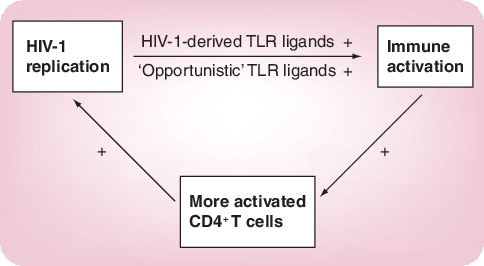
Chronic immune activation is a hallmark of progressive HIV-1 infection. Pattern recognition receptors have recently been proposed to contribute to the persistent immune activation in HIV-1 infection. Interfering with Toll-like receptor (TLR) signaling might, therefore, help to disrupt the vicious cycle between viral replication and enhanced immune activation that leads to CD4+ T-cell loss in HIV-1 infection.
Immune activation in HIV-1 infection
Approximately 40 million individuals worldwide are currently infected with HIV/AIDS and more than 20 million have died as a result of the epidemic. Understanding the mechanisms through which HIV-1 evades the human immune system or actually uses the immune response to propagate its replication provides the key for developing new therapeutic approaches.
Persistent activation of the immune system is a central characteristic of chronic HIV-1 disease and markers of immune cell activation strongly predict the rate of CD4+ T-cell loss and progression to AIDS in untreated infection Citation[1]. A widely accepted model of HIV-1 immunopathogenesis postulates that the accelerated immune activation results in proliferation, functional impairment and eventual exhaustion of the immune system. Furthermore, persistent immune activation may facilitate viral infection of activated CD4+ T cells, the preferential targets of HIV-1 replication Citation[2], as well as subsequent loss of T cells.
Further support for the central role that immune activation plays in HIV-1 pathogenesis and disease progression comes from several studies comparing disease progression following infection with SIV in two monkey models: the natural host of SIV, the sooty mangabeys, which do not progress to AIDS despite high viral replication, and the rhesus macaques, which progress to AIDS in a manner similar to HIV-1-infected humans. SIV-infected rhesus macaques exhibit immediate and persistent immune activation and proliferation, as well as subsequent CD4+ T-cell depletion and progression to AIDS Citation[3]. By contrast, sooty mangabeys display very little immune activation and do not progress to AIDS, despite high viral loads Citation[3]. These studies suggest that the limited immune activation, that is, the ability of sooty mangabeys to ‘ignore’ the replicating virus, might display a key mechanism in the natural host of SIV to avoid immunodeficiency, despite high viral replication Citation[4]. The underlying mechanisms leading to persistent immune activation in viremic HIV-1 infection, however, remain poorly understood.
TLRs sense infections & initiate the immune response against invading microorganisms
TLRs are highly conserved pattern-recognition receptors specialized in detecting foreign material Citation[5]. These receptors allow TLR-expressing cells to respond to patterns associated with non-self (pathogen-associated molecular patterns [PAMPs]) and thereby recognize and respond to invading pathogens. TLRs therefore play a crucial role in initiating the innate immune response to infections, and also help to shape the subsequent adaptive immune responses. To date, a total of 13 TLR molecules are known and ligands have been defined for most of them in animal models and humans Citation[5,6]. TLR ligation initiates complex signaling cascades ultimately leading to activation of transcription factors, such as nuclear factor-κB (NF-κB), as well as subsequent differential gene expression Citation[7], which can vary depending on which TLR has been activated. The activation of TLR3, 7, 8 and 9, which can sense viral RNA and DNA, leads to the expression of type I interferons, whereas bacterial PAMPs induce secretion of proinflammatory cytokines and chemokines via TLR2, 4 and 5.
A variety of different studies have demonstrated the essential role of TLRs for detecting viruses and subsequently eliciting antiviral responses Citation[5]. TLR signaling was shown to be essential in clearing viral infections, such as vesicular stomatitis virus, cytomegalovirus and hepatitis B virus in vivoCitation[8–10]. Mice lacking the TLR adaptor molecules MyD88 or IRAK-4 were shown to be more susceptible to viral infections Citation[11,12]. Furthermore, knockout mice for certain TLRs or mice with mutations within TLR genes were shown to be highly susceptible to a variety of viral infections, including respiratory syncytial virus and cytomegalovirus, compared with wild-type mice Citation[9,13]. Several studies have shown that, in order to overcome recognition by TLRs and to evade eventual elimination by the immune response, some viruses, including Ebola virus, Marburg virus, respiratory syncytial virus and measles virus, have developed mechanisms to specifically inhibit TLR-mediated antiviral responses Citation[14–17], further demonstrating the importance of TLR signaling for defense against viral infections.
