(A) In patients with primary prevention indications, Kaplan-Meier survival curves for freedom from first appropriate ICD shock are plotted and compared according to risk score classification. (B) Risk score, corresponding risk category, number of patients and annualized rate of appropriate shocks are summarized below.
ICD: Implantable cardioverter-defibrillator.
Redrawn with permission from Citation[5].
![Figure 1. Freedom from appropriate ICD shocks in primary prevention patients with tetralogy of Fallot according to their risk category.(A) In patients with primary prevention indications, Kaplan-Meier survival curves for freedom from first appropriate ICD shock are plotted and compared according to risk score classification. (B) Risk score, corresponding risk category, number of patients and annualized rate of appropriate shocks are summarized below.ICD: Implantable cardioverter-defibrillator.Redrawn with permission from Citation[5].](/cms/asset/db2944d1-9b73-42c7-9bbf-142efa8961ac/ierd_a_11207021_f0001_b.jpg)
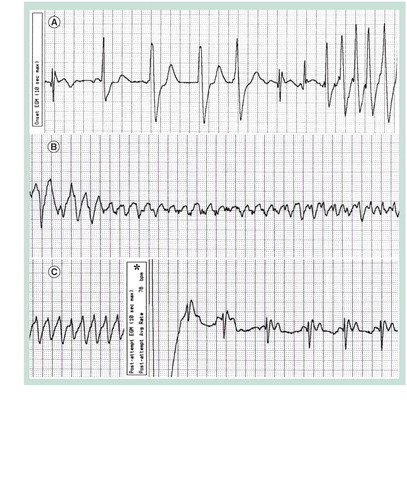
A stored ventricular electrogram is depicted with a continuous recording at 25 mm per second. (A) Premature ventricular beats are seen, followed by onset of a rapid ventricular tachyarrhythmia. (B) The ventricular tachycardia degenerated into a polymorphic form. (C) An appropriate implantable cardioverter-defibrillator discharge, marked by the asterisk, restored normal sinus rhythm at 78 bpm.
A postero–anterior chest x-ray is shown in a patient with congenital heart disease, a mechanical mitral valve and epicardial cardiac resynchronization therapy. Note the presence and configuration of the three bipolar epicardial leads, positioned on the right atrial surface, at the level of the basal to mid-right ventricular free-wall segments, and on the left ventricular lateral free wall. Bipoles were placed 1 cm apart.
Reproduced with permission from Citation[11].
![Figure 3. Chest x-ray in a patient with congenital heart disease and epicardial cardiac resynchronization therapy.A postero–anterior chest x-ray is shown in a patient with congenital heart disease, a mechanical mitral valve and epicardial cardiac resynchronization therapy. Note the presence and configuration of the three bipolar epicardial leads, positioned on the right atrial surface, at the level of the basal to mid-right ventricular free-wall segments, and on the left ventricular lateral free wall. Bipoles were placed 1 cm apart.Reproduced with permission from Citation[11].](/cms/asset/dc672ff9-1280-40cf-ac17-688b65eedacc/ierd_a_11207021_f0003_b.jpg)
A rapidly expanding population of patients with congenital heart disease has arisen from successes in pediatric cardiac care Citation[1]. This extraordinary triumph is, however, tempered by the recognition that early surgical interventions were ‘reparative’, not ‘curative’. Following surgical palliation, patients characteristically experience relatively uneventful childhood courses, with arrhythmias that emerge in adulthood Citation[2]. Indeed, sudden cardiac death of presumed arrhythmic etiology is the most common cause of mortality in adults with congenital heart disease, with up to a 100-fold increased risk compared with age-matched controls Citation[3,4]. Moreover, sudden deaths often transpire at a young age, commonly in the third and fourth decades of life Citation[3–5]. As a result, implantable cardioverter-defibrillators (ICDs) are increasingly utilized in this patient population. Importantly, this practice is not supported by prospective, randomized clinical trials specific to congenital heart disease. However, retrospective, multicenter cohort studies have begun characterizing patient profiles most likely to benefit from this technology Citation[6,7]. Complexities in underlying anatomy, associated extracardiac malformations, underdeveloped or obstructed vascular access and intracardiac shunts can pose unique challenges to transvenous ICD implantation Citation[8] and lead extraction Citation[9], if required.
Paralleling the high incidence of cardiac arrhythmias, heart failure remains a common long-term complication of congenital heart disease and is a major source of morbidity and mortality. Issues specific to this patient population limit the generalization of studies conducted in patients without congenital heart defects. This is true for pharmacological agents Citation[10] and cardiac resynchronization therapy (CRT). For example, in contrast to adults with heart failure and left bundle branch block (LBBB), right ventricular conduction delay and right bundle branch block (RBBB) are more common in congenital heart disease. The morphologic right ventricle may be systemically positioned and more prone to dysfunction. Moreover, only one functional yet failing ventricle may be present, precluding biventricular pacing but not CRT by multisite pacing Citation[11]. Current evidence supporting CRT in congenital heart disease remains sparse, yet the initial collective experience is promising Citation[11,12].
