Abstract
Aromatase excess syndrome is characterized by pre- or peripubertal onset of gynecomastia due to estrogen excess because of a gain-of-function mutation in the aromatase gene (CYP19A1). Subchromosomal recombination events including duplication, deletion, and inversion has been identified. The latter two recombinations recruit novel promoters for CYP19A1 through a unique mechanism. Gynecomastia continues for life, and although the general condition is well preserved, it may cause psychological issues. Minor symptoms (variably advanced bone age and short adult height), if present, are exclusively because of estrogen excess. Serum estradiol levels are elevated in 48% of affected males, but are not necessarily useful for diagnosis. Molecular analysis of CYP19A1 mutations is mandatory to confirm aromatase excess syndrome diagnosis. Furthermore, the use of an aromatase inhibitor can ameliorate gynecomastia.
Aromatase excess syndrome (AEXS; Mendelian Inheritance in Man database no. 139300), formerly known as familial gynecomastia, is a rare genetic disease characterized by the pre- or peripubertal onset of gynecomastia. Symptoms are exclusively related to estrogen excess and are not life-threatening unlike those of common gynecomastia, which has a variety of well-established etiologies, especially secondary gynecomastia. Only 10 years have passed since the initial discovery of genetic recombination events relevant to AEXS Citation[1].
Although AEXS is rare, this condition has been alluded to in the literature since the early 1960s Citation[2,3] as familial gynecomastia without hypogonadism. By the mid-1980s, researchers established that massive extraglandular conversion of plasma androgens caused hyperestrogenemia in affected boys Citation[4,5]. Following the molecular cloning of the aromatase (estrogen-synthesizing enzyme) gene (CYP19A1) in the early 1990s, aberrant aromatase expression was confirmed in the lymphocytes of an affected young male patient Citation[6]. In 2003, a subchromosomal inversion of CYP19A1 as a causative mutation of AEXS was identified Citation[1,7]. Familial gynecomastia was renamed AEXS because it was established that this disorder is an independent entity of an autosomal genetic disease caused by a gain-of-function mutation in CYP19A1.
Since 2003, 12 mutant alleles have been identified in 15 families composed of 30 affected males. After a brief introduction to aromatase, we review the clinical features of AEXS with regard to its pathophysiology, diagnosis and treatment and briefly discuss the characteristics of this disease, primarily based on the clinical features of genetically defined cases Citation[1,7–10].
Aromatase: gene structure, expression & enzymatic activities
Enzymatic activities & gene structure
Aromatase is a key player in estrogen synthesis and converts androgen to estrogen. Aromatase is a member of the cytochrome P450 superfamily that catalyzes the aromatization of the A-ring of androstenedione to produce estrone as well as the aromatization of the A-ring of testosterone to produce estradiol. The reaction proceeds in collaboration with nicotinamide adenine dinucleotide phosphate-cytochrome P450 reductase, which is conjugated with aromatase and supplies nicotinamide adenine dinucleotide phosphate, an essential co-enzyme for hydroxylation to aromatase.
Aromatase is composed of 503 amino acids and an iron-containing heme group. The enzyme is found in the cytoplasm or endoplasmic membrane with the glycosylated N-terminal residue inside the lumen of the endoplasmic reticulum.
Aromatase is encoded by CYP19A1, which is located on chromosome 15q21.1. The entire gene spans 123 kb and is composed of at least 10 coding exons and upstream 5′ noncoding exons (exon Is) Citation[11–14]. Each exon I possesses a unique upstream promoter sequence that allows tissue-specific alternative use, and therefore, tissue-specific regulation. For example, the most proximal promoter (PII) is almost exclusively expressed in the gonadal tissues, whereas exons 1f and I.4, which are located upstream of PII, are almost exclusively expressed in the brain and adipose tissues, respectively. The most upstream promoter (I.1) is exclusively expressed in the placenta.
Figure 1. CYP19A1 structure. Open boxes represent aromatase exon Is and L-shaped arrows in front of the boxes represent corresponding promoters. Closed boxes represent aromatase exons II–X encoding the open reading frame. Broken lines represent the splicing patterns.