As a consequence of their crucial role in antiviral immunity, TLR agonists have been tested clinically and used successfully as therapeutics in a diversity of viral infections, such as hepatitis C virus, herpes simplex virus (HSV), human papillomavirus and influenza Citation[18–21]. UC-1V150/MSA, a TLR7 agonist conjugate, was demonstrated to lower mortality significantly in mice challenged with H1N1 influenza virus Citation[21]. In humans, isatoribine, a TLR7 agonist, was shown to lower viral loads significantly in chronic hepatitis C virus infection Citation[20]. In a recent controlled trial, a topical TLR7 agonist reduced HSV shedding and reduced the frequency of mucosal HSV-2 reactivation Citation[22]. Furthermore, TLR agonists provide an important and clinically established role in treatment for genital warts caused by human papillomavirus Citation[23]. Taken together, these studies have demonstrated that TLR ligands can play a crucial role in enhancing virus-specific immunity.
TLR stimulation might contribute to immune activation in HIV-1 infection
While the activation of the immune system through TLRs provides a powerful tool contributing to the elimination of the invading pathogen, continuous immune activation through TLRs might contribute to the pathogenesis of other persistent infections. In HIV-1 infection, the immune system fails to eliminate the infecting virus and full containment of viral replication by the immune system is only achieved in a very small fraction of infected individuals Citation[24]. By contrast, the majority of HIV-1-infected individuals present with persistent viral replication and chronic activation of the immune system, resulting in both the continuous loss of CD4+ T cells and the progression of disease and death in the absence of highly active antiviral drug therapy. Some recent studies have proposed TLR signaling as an underlying cause for the persistent immune activation observed in progressive HIV-1 disease.
Brenchley and colleagues demonstrated that plasma levels of the TLR4 ligand lipopolysaccharide (LPS), which is part of the cell wall of Gram-negative bacteria, is elevated in chronic HIV-1 infection, as well as in chronically SIV-infected rhesus macaques Citation[25]. In SIV-infected macaques, LPS levels and immune activation were reduced by eliminating bacteria from the gut using antibiotic treatment, suggesting that the source of systemic LPS was the gut of these monkeys Citation[25]. In primary HIV-1 and SIV infection, the gut mucosa is the major site of viral replication and CD4+ T-cell depletion Citation[26,27], resulting in a severe impairment of the gut-associated lymphoid tissue and potentially allowing resident bacteria of the gastrointestinal flora to translocate into the blood stream. Brenchley and colleagues also demonstrated that levels of plasma LPS were closely correlated with markers of T-cell activation and elevated cytokine levels in the peripheral blood of chronically HIV-1-infected humans. These data suggest that bacterial TLR ligands reaching the circulation following translocation through a severely immunocompromised, ‘leaky’ gut might be a cause of systemic immune activation in chronic HIV-1 infection Citation[25,28].
In addition to these bacterial PAMPs that can reach the circulation as a consequence of the destruction of the host’s immune response by HIV-1, PAMPs encoded by HIV-1 itself may also contribute to the immune activation observed in HIV-1 infection. Immune activation declines rapidly with the initiation of highly active antiretroviral therapy (HAART) and increases within days once HIV-1 viremia rebounds following interruption of HAART Citation[29], suggesting that viral replication contributes directly to the high level of immune cell activation. The intracellularly located TLRs TLR7 and 8 can detect viral ssRNA Citation[30], and a 20-nucleotide-long sequence in the TLR region of HIV-1 has been shown to signal through TLR8 Citation[31]. We recently identified a number of additional TLR7/8 ligands within the ssRNA of HIV-1 and demonstrated that these HIV-1-encoded TLR7/8 ligands have a strong MyD88-dependent immune stimulatory activity Citation[32]. Furthermore, TLR7 has been demonstrated to be crucial for the detection of HIV-1 by human plasmacytoid dendritic cells Citation[33]. TLR7/8 recognition of HIV-1-encoded PAMPs might, therefore, contribute to the strong immune activation observed in viremic HIV-1 infection .
Other human diseases that are characterized by an overactive immune response potentially triggered by TLR signaling, such as systemic lupus erythematosus and rheumatoid arthritis, have been suggested to benefit from anti-TLR signaling treatment, such as blocking the TLR pathway Citation[34], and several companies are developing compounds that can interfere with TLR ligation or signaling in order to better and more specifically treat autoimmune diseases in the future. It will be of interest to explore the ability of these TLR antagonists to reduce immune activation, loss of CD4+ T cells and potentially even activation-induced viral replication in HIV-1-infected individuals.
Successful antiretroviral treatment suppressing HIV-1 replication significantly lowers immune activation and disease progression; however, viral resistance to commonly used antiretroviral therapies has become an increasing problem and, therefore, additional therapeutic approaches are needed. In the past, several studies have aimed to nonspecifically suppress immune activation in early HIV-1 infection, using immunosuppressive drugs Citation[35,36]. However, long-term therapy with immunosuppressive compounds harbors the risk of serious side effects. A more specific blockade of the receptors mediating the chronic immune activation in HIV-1 infection would, therefore, be highly desirable. Pilot studies are now needed to initially test the ability of specific TLR7/8 agonists to reduce immune activation in SIV-infected macaques. If successful and safe, this approach might represent an interesting future avenue for the treatment of HIV-1 infection and might ultimately delay the need for initiating HAART.