Two typical cases are briefly presented to illustrate some issues and successes regarding evolving indications for ICDs and CRT in congenital heart disease.
Case 1: primary prevention ICD in tetralogy of Fallot
A 27-year-old woman with tetralogy of Fallot (TOF) presented with syncope, briefly preceded by palpitations of sudden onset. She had a right Blalock–Taussig shunt at 10 months of age and corrective surgery at 6 years, with a right ventriculotomy incision, closure of a subaortic ventricular septal defect and a subannular patch to relieve right ventricular outflow tract obstruction. At 16 years of age, additional surgery alleviated residual right ventricular outflow tract obstruction. She was on no medical therapy and was previously asymptomatic. Her 12-lead electrocardiogram showed normal sinus rhythm, first-degree atrioventricular (AV) block and a typical RBBB pattern with a QRS duration of 170 ms. Echocardiography revealed an 18 mmHg gradient across the right ventricular outflow tract, mild-to-moderate pulmonary regurgitation, trivial tricuspid regurgitation, mild right ventricular dilation, no residual ventricular septal defect and normal biventricular function. A 24-h Holter monitor, 1 year prior, recorded occasional single premature atrial beats, an average of 10 premature ventricular beats per hour, and a 5-beat run of asymptomatic nonsustained monomorphic ventricular tachycardia (210 bpm).
Case discussion
Syncope occurred in the context of normal biventricular function and otherwise stable hemodynamics. In the absence of sinus node dysfunction or more advanced forms of conduction system disease, a bradyarrhythmic event is improbable. Notably, RBBB often appears after corrective surgery for TOF due to injury to the right bundle branch and myocardium Citation[13], whereas later broadening reflects right ventricular dilation Citation[14]. It is more likely that syncope, accompanied a transient tachyarrhythmia, be it atrial with rapid ventricular conduction or a primary ventricular source. Both arrhythmias are prevalent in TOF, with atrial tachyarrhythmias noted in 10%, sustained ventricular tachycardia in 12% and sudden death in 8% over a 10-year follow-up Citation[15].
The patient did not have a transannular patch or electrocardiographic markers (e.g., QRS duration ≥ 180 ms) associated with ventricular tachycardia or sudden death Citation[15]. However, several characteristics that independently predict risk for inducible sustained ventricular tachycardia were present, including age of 18 years or over, palpitations, prior palliative surgery and nonsustained ventricular tachycardia Citation[16]. Electrophysiology studies appear most valuable in patients at moderate risk Citation[17]. Inducible sustained ventricular tachycardia is associated with a nearly fivefold increased risk of clinical sustained ventricular tachycardia or sudden death Citation[16]. Although imperfect, it affords predictive value above and beyond known noninvasive risk factors. An electrophysiological study may also allow more thorough assessment of other potential arrhythmic triggers, including atrial tachyarrhythmias with rapid ventricular conduction.
In our patient, programmed ventricular stimulation was, therefore, performed. No atrial tachyarrhythmia was inducible. However, sustained monomorphic ventricular tachycardia (220 bpm) with hemodynamic compromise followed two ventricular extrastimuli. Ventricular tachycardia had a LBBB morphology and superior QRS axis, consistent with a right outflow tract origin. An ICD was, therefore, implanted for primary prevention against sudden death and β-blockers were empirically initiated.
A high rate of appropriate ICD shocks has been observed in patients with TOF and primary prevention indications Citation[6]. Independent risk factors include a higher left ventricular end diastolic pressure and nonsustained ventricular tachycardia. summarizes a risk score to predict appropriate ICD shocks in TOF patients with prophylactic ICDs Citation[6]. In our patient, eight of 12 points were tabulated, placing her in the high-risk category (i.e., 17.5% annual risk of appropriate shocks) shown in . Indeed, during follow-up, an appropriate and likely life-saving ICD shock was delivered for rapid ventricular tachycardia that degenerated into the polymorphic form captured in .