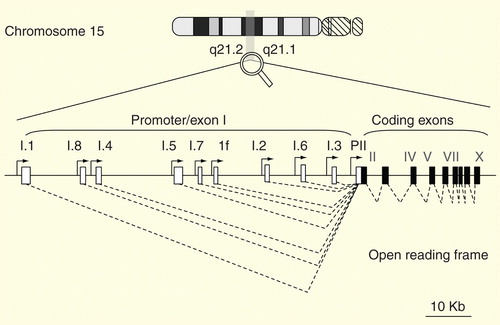
Primary transcripts from exon Is are spliced to a common splicing acceptor site located in exon II so that the secondary mRNAs share exons II–X, which encode the full-length aromatase enzyme. Thus, all CYP19A1 mRNAs encode the same protein, irrespective of exon I, which contains the transcriptional start site.
Aromatase expression in heath & disease
Aromatase is highly expressed in the gonadal tissue, and the resulting estrogen plays an essential role in reproduction through modulation of endocrine action on the uterine, breast and brain tissues. The brain itself expresses aromatase, and locally synthesized estrogen plays a role in controlling gonadotropin secretion as well as other functions in the brain.
It is well known that aromatase is expressed more widely outside the gonadal tissue than previously believed, including the adipose, breast, bone and uterine tissues, as well as vessels Citation[15,16]. Aromatase is expressed in these tissues at low basal levels and induced by local factors (cytokines, prostaglandins and steroids) in a limited temporospatial manner. Unlike the gonadal tissues, these tissues do not express CYP17A1, a key enzyme that synthesizes steroid hormones from cholesterol de novo, and thus, circulating androgen is the sole source of in situ estrogen synthesis.
Peripheral tissues expressing aromatase are also positive for the estrogen receptor, which is the target of estrogen. Estrogen synthesized in these tissues in situ acts directly on estrogen-producing or neighboring cells in an autocrine/paracrine manner, so that the biological effect in situ is more potent than expected from the amount of synthesis. In situ estrogen secretion in these tissues possibly plays physiological roles on breast development and bone closure. More importantly, over- and/or dysregulated expression of in situ aromatase plays a role in the pathogenesis of various diseases, such as breast cancer, endometrial cancer, endometriosis and uterine fibroid development Citation[16–19].
The placenta is considered an endocrine organ because it synthesizes massive amounts of estrogen. Examinations of aromatase deficiency have revealed that placental aromatase protects the fetus from virilization through the clearance of potentially hazardous adrenal androgen Citation[20]. In line with this concept, aromatase is expressed in the placenta of only those higher primates that secrete adrenal androgens.
Clinical features of AEXS
The most characteristic feature of AEXS is the pre- or peripubertal onset of gynecomastia in response to increasing estrogen production. Additional symptoms, which may or may not be associated, are also related to estrogen excess and include accelerated bone growth during the peripubertal period, resulting in reduced adult height and hypogonadotropic hypogonadism. Sparse facial hair and a high-pitched voice are also characteristics in some cases.
Gynecomastia
The source of estrogen excess in gynecomastia is circulating androgens from the adrenal glands, and the earliest onset of gynecomastia occurs around the adrenarche period (7–14 years) Citation[4]. No case of adult-onset gynecomastia has ever been reported. Onset of gynecomastia somewhat varies among individuals , but shows an apparent consistency within the same family, indicating genotypic influence on disease severity Citation[8].
Figure 2. Clinical features of 30 male patients with molecularly diagnosed aromatase excess syndrome. (A) Distribution of gynecomastia onset. (B) Distribution of developmental stage of gynecomastia at the time of the initial visit. Severity of gynecomastia is expressed using the Tanner staging system for morphological description of the female breast. (C) Chronological change in height. Height expressed in standard deviation for age is plotted against chronological age. Closed circles, deletion-type mutations; open circles, duplication-type mutations; open triangles, inversion-type mutations. (D) Acceleration of bone growth. Differences between bone age and chronological age (years) are plotted against chronological age. Acceleration of bone growth becomes evident before 10 years of age.
