- Sethi S, Golparian D, Bala M et al. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from India, Pakistan and Bhutan in 2007-2011. BMC Infect. Dis. 13, 35 (2013).
- Kacmar J, Cheh E, Montagno A and Peipert JF. A randomized trial of azithromycin versus amoxicillin for the treatment of Chlamydia trachomatis in pregnancy. Infect. Dis. Obstet. Gynecol. 9(4), 197–202 (2001).
- Jacobson GF, Autry AM, Kirby RS, Liverman EM, Motley RU. A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy. Am. J. Obstet. Gynecol. 184(7), 1352–1354, discussion 1354–1356 (2001).
- Wehbeh HA, Ruggeirio RM, Shahem S, Lopez G, Ali Y. Single-dose azithromycin for Chlamydia in pregnant women. J. Reprod. Med. 43(6), 509–514 (1998).
- Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstet. Gynecol. 91(2), 165–168 (1998).
- Gunter ME AC, Ernest JM, McElroy G. Azithromycin powder versus erythromycin in the treatment of chlamydial cervicitis in pregnancy. Infect. Dis. Obstet. Gynecol. 4(53) (1996).
- Edwards MS, Newman RB, Carter SG, Leboeuf FW, Menard MK, Rainwater KP. Randomized clinical trial of azithromycin vs. erythromycin for the treatment of chlamydia cervicitis in pregnancy. Infect. Dis. Obstet. Gynecol. 4(6), 333–337 (1996).
- Rosenn MF, Macones GA and Silverman NS. Randomized trial of erythromycin and azithromycin for treatment of chlamydial infection in pregnancy. Infect Dis Obstet Gynecol, 3(6), 241-244 (1995).
- Bush MR and Rosa C. Azithromycin and erythromycin in the treatment of cervical chlamydial infection during pregnancy. Obstet. Gynecol. 84(1), 61–63 (1994).
- Golden MR, Whittington WL, Handsfield HH et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N. Engl. J. Med. 352(7), 676–685 (2005).
- Katz BP FD, Orr DP. Factors affecting chlamydial persistence or recurrence one and three months after treatment. Chlamydial infections. Proceedings of the ninth international symposium on human chlamydial infection. Stephens RS, Byrne GI, Christiansen G et al.( Eds). International Chlamydia Symposium, USA 35–38 (1998).
- Horner P. The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection. Sex. Transm. Infect. 82(4), 340–343 (2006).
- Samra Z, Rosenberg S, Soffer Y, Dan M. In vitro susceptibility of recent clinical isolates of Chlamydia trachomatis to macrolides and tetracyclines. Diag. Microbiol. Infect. Dis. 39(3), 177–179 (2001).
- Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181(4), 1421–1427 (2000).
- Lefevre JC and Lepargneur JP. Comparative in vitro susceptibility of a tetracycline-resistant Chlamydia trachomatis strain isolated in Toulouse (France). Sex. Transm. Dis. 25(7), 350–352 (1998).
- Jones RB, Van der Pol B, Martin DH, Shepard MK. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J. Infect. Dis. 162(6), 1309–1315 (1990).
- Dreses-Werringloer U, Padubrin I, Zeidler H, Kohler L. Effects of azithromycin and rifampin on Chlamydia trachomatis infection in vitro. Antimicrob. Agents Chemother. 45(11), 3001–3008 (2001).
- Donati M, Rodriguez Fermepin M, Olmo A, D'Apote L, Cevenini R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J. Antimicrob. Chemother. 43(6), 825–827 (1999).
- Misyurina OY, Chipitsyna EV, Finashutina YP et al. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides. Antimicrob. Agents Chemother. 48(4), 1347–1349 (2004).
- Rice RJ, Bhullar V, Mitchell SH, Bullard J, Knapp JS. Susceptibilities of Chlamydia trachomatis isolates causing uncomplicated female genital tract infections and pelvic inflammatory disease. Antimicrob. Agents Chemother. 39(3), 760–762 (1995).
