Abstract
Stereotactic biopsies are frequently performed to secure definitive diagnosis for brain tumor patients. Fundamentally, there are two major difficulties in these endeavors. First, because of intra-tumoral heterogeneity inherent in many forms of brain cancer, biopsies taken from one region may yield a different diagnosis than if another area is biopsied. Second, stereotactic needle biopsies inherently rely on mathematical algorithms for targeting, without real-time visualization of the actual biopsy site. This article describes the novel MRI-based technologies that can potentially afford neurosurgeons the opportunity to address these challenges.
For most solid tumors, definitive diagnosis requires securement of abnormal tissue and careful pathologic examination of this tissue. This tissue diagnosis remains the cornerstone of subsequent therapy Citation[1]. However, there are a number of cancers where intrinsic intra-tumoral histologic heterogeneity render definitive diagnosis challenging. Glioblastoma multiforme, the common form of primary brain cancer, is one such cancer Citation[2].
Glioblastoma are derived from astrocytes, star-shaped cells responsible for mediating maintaining homeostasis of the neuronal microenvironment Citation[3]. Astrocytic tumors are classified histologically based on WHO criteria Citation[4,5]. Grade I tumors are biologically benign and complete surgical excision is typically curative. Grade II astrocytomas are characterized by hypercellularity with diffuse infiltration into the surrounding cerebral parenchyma. Complete surgical excision of grade II tumors cannot generally be achieved. The median survival for patients afflicted with grade II astrocytomas range from 5 to 8 years Citation[6]. Grade III or IV astrocytomas are considered malignant. In addition to hypercellularity, grade III astrocytomas exhibit nuclear atypia and increased mitotic figures Citation[7]. The median survival for grade III tumor is approximately 3 years. Grade IV astrocytomas, or glioblastomas, are characterized by histologic findings of angiogenesis and necrosis. Grade IV tumors are extremely aggressive and are associated with a median survival of 12–18 months Citation[8].
As the name ‘multiforme’ implies, glioblastoma is notoriously heterogeneous in terms of the histologic appearances Citation[5]. This heterogeneity exists at a cellular and a regional level, such that biopsy of tissue secured from one region of the tumor may yield a diagnosis of grade III tumor, while specimens secured from another region of the same tumor can render a grade IV diagnosis. In one study, 81 patients afflicted with astrocytomas of differing grades underwent stereotactic biopsy followed by surgical resection (within 30 days), 38% of the initial diagnosis secured through stereotactic needle biopsy were of a grade that differed from diagnosis achieved through surgical resection Citation[9]. In a second study, misdiagnosis occurs in 25% of stereotactic needle biopsies involving lesions <1 cc in volume Citation[10]. These studies demonstrate that misdiagnosis from stereotactic needle biopsies represent a genuine challenge in neurosurgery.
Brain biopsies: challenges
The difficulty of the tissue sampling is confounded by the complexity of the brain, where hundreds of trillions of neuronal connections define those qualities that we consider human Citation[11]. The notion of eloquence is often applied by neurosurgeons to delineate regions of the brain that are amenable to surgical manipulation. In neurosurgery, eloquent cortex is defined by regions where injuries result in obvious neurologic deficits, such as weakness or paralysis. However, there is a large body of literature demonstrating that injuries in regions not considered eloquent, nevertheless, cause deficits that are detectable on sophisticated neurocognitive testing Citation[12,13]. As such, it is our contention that any neurosurgical procedure should be performed with the intent of minimizing injuries to any portion of the cerebrum. As such, it is highly desirable to achieve definitive diagnosis with minimal disruption of the cerebrum.
Another major hurdle in biopsy of the human cerebrum is that it is effectively a ‘blind’ procedure. While sophisticated methods have been developed to triangulate the intended region of the biopsy and to deliver the biopsy needle to this region Citation[10], the neurosurgeon performing the procedure has limited means of visually confirming the location of the biopsy needle. In other words, the accuracy of the biopsy is entirely dependent on precision of the instrument, and the neurosurgeon has little means of validating the accuracy with human aptitudes, such as the surgeon’s experience and intuition. For instance, the stereotactic frame may incur subtle deformation with repeated use. If not properly serviced, utilization of this sub-optimal frame introduces inaccuracies. For this and other well-described factors that influence the accuracy of stereotactic biopsies Citation[14,15], most experienced neurosurgeon will have experienced situations in the operating room (OR) where the frozen specimen analysis is non-diagnostic and the doubts are raised as to the actual location of the biopsy. For the most part, the neurosurgeon makes educated ‘guesses’ in these situations to decide on the subsequent course of action.
The blind nature of brain biopsies additionally impede the surgeon’s ability to react to intraoperative events. For instance, most surgeons would terminate the surgery if they know that the biopsy had triggered significant hemorrhage. However, the way that brain biopsies are currently performed, the surgeons are effectively blind to these events. The surgeon effectively relies on the patient’s neurologic examination as she/he emerges from anesthesia to assess whether adverse events were incurred during the biopsy. If the examination is concerning, the surgeon would then rush the patient to a CT scanner for imaging. The delay between the timing of the actual hemorrhage and the timing of detection on CT can be on the order of hours. Based on the available literature, the risk of biopsy-related hemorrhage ranged between 1 and 9% Citation[16–21].