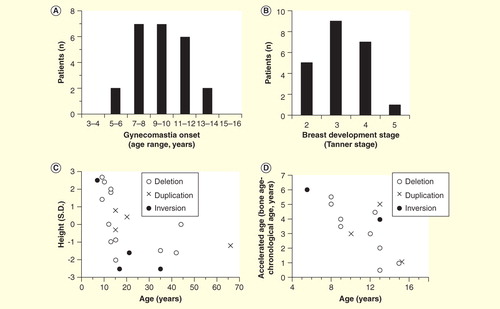
Gynecomastia is not only a physical but also a mental health problem for patients and their families, even in mild cases. In a cohort of 30 males, 20 underwent mastectomy between the ages of 12 and 19 years, with most cases undergoing surgery by the age of 16 years. Among patients aged ≥12 years only, mastectomy was performed in 81% cases, including cases with relatively mild gynecomastia. Early diagnosis and prophylactic treatment can avoid the need for surgical intervention. Therefore, genetic screening is warranted for members of affected families before symptom onset.
Bone growth/height
Estrogen excess during the prepubertal period initially accelerates bone growth and bone age, and subsequently, induces premature epiphyseal closure . As seen in other instances of precocious puberty, patients are taller than their age-matched peers until the early teenage period, after which height is in the subnormal adult range between −2.5 and 0 standard deviations of normal.
Fertility
Patients with AEXS may show mild hypogonadotropic hypogonadism. Decreased testosterone levels are consistent in patients with AEXS of all ages. Testicular volume is subnormal in teens, but normal in adults. Gender identity is not compromised. Although infertility has not been reported, mild oligozoospermia was noted in one patient, but fecundity in this patient remains unknown because he had no desire to have children at the time of examination.
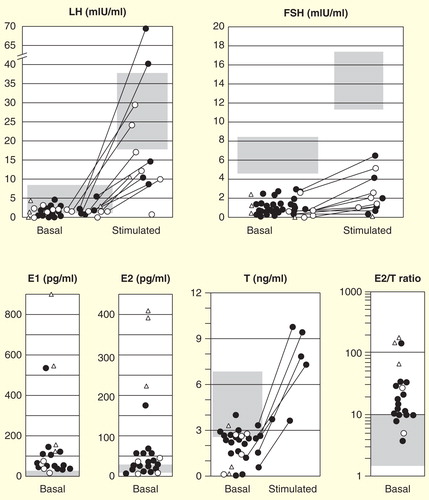
Serum follicle-stimulating hormone (FSH) levels are consistently suppressed , but luteinizing hormone (LH) and testosterone production in response to human chorionic gonadotropin is fairly preserved. The low basal testosterone levels do not seem to be a consequence of compromised testicular function, but rather a consequence of enhanced conversion to estrogen.
Figure 3. Hormonal profiles of 30 male patients with molecularly diagnosed aromatase excess syndrome. Isolated marks represent basal luteinizing hormone, follicle-stimulating hormone, estradiol, and testosterone levels and estradiol/testosterone ratio in each individual. Closed circles, deletion-type mutations; open circles, duplication-type mutations; open triangles, inversion-type mutations. The gray zones represent approximate normal reference ranges of adolescents. Paired marks tethered by a solid bar represent the gonadotropin levels before and 30 min after 100 µg of LH-releasing hormone loading. Paired marks in the testosterone chart represent levels before and after injection of human chorionic gonadotropin.
Female patients
Phenotypic characteristics of females with AEXS are not well defined Citation[21], since only eight women among seven families have been molecularly diagnosed with AEXS Citation[7,8,10,21,22]. Six of these women had one or more of the following symptoms: premature thelarche, early menarche, accelerated bone age at puberty, short adult stature, macromastia, irregular uterine bleeding and an enlarged uterus, while two women were phenotypically normal. One woman underwent reductive surgery for macromastia. Thus, symptoms of gynecomastia in females seem relatively mild compared to those in males.