- Bhengraj AR, Vardhan H, Srivastava P, Salhan S, Mittal A. Decreased susceptibility to azithromycin and doxycycline in clinical isolates of Chlamydia trachomatis obtained from recurrently infected female patients in India. Chemotherapy 56(5), 371–377 (2010).
- Kaul R, Kimani J, Nagelkerke NJ et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 291(21), 2555–2562 (2004).
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am. J. Trop. Med. Hyg. 83(6), 1212–1220 (2010).
- Wawer MJ, Sewankambo NK, Serwadda D et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 353(9152), 525–535 (1999).
- Cowan FM, Hargrove JW, Langhaug LF et al. The appropriateness of core group interventions using presumptive periodic treatment among rural Zimbabwean women who exchange sex for gifts or money. J. Acquir. Immune Defic. Syndr. 38(2), 202–207 (2005).
- Hay PE. Therapy of bacterial vaginosis. J. Antimicrob. Chemother. 41(1), 6–9 (1998).
- Eschenbach DA. Bacterial vaginosis: resistance, recurrence, and/or reinfection? Clin. Infect. Dis. 44(2), 220–221 (2007).
- Schwebke JR and Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin. Infect. Dis. 44(2), 213–219 (2007).
- Cohen MS, Hoffman IF, Royce RA et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 349(9069), 1868–1873 (1997).
- McClelland RS, Wang CC, Mandaliya K et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS (Lond., Engl.), 15(1), 105–110 (2001).
- Wang CC, McClelland RS, Reilly M et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J. Infect. Dis. 183(7), 1017–1022 (2001).
- Price MA, Zimba D, Hoffman IF et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex. Transm. Dis. 30(6), 516–522 (2003).
- Gray RH, Wabwire-Mangen F, Kigozi G et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am. J. Obstet. Gynecol. 185(5), 1209–1217 (2001).
- Aires FT, Soares RP and Bernardo WM. Efficacy of azithromycin on the treatment of syphilis. Rev. Assoc. Med. Bras. 56(5), 496 (2010).
- Zhou P, Qian Y, Xu J, Gu Z and Liao K. Occurrence of congenital syphilis after maternal treatment with azithromycin during pregnancy. Sex. Transm. Dis. 34(7), 472–474 (2007).
- Luntamo M, Kulmala T, Mbewe B, Cheung Y B, Maleta K, Ashorn P. Effect of Repeated Treatment of Pregnant Women with Sulfadoxine-Pyrimethamine and Azithromycin on Preterm Delivery in Malawi: A Randomized Controlled Trial. Am. J. Trop. Med. Hyg. 83, 1212–1220 (2010).
- Kigozi GG, Brahmbhatt H, Wabwire-Mangen F et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am. J. Obstet. Gynecol. 189(5), 1398–1400 (2003).
- Klebanoff MA, Carey JC, Hauth JC et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N. Engl. J. Med. 345(7), 487–493 (2001).
- Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth. Samj South Afr. Med. J. 100(1), 58–64 (2010).
- Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 1, CD000262 (2013).
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29(2), 297–301 (1991).
- World Health Organization and Special Programme for Research and Training in Tropical Diseases. The use of rapid syphilis tests. WHO, Geneva (2006).
- World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. World Health Organization, Geneva (2001).
- Mayaud P, ka-Gina G, Cornelissen J et al. Validation of a WHO algorithm with risk assessment for the clinical management of vaginal discharge in Mwanza, Tanzania. Sex. Transm. Infect. 74( Suppl. 74), S77–S84 (1998).
- Vuylsteke B, Laga M, Alary M et al. Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire. Clin. Infect. Dis. 17(1), 82–88 (1993).
- Costello Daly C, Wangel AM, Hoffman IF et al. Validation of the WHO diagnostic algorithm and development of an alternative scoring system for the management of women presenting with vaginal discharge in Malawi. Sex. Transm. Infect. 74( Suppl. 74), S50–S58 (1998).