Improving brain biopsies
Restriction spectrum imaging
The development of diffusion weighted magnetic resonance imaging (DWI) has allowed visualization of microstructural and physiological changes within the brain Citation[22]. The physical principle underlying DWI involves assessing molecular diffusion by the imposition of two radiofrequency pulses that are equal in magnitude and 180 degrees out of phase. The amplitude of the radiofrequency perturbation is characterized by the b value, and most conventional DWI images are acquired using a single b value Citation[23]. Using diffusion imaging, cellularity maps can be generated using DWI to guide target planning for stereotactic brain biopsies Citation[24].
Restriction spectrum imaging (RSI) is an advanced form of DWI technique that integrates multiple amplitudes of radiofrequency perturbations (hence a spectrum of b values) to assess molecular motion Citation[23]. We have previously shown that RSI afford finer resolution of molecular diffusion relative to conventional DWI, affording assessment of a gradation of diffusional restrictions, ranging from free to partial restriction to absolute restriction Citation[25]. With RSI, we were able to determine regions of high cellularity within the tumor that failed detection by conventional DWI Citation[26]. Incorporation of this information into surgical planning can potentially enhance the surgeon’s ability to select region of disparate cellularity for biopsy .
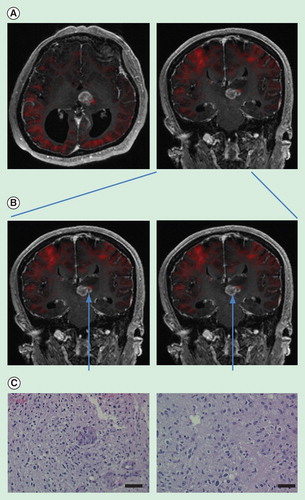
Figure 1. Restriction spectrum imaging (RSI)-guided selection of biopsy sites. (A) RSI signal imposed onto conventional MR imaging. Red color indicates regions of increased cellularity. Expectedly, the cortex of the cerebrum exhibits increased RSI signal. There is an increased RSI signal on the lateral edge of the tumor mass. (B) Biopsy of a region of tumor with increased RSI signal and another adjacent region without increased RSI signal. (C) Pathologic specimens secured from respective regions. The region of increased RSI signal revealed increased cellularity as well as increased microvascular proliferation. The region without increased RSI signal showed moderate cellularity. Both slides were taken at 20×. Bar = 50 μm. The specimens were stained by H&E.
Intra-MRI biopsies
With the development of MRI-compatible equipment such as the ClearPoint device Citation[27] and Ad-Tech biopsy needle Citation[28], brain biopsies can now be performed within the MRI. In doing so, the surgeons will have a real-time view of the lesion as it is being biopsied. Adjustments in trajectory can be made in real time to sample the regions of interest.
The ClearPoint device is an integrated system of hardware, software and disposable MRI compatible instruments that afford surgeons a real-time view of the surgical lesion and the biopsy needle in real time as the biopsy is being performed. To the best of our knowledge, it is the only commercial device that allows for real-time MRI-guided neuronavigation. The patients undergoing a ClearPoint procedure are placed under general anesthesia. A set of MRI images were then taken and used for planning surgical trajectory. Based on this trajectory, an incision is made followed by a dime-sized burr hole through the skull. A tripod device (termed SmartFrame) is mounted over the incision . This frame is synchronized to the hardware and software such that the trajectory of the biopsy needle inserted through the center of the tripod can be calculated. Based on this calculation, the needle is advanced slowly to the intended target. MRIs are performed during this advancement as well as during the actual biopsy to visualize the trajectory in real time. Because actual views of the process are available in real time, the surgeon can use his/her judgment to adjust to any inaccuracies related to the surgical equipment and react to any intraoperative events encountered.
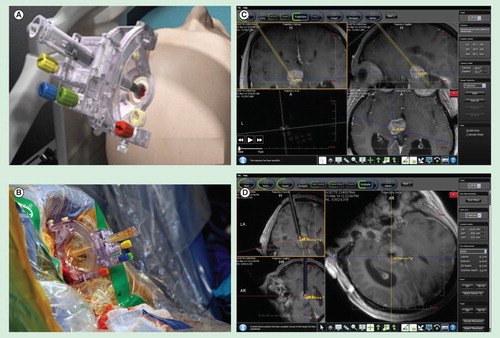
Figure 2. ClearPoint system for real-time MRI-guided biopsy. (A) Schematic of SmartFrame. (B) SmartFrame placement during surgery. (C) Pre-operative trajectory planning. Trajectory planning can be determined based on real-time information as to target the region of interest. The trajectory is indicated by the yellow line. The target is indicated by the orange circle. (D) Real-time monitoring of surgical trajectory. The needle tract (indicated by the T1-hypointense tract) can be clearly visualized in real-time throughout the surgery. Precise overlap of the intended target (orange circle) with the biopsy needle was observed.