Pathophysiology
Estrogen & androgen metabolism
The rate of plasma androgen conversion to estrogen in the entire body was measured using radioactive tracers. In four boys with clinically defined familial gynecomastia, as much as 16–48% of plasma androstenedione was converted to estrone, which was 15- to 50-fold greater than that in age-matched controls Citation[4,5]. Moreover, this conversion essentially occurred in extrahepatic sites, namely subcutaneous adipose tissue and skin. Similarly, 55 and 59% of androstenedione was converted to estrone in two boys with genotype-defined AEXS, which was 40-fold greater than that in controls Citation[1]. The conversion from testosterone to estradiol was also enhanced by 50-fold in these boys. The interconversion between androgens (androstenedione to testosterone and vice versa) was 8 and 5%, respectively, whereas that between estrogens (estrone to estradiol and vice versa) was 50 and 90%, respectively, but they were not statistically different from those of controls.
These studies demonstrated that excessive aromatization is responsible for the increase in estrogen associated with the decrease in androgen in circulating blood. These studies also demonstrated that a major source of estrogen is adrenal androgen (androstenedione), and therefore, the primary product is estrone. The contribution of testicular androgen (testosterone) and its product (estradiol) is small (<0.04% of net estradiol production is formed by the testes).
Aromatase overexpression
The initial metabolic studies excluded the possibility that the testis, adrenal gland and liver are responsible for excess aromatization Citation[4,5,23]. Instead, peripheral tissues, including the skin and subcutaneous fat, were considered as a possible site of conversion Citation[24–26], partly because of the curious resemblance to henny-feathered male Sebright Bantam chickens, which synthesize estrogen in all body tissues at high levels, especially the chest skin, resulting in female-type feathering Citation[5,27–29].
Aberrant expression of aromatase has been demonstrated in humans Citation[1,6–8,30]. Patient-derived skin fibroblasts showed an increase in aromatase activity of 11- to 24-fold and an increase in aromatase mRNA levels of 14- to 24-fold compared to normal controls Citation[1].
Recent studies have revealed that promoters of so-called housekeeping genes are recruited for the aberrant expression of aromatase in AEXS Citation[1,7,8]. Thus, aromatase is probably expressed continuously and ubiquitously in all somatic cells, as are housekeeping genes, which is in contrast to the continuous downregulation and temporospatial upregulation of aromatase in normal subjects Citation[12,14,16]. Prolonged and wide expression can explain why even a slight increase in the basal expression level causes detectable changes in androgen and estrogen levels in circulating blood.
Epstein–Barr virus-transformed lymphocytes have been used as an alternate to primary cells for DNA analysis. Lymphocytes can also serve as research material for mRNA analysis because they may express aromatase through aberrant promoters Citation[1,6–8].
Genetics
Genotypes
AEXS is a genetic disease exclusively caused by CYP19A1 mutations. No other genes so far have been reported to cosegregate with CYP19A1 in AEXS. A total of 12 types of gain-of-function mutations in CYP19A1 have been identified among 15 families Citation[1,7–10]. In the literature, there are at least four reports of families with suspected CYP19A1 mutations: two were clinically defined cases reported before the molecular cloning of CYP19A1 and, therefore, lack information regarding genomic mutations Citation[4,5], whereas, in the other two cases, aberrant aromatase transcripts were detected and a genetic link to CYP19A1 polymorphism was evident, but no corresponding CYP19A1 mutation was found Citation[6,22].
All 12 mutations were recombinations of one allele, which gave rise to a gain-of-function mutation and were responsible for autosomal dominant transmission of the disease. To date, gene amplification and adoption of a novel promoter have been identified as a gain-of-function mechanism responsible for aromatase overexpression .
Figure 4. Schematic representations of DNA recombination events that give rise to gain-of-function mutations in familial aromatase excess syndrome. CYP19A1 exons II–X are expressed in one box. The arrows and following closed boxes represent one of multiple CYP19A1 promoters. The arrows indicate transcriptional direction. Similarly, the structure of DMXL2, a neighboring gene of CYP19A1 on the same (minus) strand as CYP19A1, is represented by a gray box (coding exons) and a shaded box (exon 1) associated with an arrow (promoter). DMXL2 exon 1 contains the 5′ end of the open reading frame. TMOD3 and SEMAD6 represent upstream and downstream neighboring genes of CYP19A1, on the opposite strand of CYP19A1, and are involved in inversion mutation. Inversion mutation may be combined with any type of other recombinations listed above to form the more complex DNA recombinations. For details, please refer to Citation[33].