- Tann CJ, Mpairwe H, Morison L et al. Lack of effectiveness of syndromic management in targeting vaginal infections in pregnancy in Entebbe, Uganda. Sex. Transm. Infect. 82(4), 285–289 (2006).
- Chico RM, Ariti C, Cano J, Chandramohan D, Greenwood B. Malaria transmission intensity and the protective effect of intermittent preventive therapy using sulphadoxine-pyrimethamine. Evidence Review Group of the World Health Organization (2013).
- Temmerman M, Njagi E, Nagelkerke N, Ndinya-Achola J, Plummer FA, Meheus A. Mass antimicrobial treatment in pregnancy. A randomized, placebo-controlled trial in a population with high rates of sexually transmitted diseases. J. Reprod. Med. 40(3), 176–180 (1995).
- Elliott B, Brunham RC, Laga M et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J. Infect. Dis. 161(3), 531–536 (1990).
- Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex. Transm. Dis. 38(3), 167–171 (2011).
- Silveira MF, Ghanem KG, Erbelding EJ et al. Chlamydia trachomatis infection during pregnancy and the risk of preterm birth: A case-control study. Int. J. STD AIDS 20(7), 465–469 (2009).
- Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: A population-based cohort study in Washington State. Sex. Transm. Infect. 83(4), 314–318 (2007).
- Kovacs L, Nagy E, Berbik I, Meszaros G, Deak J, Nyari T. The frequency and the role of Chlamydia trachomatis infection in premature labor. Int. J. Gynaecol. Obstet. 62(1), 47–54 (1998).
- Investigators of the Johns Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Association of Chlamydia trachomatis and Mycoplasma hominis with intrauterine growth retardation and preterm delivery. Am. J. Epidemiol. 129, 1247–1257 (1989).
- Meis PJ, Goldenberg RL, Mercer B et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal M edicine Units Network. Am. J. Obstet. Gynecol. 173(4), 1231–1235 (1995).
- Minkoff H, Grunebaum AN, Schwarz RH et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150(8), 965–972 (1984).
- Hillier SL, Nugent RP, Eschenbach DA et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 333(26), 1737–1742 (1995).
- Kiddugavu MG, Kiwanuka N, Wawer MJ et al. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex. Transm. Dis. 32(1), 1–6 (2005).
- Hook EW 3rd, Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann. Int. Med. 131(6), 434–437 (1999).
- Riedner G, Rusizoka M, Todd J et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N. Engl. J. Med. 353(12), 1236–1244 (2005).
- Chen CY, Chi KH, Pillay A, Nachamkin E, Su JR, Ballard RC. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay. J. Clin. Microbiol. 51(3), 908–913 (2013).
- Muller EE, Paz-Bailey G and Lewis DA. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex. Transm. Infect. 88(6), 470–474 (2012).
- The A2058G Prevalence Workgroup. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex. Transm. Dis. 39(10), 794–798 (2012).
- Matejkova P, Flasarova M, Zakoucka H et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J. Med. Microbiol. 58(Pt 6), 832–836 (2009).
- Martin IE, Gu W, Yang Y, Tsang RS. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin. Infect. Dis. 49(4), 515–521 (2009).
- Tipple C, McClure MO, Taylor GP. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex. Transm. Infect. 87(6), 486–488 (2011).
- Rekart ML, Patrick DM, Chakraborty B et al. Targeted mass treatment for syphilis with oral azithromycin. Lancet 361(9354), 313–314 (2003).
- Muldoon EG, Walsh A, Crowley B, Mulcahy F. Treponema pallidum azithromycin resistance in Dublin, Ireland. Sex. Transm. Dis. 39(10), 784–786. (2012).
- Martin IE, Tsang RS, Sutherland K et al. Molecular typing of Treponema pallidum strains in western Canada: predominance of 14d subtypes. Sex. Transm. Dis. 37(9), 544–548 (2010).
- Morshed MG and Jones HD. Treponema pallidum macrolide resistance in BC. CMAJ 174(3), 349 (2006).