References
- Fahey JL, Taylor JM, Detels R et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med.322(3), 166–172 (1990).
- Douek DC, Brenchley JM, Betts MR et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature417(6884), 95–98 (2002).
- Silvestri G, Sodora DL, Koup RA et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity18(3), 441–452 (2002).
- Silvestri G, Fedanov A, Germon S et al. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol.79(7), 4043–4054 (2005).
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol.21, 335–376 (2003).
- Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol.5(10), 975–979 (2004).
- Takeda K, Akira S. TLR signaling pathways. Semin. Immunol.16(1), 3–9 (2004).
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo.J. Virol.79(11), 7269–7272 (2005).
- Tabeta K, Georgel P, Janssen E. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl Acad. Sci. USA101(10), 3516–3521 (2004).
- Zhou S, Kurt-Jones EA, Fitzgerald KA et al. Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J. Immunol.178(8), 5173–5181 (2007).
- Suzuki N, Suzuki S, Duncan GS et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature416(6882), 750–756 (2002).
- Mansur DS, Kroon EG, Nogueira ML et al. Lethal encephalitis in myeloid differentiation factor 88-deficient mice infected with herpes simplex virus 1. Am. J. Pathol.166(5), 1419–1426 (2005).
- Kurt-Jones EA, Popova L, Kwinn L et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol.1(5), 398–401 (2000).
- Kash JC, Muhlberger E, Carter V et al. Global suppression of the host antiviral response by Ebola- and Marburg viruses: increased antagonism of the type I interferon response is associated with enhanced virulence. J. Virol.80(6), 3009–3020 (2006).
- Stack J, Haga IR, Schroder M et al. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med.201(6), 1007–1018 (2005).
- Hahm B, Arbour N, Oldstone MB. Measles virus interacts with human SLAM receptor on dendritic cells to cause immunosuppression. Virology323(2), 292–302 (2004).
- Hahm B, Cho JH, Oldstone MB. Measles virus-dendritic cell interaction via SLAM inhibits innate immunity: selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology358(2), 251–257 (2007).
- Ashkar AA, Bauer S, Mitchell WJ, Vieira J, Rosenthal KL. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J. Virol.77(16), 8948–8956 (2003).
- Herbst-Kralovetz MM, Pyles RB. Quantification of poly(I:C)-mediated protection against genital herpes simplex virus type 2 infection. J. Virol.80(20), 9988–9997 (2006).
- Horsmans Y, Berg T, Desager JP et al. Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology42(3), 724–731 (2005).
- Wu CC, Hayashi T, Takabayashi K et al. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc. Natl Acad. Sci. USA104(10), 3990–3995 (2007).
- Mark KE, Corey L, Meng TC et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J. Infect. Dis.195(9), 1324–1331 (2007).
- Syed TA, Hadi SM, Qureshi ZA, Ali SM, Kwah MS. Treatment of external genital warts in men with imiquimod 2% in cream. A placebo-controlled, double-blind study. J. Infect.41(2), 148–151 (2000).
- Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science272(5265), 1167–1170 (1996).
- Brenchley JM, Price DA, Schacker TW et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med.12(12), 1365–1371 (2006).
- Brenchley JM, Schacker TW, Ruff LE et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med.200(6), 749–759 (2004).
- Mehandru S, Poles MA, Tenner-Racz K et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med.200(6), 761–770 (2004).
- Haynes BF. Gut microbes out of control in HIV infection. Nat. Med.12(12), 1351–1352 (2006).
- Libois A, Lopez A, Garcia F et al. Dynamics of T cells subsets and lymphoproliferative responses during structured treatment interruption cycles and after definitive interruption of HAART in early chronic HIV type-1-infected patients. AIDS Res. Hum. Retroviruses22(7), 657–666 (2006).
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science303(5663), 1529–1531 (2004).
- Heil F, Hemmi H, Hochrein H et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science303(5663), 1526–1529 (2004).
- Meier A, Alter G, Frahm N et al. MyD88-dependent Immune Activation mediated by HIV-1-encoded TLR Ligands. J. Virol. DOI: 10.1128/JVI.00421-07 (2007).
- Beignon AS, McKenna K, Skoberne M et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor- viral RNA interactions. J. Clin. Invest.115(11), 3265–3275 (2005).
- Barrat FJ, Meeker T, Gregorio J et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med.202(8), 1131–1139 (2005).
- Martin LN, Murphey-Corb M, Mack P et al. Cyclosporin A modulation of early virologic and immunologic events during primary simian immunodeficiency virus infection in rhesus monkeys. J. Infect. Dis.176(2), 374–383 (1997).
- Rizzardi GP, Harari A, Capiluppi B et al. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J. Clin. Invest.109(5), 681–688 (2002).