Case 2: CRT in transposition of the great arteries
A 12-year-old boy with congenitally corrected transposition of the great arteries (TGA), an intact ventricular septum and Ebstein’s malformation of the left-sided tricuspid valve, experienced increasing dyspnea and fatigue. No history of palpitations was elicited. Medical therapy consisted of an angiotensin-converting enzyme inhibitor, digoxin, and diuretics. On physical examination, S1 was normal and S2 single, with no third or fourth heart sound. A grade 3/6 holosystolic murmur was best heard at the apex, with transmission into the lower left part of the thorax. The electrocardiogram showed sinus rhythm with first-degree AV block (PR interval 270 ms), left-axis deviation, and intraventricular conduction delay of the LBBB type with Q waves in right precordial and inferior leads, no Q waves in left precordial leads, and a QRS duration of 155 ms. Echocardiography confirmed the inverted nature of the ventricles, mitral to pulmonary valve fibrous continuity and Ebstein’s malformation of the tricuspid valve. Severe tricuspid regurgitation was noted, with a systemic right ventricular ejection fraction of 34%.
Case discussion
In patients with complete (D-) and congenitally corrected (L-) TGA, the morphological right ventricle is systemically positioned and prone to failure over time Citation[18,19]. In addition to systemic ventricular dysfunction, severe tricuspid regurgitation was identified in our patient. In L-TGA, tricuspid valve anomalies include a spectrum of disease that commonly involves valve leaflets and the subvalvar apparatus. High pressures probably contribute to progressive regurgitation with increasing age. Also noteworthy is the patient’s marked first-degree AV block. In L-TGA, a fragile AV node is displaced anterolaterally Citation[20]. Complete supra- or intra-Hisian AV block Citation[21] has been reported in over 20% of patients, with an estimated annual incidence of 2% Citation[22]. Complete permanent AV block commonly occurs postoperatively Citation[22,23].
In light of the severely regurgitant deformed tricuspid valve, surgery was planned. In addition to tricuspid valve replacement, access via the thoracotomy permitted adjuvant epicardial CRT. This was justified on the basis of the depressed systemic ventricular ejection fraction (34%), intraventricular conduction delay (QRS 155 ms), first-degree AV block (PR 270 ms) with high likelihood of eventual progression to complete block (if not immediately postoperatively), difficulties with transvenous coronary sinus lead insertion in L-TGA and epicardial access.
Cardiac resynchronization therapy for systemic right ventricular dysfunction was first reported in a 24-year-old man with L-TGA and complete heart block Citation[24]. Technical feasibility and hemodynamic benefits were more formally assessed in eight patients with systemic right ventricles Citation[25]. Encouragingly, change from baseline rhythm to CRT was accompanied by a decrease in QRS duration, reduction in interventricular mechanical delay and immediate improvement in right ventricular filling time, Tei index and dP/dt Citation[25]. The right ventricular ejection fraction increased by 10% on average Citation[25]. However, no reduction in tricuspid regurgitation was noted. Concurrent tricuspid valve interventions may, therefore, be indicated in patients with severe regurgitation.
Our general approach to epicardial CRT in congenital heart disease is to place three bipolar epicardial leads on the right atrial surface – at the level of the basal to mid-subpulmonary ventricular free-wall segment – and on the systemic ventricular lateral-free wall, as shown in Citation[11]. While strategies remain empiric, we systematically optimize interventricular and AV delays by tissue Doppler imaging. In this particular patient, the optimal interventricular delay consisted of right preceding left ventricular pacing by 4 ms. In our experience with CRT in a heterogeneous group of patients with congenital heart disease, the ejection fraction significantly increased from 31.4 ± 13.5% at baseline to 50.6 ± 15.2% on follow-up Citation[11]. Hemodynamic improvement was similar whether patients had concomitant surgery or not, and whether the systemic ventricle was right or left Citation[11].
At 1-year follow-up post-tricuspid valve replacement and CRT, our patient’s dyspnea and fatigue had resolved and the systemic right ventricular ejection fraction normalized to 60%.
Expert commentary & five-year view
Unlike standard ICD and CRT indications based on multiple randomized clinical trials, evidence supporting this technology in congenital heart disease is limited but growing. Multicenter international studies on ICDs in TOF and TGA have better defined the patient population most likely to derive benefit Citation[6,7]. Whereas secondary prevention indications are rarely disputed, the challenge lies in identifying the subgroup of patients without clinical sustained ventricular tachyarrhythmias or resuscitated cardiac arrest at high risk for sudden death. In TOF, the most common form of congenital heart disease in ICD recipients, risk of developing ventricular tachyarrhythmias is modulated by a combination of surgical, hemodynamic, electrocardiographic and electrophysiological factors Citation[6]. Clinical characteristics are helpful in identifying candidates for further risk stratification by programmed ventricular stimulation Citation[16,17] and, ultimately, for ICD implantation Citation[6].