- Khaki P, Bhalla P, Sharma A, Kumar V. Correlation between In vitro susceptibility and treatment outcome with azithromycin in gonorrhoea: a prospective study. Indian J. Med. Microbiol. 25(4), 354–357 (2007).
- Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect. Dis. 13, 40 (2013).
- Lahra MM. Annual report of the Australian Gonococcal Surveillance Programme, 2011. Commun. Dis. Intell. Q Rep. 36(2), E166–E173. (2012).
- Lo JY, Ho KM, Lo AC. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance. J. Antimicrob. Chemother. 67(6), 1422–1426 (2012).
- Lefebvre B, Bourgault AM. P1-S1.44 Antimicrobial susceptibility profile of Neisseria gonorrhoeae isolates in the Province of Quebec - 2010. Sex. Transm. Infect. 87( Suppl. 87), A117 (2011).
- Hottes TS, Lester RT, Hoang LM et al. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011. Sex. Transm. Dis. 40(1), 46–51 (2013).
- Yuan LF, Yin YP, Dai XQ et al. Resistance to azithromycin of Neisseria gonorrhoeae isolates from 2 cities in China. Sex. Transm. Dis. 38(8), 764–768 (2011).
- Takahashi S, Kurimura Y, Hashimoto J et al. Antimicrobial susceptibility and penicillin-binding protein 1 and 2 mutations in Neisseria gonorrhoeae isolated from male urethritis in Sapporo, Japan. J. Infect. Chemother. 19(1), 50–56 (2013).
- Herchline TE, Inkrott BP. Resistance trends in Neisseria gonorrhoeae in southwestern Ohio. Sex. Transm. Dis. 37(2), 121–122 (2010).
- Olsen B, Mansson F, Camara C et al. Phenotypic and genetic characterisation of bacterial sexually transmitted infections in Bissau, Guinea-Bissau, West Africa: a prospective cohort study. BMJ Open. 2(2), e000636 (2012).
- Tanaka M, Koga Y, Nakayama H et al. Antibiotic-resistant phenotypes and genotypes of Neisseria gonorrhoeae isolates in Japan: identification of strain clusters with multidrug-resistant phenotypes. Sex. Transm. Dis. 38(9), 871–875 (2011).
- Bala M. Characterization of profile of multidrug-resistant Neisseria gonorrhoeae using old and new definitions in India over a decade: 2000–2009. Sex. Transm. Dis. 38(11), 1056–1058 (2011).
- Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J. Antimicrob. Chemother. 64(2), 353–358 (2009).
- Khaki P, Bhalla P, Sharma P, Chawla R, Bhalla K. Epidemilogical analysis of Neisseria gonorrhoeae isolates by antimicrobial susceptibility testing, auxotyping and serotyping. Indian J. Med. Microbiol. 225–229 (2007).
- Enders M, Turnwald-Maschler A, Regnath T. Antimicrobial resistance of Neisseria gonorrhoeae isolates from the Stuttgart and Heidelberg areas of southern Germany. Eur. J. Clin. Microbiol. Infect. Dis. 25(5), 318–322 (2006).
- Vorobieva V, Firsova N, Ababkova T et al. Antibiotic susceptibility of Neisseria gonorrhoeae in Arkhangelsk, Russia. Sex. Transm. Infect. 83(2), 133–135 (2007).
- Sutrisna A, Soebjakto O, Wignall FS et al. Increasing resistance to ciprofloxacin and other antibiotics in Neisseria gonorrhoeae from East Java and Papua, Indonesia, in 2004 - implications for treatment. J. Clin. Pathol. 60(1), 90–91 (2007).
- Martin IM, Hoffmann S, Ison CA. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J. Antimicrob. Chemother. 58(3), 587–593 (2006).
- Chaudhary C, Hasan Chaudhary FA, Pandy AR et al. A pilot study on antimicrobial susceptibility of Neisseria gonorrhoeae isolates from Nepal. Sex. Transm. Dis. 32(10), 641–643 (2005).