Importantly, performing the biopsy in the MRI allows the neurosurgeon to more quickly react to the situation of an enlarging hematoma. For deep-seated tumors, the management strategy for an expanding hematoma involves termination of biopsy, correction of aberrant coagulation parameters and blood-pressure control, with surgical evacuation in the case of hematoma exerting significant mass effect. To determine whether hemorrhage has occurred and the size of the hematoma, the patients are typically emergently transported from the OR (where conventional biopsies are performed) to the imaging suite. If the hematoma size is significant, the patient is then brought back to the OR. In the case of MRI-guided biopsies, the patient is already under surveillance by MRI and an abbreviated T2 sequence would determine whether the hematoma is of a size that requires evacuation, thereby bypassing the trip from the OR to the CT. Thus, the proper course of action can be more quickly determined for a patient biopsied in the MRI relative to the patient who was biopsied in the OR.
A link to a video describing the integration of pre-operative RSI planning and intra-MRI biopsy can be found in Reference Citation[29]. The video describes a case where regions of differing RSI signals were biopsied with the MRI. The localization of the regions of biopsies was visually confirmed in real time. The biopsy derived from a region of lower cellularity (or low RSI signal) yielded the diagnosis of anaplastic astrocytoma. In contrast, the biopsy derived from a region of high cellularity (or high RSI signal) yielded the diagnosis of glioblastoma . The case illustrates the potential of RSI imaging and real-time MRI in improving the accuracy of stereotactic needle biopsies.
Conclusion
Technological advances have now conferred neurosurgeons with the ability to pre-operatively define the regional heterogeneity of brain tumors as well as real-time visualization of biopsy as it is performed. Adaptation of these technologies can potentially improve the safety and accuracy of brain biopsies. However, assessment of efficacy, cost–benefit analysis and clinical experience from larger cohorts are needed before clinical adaptation of these technologies.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- NCCN Clinical Practice Guidelines in Oncology. www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359(5):492-507
- Ehmsen JT, Ma TM, Sason H, et al. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci 2013;33(30):12464-9
- Kleihues P, Soylemezoglu F, Schäuble B, et al. Histopathology, classification, and grading of gliomas. Glia 1995;15(3):211-21
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114(2):97-109
- Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol 1997;15(9):3129-40
- Curran WJJr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85(9):704-10
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987-96
- Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 2001;3(3):193-200
- Waters JD, Gonda DD, Reddy H, et al. Diagnostic yield of stereotactic needle-biopsies of sub-cubic centimeter intracranial lesions. Surg Neurol Int 2013;4(Suppl 3):S176-81
- Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009;513(5):532-41
- Hillis AE, Anderson N, Sampath P, Rigamonti D. Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. J Neurol Neurosurg Psychiatry 2000;69(5):608-15
- Tuffiash E, Tamargo RJ, Hillis AE. Craniotomy for treatment of unruptured aneurysms is not associated with long-term cognitive dysfunction. Stroke 2003;34(9):2195-9
- Fitzpatrick JM, West JB. The distribution of target registration error in rigid-body point-based registration. IEEE Trans Med Imaging 2001;20(9):917-27
- West JB, Fitzpatrick JM, Toms SA, et al. Fiducial point placement and the accuracy of point-based, rigid body registration. Neurosurgery 2001;48(4):810-16. discussion 816-7
- Apuzzo ML, Chandrasoma PT, Cohen D, et al. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery 1987;20(6):930-7
- Chen CC, Hsu PW, Erich Wu TW, et al. Stereotactic brain biopsy: single center retrospective analysis of complications. Clin Neurol Neurosurg 2009;111(10):835-9
- Ersahin M, Karaaslan N, Gurbuz MS, et al. The safety and diagnostic value of frame-based and ct-guided stereotactic brain biopsy technique. Turk Neurosurg 2011;582-90
- Feiden W, Steude U, Bise K, Gündisch O. Accuracy of stereotactic brain tumor biopsy: comparison of the histologic findings in biopsy cylinders and resected tumor tissue. Neurosurg Rev 1991;14(1):51-6
- Silva EU, de Vasconcellos LP, Lara NA Jr, et al. Stereotactic biopsy for intracranial lesions. Arq Neuropsiquiatr 2009;67(4):1062-5
- Tilgner J, Herr M, Ostertag C, Volk B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis–influence of clinical factors. Neurosurgery 2005;56(2):257-65. discussion 257-65
- Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217(2):331-45
- McDonald CR, White NS, Farid N, et al. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am J Neuroradiol 2013;34(6):1157-63
- Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001;22(6):1081-8
- White NS, Leergaard TB, D’Arceuil H, et al. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp 2013;34(2):327-46
- Farid N, Almeida-Freitas DB, White NS, et al. Restriction-spectrum imaging of bevacizumab-related necrosis in a patient with GBM. Front Oncol 2013;3:258
- Richardson RM, Kells AP, Martin AJ, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg 2011;89(3):141-51
- Dujovny M, Misra M, Alp MS. The AD-TECH disposable brain biopsy needle. Surg Neurol 1996;46(6):597-8
- www.youtube.com/watch?v=hALKyJL9e_c