![Figure 4. Schematic representations of DNA recombination events that give rise to gain-of-function mutations in familial aromatase excess syndrome. CYP19A1 exons II–X are expressed in one box. The arrows and following closed boxes represent one of multiple CYP19A1 promoters. The arrows indicate transcriptional direction. Similarly, the structure of DMXL2, a neighboring gene of CYP19A1 on the same (minus) strand as CYP19A1, is represented by a gray box (coding exons) and a shaded box (exon 1) associated with an arrow (promoter). DMXL2 exon 1 contains the 5′ end of the open reading frame. TMOD3 and SEMAD6 represent upstream and downstream neighboring genes of CYP19A1, on the opposite strand of CYP19A1, and are involved in inversion mutation. Inversion mutation may be combined with any type of other recombinations listed above to form the more complex DNA recombinations. For details, please refer to Citation[33].](/cms/asset/f526da24-1490-4a41-a56c-b2f3dff8ac31/iere_a_926810_f0004_b.jpg)
Gene amplification
There are two modes of CYP19A1 amplification: tandem duplication encompassing both promoters and coding exons and tandem duplication of the promoter region only . In both types of mutation, the promoter sequence was intact, so tissue-specific gene regulation was probably functional. Thus, a regulated, but enhanced, transcriptional response to physiological stimuli is probably the mechanism of aromatase excess in these mutations.
Adoption of cryptic promoters
Another mode of gain-of-function mutation, namely adoption of novel promoters from unrelated genes, causes eccentric aromatase expression. There exist two different mechanisms for promoter adoption: deletion and inversion.
Among deletion-type mutations, five different mutations have been identified in five families and two sporadic cases, which share a minimal deletion region between DMXL2 exon 1 and CYP19A1, resulting in recombination, although the precise size and location of the deleted regions differed . The insulator between the two genes is probably removed and the DMXL2 promoter, relocated just upstream of CYP19A1, drives transcription all the way downstream to CYP19A1, resulting in an increase in the recombinant DMXL2 exon 1-CYP19A1 transcript. The DMXL2 promoter drives aromatase transcription in an unlimited spatiotemporal manner compared to tissue- and time-limited expression of authentic promoters of CYP19A1. Constitutive expression may explain why the DMXL2-CYP19A1 recombinant, albeit a low translational efficacy as described later, causes an increase in net estrogen production.
In the last type of gain-of-function mutation, CYP19A1 recruits a cryptic promoter through inversion , which is a truly original mechanism of a gain-of-function mutation Citation[1]. There exist several genes upstream of CYP19A1, but on the opposite strand of CYP19A1, so that the promoters of these neighboring genes are in the opposite direction to CYP19A1 and are transcribed unrelatedly to CYP19A1. If the promoter is inverted upstream of CYP19A1 and directs to CYP19A1, it in turn can drive CYP19A1 transcription. Four genes (TMOD3, MAPK6, TLN2 and CGML1) are reportedly involved in this mechanism. Conversely, inversion of CYP19A1 itself, instead of the neighboring genes, creates the same situation. CYP19A1 is inverted in situ and ligated just downstream of the neighboring gene promoter in the same direction. Now, the neighboring gene promoter in the original location can drive the transcription of the inverted CYP19A1. SEMAD6, located downstream and on the opposite strand of CYP19A1, has been shown to sacrifice its promoter to CYP19A1 by this mechanism Citation[31].
Mechanisms of genetic recombinations
Extensive studies on the break points of recombinations revealed that various mechanisms are involved in the development of rearrangements found in AEXS Citation[7,8,31]. Simple deletions are caused by nonallelic homologous recombination or nonhomologous end-joining occurring after double-strand DNA breakage Citation[8]. Other recombinations, including deletion, duplication and inversion, are likely to be replication-based errors caused by aberrant template switching during replication (Citation[31–33]; reviewed in detail in Citation[33]).