- Chen PL, Hsieh YH, Lee HC et al. Suboptimal therapy and clinical management of gonorrhoea in an area with high-level antimicrobial resistance. Int. J. STD AIDS 20(4), 225–228 (2009).
- Hsueh PR, Tseng SP, Teng LJ, Ho SW. High prevalence of ciprofloxacin-resistant Neisseria gonorrhoeae in Northern Taiwan. Clin. Infect. Dis. 40(1), 188–192 (2005).
- Aydin D, Kucukbasmaci O, Gonullu N, Aktas Z. Susceptibilities of Neisseria gonorrhoeae and Ureaplasma urealyticum isolates from male patients with urethritis to several antibiotics including telithromycin. Clin. Infect. Dis. 40(11), 1608–1616 (2005).
- Moodley P, Pillay C, Goga R, Kharsany AB, Sturm AW. Evolution in the trends of antimicrobial resistance in Neisseria gonorrhoeae isolated in Durban over a 5 year period: impact of the introduction of syndromic management. J. Antimicrob. Chemother. 48(6), 853–859 (2001).
- Kobayashi I, Kanayama A, Saika T et al. Tendency toward increase in the frequency of isolation of beta-lactamase-nonproducing Neisseria gonorrhoeae exhibiting penicillin resistance, and recent emergence of multidrug-resistant isolates in Japan. Pediatrics 112(1 Pt 1), 87–95 (2003).
- Llanes R, Sosa J, Guzman D et al. Antimicrobial susceptibility of Neisseria gonorrhoeae in Cuba (1995-1999): implications for treatment of gonorrhea. Sex. Transm. Dis. 30(1), 25–29 (2003).
- Sosa J, Ramirez-Arcos S, Ruben M et al. High percentages of resistance to tetracycline and penicillin and reduced susceptibility to azithromycin characterize the majority of strain types of Neisseria gonorrhoeae isolates in Cuba, 1995-1998. Sex. Transm. Dis. 30(5), 443–448 (2003).
- Dillon JA, Rubabaza JP, Benzaken AS et al. Reduced susceptibility to azithromycin and high percentages of penicillin and tetracycline resistance in Neisseria gonorrhoeae isolates from Manaus, Brazil, 1998. Sex. Transm. Dis. 28(9), 521–526 (2001).
- Zarantonelli L, Borthagaray G, Lee EH, Shafer WM. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. J. Antimicrob. Chemother. 44(3), 411–414 (1999).
- Young H, Moyes A, McMillan A. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. J. Antimicrob. Chemother. 39(5), 623–630. (1997).
- Mehaffey PC, Putnam SD, Barrett MS, Jones RN. Evaluation of in vitro spectra of activity of azithromycin, clarithromycin, and erythromycin tested against strains of Neisseria gonorrhoeae by reference agar dilution, disk diffusion, and Etest methods. Clin. Infect. Dis. 22(2), 233–239. (1996).
- Dillon JA, Li H, Sealy J, Ruben M, Prabhakar P. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from three Caribbean countries: Trinidad, Guyana, and St. Vincent. Sex. Transm. Dis. 28(9), 508–514 (2001).
- van Rijsoort-Vos JH, Stolz E, Verbrugh HA, Kluytmans JA. In-vitro activity of a new quinolone (CP-99,219) compared with ciprofloxacin, pefloxacin, azithromycin and penicillin against Neisseria gonorrhoeae. J. Antimicrob. Chemother. 36(1), 215–218 (1995).
- Ison CA, Roope NS, Dangor Y, Radebe F, Ballard R. Antimicrobial susceptibilities and serotyping of Neisseria gonorrhoeae in southern Africa: influence of geographical source of infection. Epidemiol. Infect. 110(2), 297–305 (1993).
- Starnino S, Stefanelli P. Azithromycin-resistant Neisseria gonorrhoeae strains recently isolated in Italy. J. Antimicrob. Chemother. 63(6), 1200–1204 (2009).
- Donegan EA, Wirawan DN, Muliawan P et al. Fluoroquinolone-resistant Neisseria gonorrhoeae in Bali, Indonesia: 2004. Sex. Transm. Dis. 33(10), 625–629 (2006).