Evidence supporting CRT in congenital heart disease has thus far been limited to case reports, case series and small experimental acute postoperative crossover studies. The heterogeneous patient population, technical limitations from patient size, vascular access issues and unique forms of ventricular dyssynchrony further obscure the selection of potential beneficiaries Citation[11]. Never theless, heart failure is common in congenital heart disease, particularly in those with single or systemic right ventricles. Surgically induced intraventricular conduction delay and bundle branch block may further contribute to dyssynchrony. Recent studies have begun unraveling the many facets of CRT in congenital heart disease, including RBBB, right (pulmonary) ventricular dysfunction, systemic right ventricular dysfunction, single ventricle dysfunction and acute postoperative ventricular failure. Encouragingly, experience thus far has been favorable. Although definitive evidence-based recommendations cannot be proposed at the current time, optimistic initial experience suggests that research in this field should be pursued.
Table 1. Risk score for appropriate implantable cardioverter-defibrillator shocks in primary prevention.
Acknowledgements
This work was supported in part by a Canada Research Chair in Electrophysiology and Adult Congenital Heart Disease.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Khairy P, Mackie AS, Ionescu-Ittu R, Pilote L, Marelli AJ. Changing age distribution of death in congenital heart disease from 1988 to 2005: a population-based study. J. Am. Coll. Cardiol.49(9 Suppl. 1), 268A (2007).
- Khairy P, Dore A, Talajic M et al. Arrhythmias in adult congenital heart disease. Expert Rev. Cardiovasc. Ther.4(1), 83–95 (2006).
- Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J. Am. Coll. Cardiol.32(1), 245–251 (1998).
- Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am. J. Cardiol.86(10), 1111–1116 (2000).
- Khairy P, Fernandes SM, Mayer JE Jr et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation117(1), 85–92 (2008).
- Khairy P, Harris L, Landzberg MJ et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation117(3), 363–370 (2008).
- Khairy P, Harris L, Landzberg MJ et al. Defibrillators in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm4(5), S95–S96 (2007).
- Khairy P, Landzberg MJ, Gatzoulis MA et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation113(20), 2391–2397 (2006).
- Khairy P, Roux JF, Dubuc M et al. Laser lead extraction in adult congenital heart disease. J. Cardiovasc. Electrophysiol.18(5), 507–511 (2007).
- Dore A, Houde C, Chan KL et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation112(16), 2411–2416 (2005).
- Khairy P, Fournier A, Thibault B et al. Cardiac resynchronization therapy in congenital heart disease. Int. J. Cardiol.109(2), 160–168 (2006).
- Dubin AM, Janousek J, Rhee E et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J. Am. Coll. Cardiol.46(12), 2277–2283 (2005).
- Norgard G, Gatzoulis MA, Moraes F et al. Relationship between type of outflow tract repair and postoperative right ventricular diastolic physiology in tetralogy of Fallot: implications for long-term outcome. Circulation94(12), 3276–3280 (1996).
- Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation92(2), 231–237 (1995).
- Gatzoulis MA, Balaji S, Webber SA et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet356(9234), 975–981 (2000).
- Khairy P, Landzberg MJ, Gatzoulis MA et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: a multicenter study. Circulation109(16), 1994–2000 (2004).
- Khairy P. Programmed ventricular stimulation for risk stratification in patients with tetralogy of Fallot: a Bayesian perspective. Nat. Clin. Pract. Cardiovasc. Med.4(6), 292–293 (2007).
- Khairy P, Landzberg MJ, Lambert J, O’Donnell CP. Long-term outcomes after the atrial switch for surgical correction of transposition: a meta-analysis comparing the Mustard and Senning procedures. Cardiol. Young14(3), 284–292 (2004).
- Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation105(10), 1189–1194 (2002).
- Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation50(5), 911–923 (1974).
- Daliento L, Corrado D, Buja G et al. Rhythm and conduction disturbances in isolated, congenitally corrected transposition of the great arteries. Am. J. Cardiol.58(3), 314–318 (1986).
- Huhta JC, Maloney JD, Ritter DG, Ilstrup DM, Feldt RH. Complete atrioventricular block in patients with atrioventricular discordance. Circulation67(6), 1374–1377 (1983).
- Hwang B, Bowman F, Malm J, Krongrad E. Surgical repair of congenitally corrected transposition of the great arteries: results and follow-up. Am. J. Cardiol.50(4), 781–785 (1982).
- Rodriguez-Cruz E, Karpawich PP, Lieberman RA, Tantengco MV. Biventricular pacing as alternative therapy for dilated cardiomyopathy associated with congenital heart disease. Pacing Clin. Electrophysiol.24(2), 235–237 (2001).
- Janousek J, Tomek V, Chaloupecky VA et al. Cardiac resynchronization therapy: a novel adjunct to the treatment and prevention of systemic right ventricular failure. J. Am. Coll. Cardiol.44(9), 1927–1931 (2004).