- Morris SR, Moore DF, Hannah PB et al. Strain typing and antimicrobial resistance of fluoroquinolone-resistant Neisseria gonorrhoeae causing a California infection outbreak. J. Clin. Microbiol. 47(9), 2944–2949 (2009).
- Ieven M, Van Looveren M, Sudigdoadi S et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains isolated in Java, Indonesia. Sex. Transm. Dis. 30(1), 25–29 (2003).
- Centers for Disease C and Prevention. Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR Morb. Mortal. Wkly Rep. 49(37), 833–837 (2000).
- Bruck PE, Robertson C, Allan PS. Management of Neisseria gonorrhoeae infection over 12 months in a genitourinary medicine setting against British Association for Sexual Health and HIV auditable outcome measures. Int. J. STD AIDS 23(3), e30–e32 (2012).
- Dan M, Mor Z, Gottliev S, Sheinberg B, Shohat T. Trends in antimicrobial susceptibility of Neisseria gonorrhoeae in Israel, 2002 to 2007, with special reference to fluoroquinolone resistance. Sex. Transm. Dis. 37(7), 451–453 (2010).
- McLean CA, Wang SA, Hoff GL et al. The emergence of Neisseria gonorrhoeae with decreased susceptibility to Azithromycin in Kansas City, Missouri, 1999 to 2000. Sex. Transm. Dis. 31(2), 73–78 (2004).
- Arreaza L, Vazquez F, Alcala B, Otero L, Salcedo C, Vazquez JA. Emergence of gonococcal strains with resistance to azithromycin in Spain. J. Antimicrob. Chemother. 51(1), 190–191 (2003).
- Rahangdale L, Guerry S, Bauer HM et al. An observational cohort study of Chlamydia trachomatis treatment in pregnancy. Sex. Transm. Dis. 33(2), 106–110 (2006).
- Miller JM. Efficacy and tolerance of single-dose azithromycin for treatment of chlamydial cervicitis during pregnancy. Infect. Dis. Obstet. Gynecol. 3(5), 189–192 (1995).
- Ljubin-Sternak S, Mestrovic T, Vilibic-Cavlek T et al. In vitro susceptibility of urogenital Chlamydia trachomatis strains in a country with high azithromycin consumption rate. Folia Microbiol. 29, 29 (2012).
- Donati M, Di Francesco A, D'Antuono A et al. In vitro activities of several antimicrobial agents against recently isolated and genotyped Chlamydia trachomatis urogenital serovars D through K. Antimicrob. Agents Chemother. 54(12), 5379–5380 (2010).
- Hong KC, Schachter J, Moncada J, Zhou Z, House J, Lietman TM. Lack of macrolide resistance in Chlamydia trachomatis after mass azithromycin distributions for trachoma. Emerg. Infect. Dis. 15(7), 1088–1090 (2009).
- Lefevre JC, Escaffre MC, Courdil M, Lareng MB. In vitro evaluation of activities of azithromycin, clarithromycin and sparfloxacin against Chlamydia trachomatis. Pathol. Biol. 41(4), 313–315 (1993).
- Agacfidan A, Moncada J, Schachter J. In vitro activity of azithromycin (CP-62,993) against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob. Agents Chemother. 37(9), 1746–1748 (1993).
- Scieux C, Bianchi A, Chappey B, Vassias I, Perol Y. In-vitro activity of azithromycin against Chlamydia trachomatis. J. Antimicrob. Chemother. 7–10 (1990).
- Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181(4), 1421–1427 (2000).
- van den Broek NR, White SA, Goodall M et al. The APPLe Study: A Randomized, Community-Based, Placebo-Controlled T rial of Azithromycin for the Prevention of Preterm Birth, with Meta-Analysis. PLoS Med. 6(12), e1000191 (2009).
- Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine-pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Trop. Med. Int. Health 18(4), 386–397 (2013).
- Chico RM, Chandramohan D. Azithromycin plus chloroquine: combination therapy for protection against malaria and sexually transmitted infections in pregnancy. Expert Opin. Drug Metab. Toxicol. 7(9), 1153–1167 (2011).
- Labbe AC, Pepin J, Khonde N et al. Periodical antibiotic treatment for the control of gonococcal and chlamydial infections among sex workers in Benin and Ghana: a cluster-randomized placebo-controlled trial. Sex. Transm. Dis. 39(4), 253–259 (2012).
- Wi T, Ramos ER, Steen R et al. STI declines among sex workers and clients following outreach, one time presumptive treatment, and regular screening of sex workers in the Philippines. Sex. Transm. Infect. 82(5), 386–391 (2006).
- Williams BG, Taljaard D, Campbell CM et al. Changing patterns of knowledge, reported behaviour and sexually transmitted infections in a South African gold mining community. AIDS 17(14), 2099–2107 (2003).
- Steen R, Vuylsteke B, DeCoito T et al. Evidence of declining STD prevalence in a South African mining community following a core-group intervention. Sex. Transm. Dis. 27(1), 1–8 (2000).
- Jones BM, Kinghorn GR, Duerden BI. In vitro activity of azithromycin and erythromycin against organisms associated with bacterial vaginosis and chancroid. Eur. J. Clin. Microbiol. Infect. Dis. 7(4), 551–553 (1988).
- Shanker S, Toohey M, Munro R. In vitro activity of seventeen antimicrobial agents against Gardnerella vaginalis. Eur. J. Clin. Microbiol. 1(5), 298–300 (1982).
- Ridgway GL. A review of the in-vitro activity of roxithromycin against genital pathogens. J. Antimicrob. Chemother. 20( Suppl. B), 7–11 (1987).
- Dubreuil L. In-vitro comparison of roxithromycin and erythromycin against 900 anaerobic bacterial strains. J. Antimicrob. Chemother. 20( Suppl. B), 13–19 (1987).
- Maskell JP, Sefton AM, Williams JD. Comparative in-vitro activity of azithromycin and erythromycin against Gram-positive cocci, Haemophilus influenzae and anaerobes. J. Antimicrob. Chemother. 25( Suppl. A), 19–24 (1990).
- Chang SC, Chen YC, Luh KT, Hsieh WC. Macrolides resistance of common bacteria isolated from Taiwan. Diag. Microbiol. Infect. Dis. 23(4), 147–154 (1995).
- Ednie LM, Spangler SK, Jacobs MR, Appelbaum PC. Antianaerobic activity of the ketolide RU 64004 compared to activities of four macrolides, five beta-lactams, clindamycin, and metronidazole. Antimicrob. Agents Chemother. 41(5), 1037–1041 (1997).
- Mikamo H, Yin XH, Ninomiya M, Tamaya T. In vitro and in vivo antibacterial activities of telithromycin. Chemotherapy 49(1–2), 62–65 (2003).
- Marina M, Ivanova M, Kantardjiev T. Antimicrobial susceptibility of anaerobic bacteria in Bulgaria. Anaerobe 15(4), 127–132 (2009).
- Chen SC, Gottlieb T, Palmer JM, Morris G, Gilbert GL. Antimicrobial susceptibility of anaerobic bacteria in Australia. J. Antimicrob. Chemother. 30(6), 811–820 (1992).
- Wexler HM, Molitoris E, Molitoris D, Finegold SM. In vitro activity of telithromycin (HMR 3647) against 502 strains of anaerobic bacteria. J. Antimicrob. Chemother. 47(4), 467–469 (2001).
- Spiegel CA. Susceptibility of Mobiluncus species to 23 antimicrobial agents and 15 other compounds. Antimicrob. Agents Chemother. 31(2), 249–252 (1987).
Website
- ReMeD (Network for Medicine and Development). Essential Medicines: Madagascar. World Health Organization www.who.int/selection_medicines/country_lists/mdg/en/.
Notice of correction
Since publication of this article the Financial & competing interests disclosure has been updated.