Genotype–phenotype correlation
Symptoms of AEXS are exclusively due to estrogen excess, with gene mutation confined to CYP19A1. Neighboring genes simultaneously involved in the recombination do not cause any symptoms, although their structures are disrupted. It remains unclear as to whether one intact allele of these genes is sufficient for function or if the function is indiscernible. No disease has yet been ascribed to any of these genes in the Online Mendelian Inheritance in Man database.
The severity of gynecomastia appears to be determined by estrogen action on the breast, which is a summation of the activities of estrogen in circulating blood (endocrine estrogen) and estrogen synthesized within the breast tissues (in situ estrogen). Accordingly, in terms of CYP19A1 mutation–gynecomastia correlation, it is necessary to consider both the transcriptional potency of the novel promoter sequence itself and the breast tissue-specific expression levels of the promoter Citation[8,31,32]. Taking this into consideration, phenotype appears to be correlated with functional and structural properties of genomic mutations, at least in mutations that have been identified.
Patients with CYP19A1 amplification-type mutation exhibit milder gynecomastia than those with the inversion-type mutation because, as described earlier, aromatase in the gene amplification-type mutation is likely still under ‘physiological’ regulation Citation[33]. In contrast, in inversion-type mutations, aromatase is expressed more broadly and continuously, reflecting the housekeeping gene-like expression profiles of the original genes.
Patients with the deletion-type mutation (DMXL2 exon 1-CYP19A1 fusion) manifest milder gynecomastia than those with the inversion-type mutation Citation[33]. The fusion genes of the deletion-type possess two transcription start sites: one within DMXL2 exon 1 and the other within CYP19A1 exon 2. The former is the natural transcription start site of DMXL2, but produces a premature termination codon in the fusion genes, resulting in nonsense-mediated mRNA decay. The transcript derived from the downstream transcription start site only produces the aromatase protein, although it is a minor species of the fusion gene transcript.
Among five deletion-type mutations (DMXL2 exon 1-CYP19A1), the largest deletion exhibits milder gynecomastia than that caused by the others Citation[9]. The largest deletion simultaneously affects seven of 10 upstream promoters of CYP19A1 in a mutant allele, whereas all promoters are intact in the remaining four deletion-type mutations. Deletion of the CYP19A1 promoters in DMXL2 exon 1–CYP19A1 recombinants ameliorates gynecomastia, indicating that, in addition to the cryptic DMXL2 promoter, upstream seven genuine CYP19A1 promoters in a recombinant allele contribute to the development of gynecomastia. However, it remains unclear whether this is just due to a gene dose effect of the promoters or unrecognized interactions among promoters in tandem position.
Diagnosis
Diagnostic scheme
AEXS is simply defined as the pre- or peripubertal onset of gynecomastia caused by estrogen excess due to a gain-of-function mutation in CYP19A1. AEXS may be associated with minor symptoms, but the general condition is well preserved, at least in the cases that have been identified until date.
A diagnostic approach has been proposed in a study supported by the Japanese government Citation[34]. AEXS is clinically suspected based on the four criteria of gynecomastia defined below. A suspected diagnosis of AEXS is not difficult in a typical case with well-developed gynecomastia for physicians who recognize this entity as a hereditary and benign disease. Conventional laboratory tests, including measurement of serum estradiol levels, are useful to reconfirm endocrinological pathology, but should not be used for exclusive diagnosis of this disease, especially in cases with indistinct symptoms. After clinical suspicion of the disease using a combination of inclusion and exclusion criteria , AEXS should be established through the identification of a CYP19A1 mutation. Genetic analysis is essential to diagnose a sporadic case of mild gynecomastia in young males and also a family with female proband only.
Figure 5. A diagnostic and therapeutic schema. Physical examination for estrogen-unrelated symptoms is crucial for exclusion of aromatase excess syndrome diagnosis.
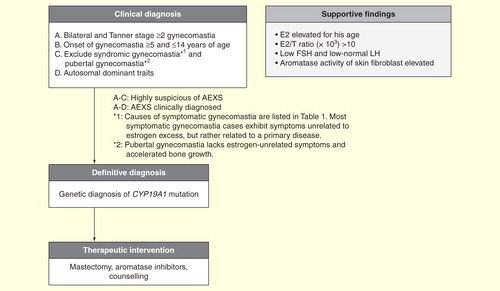
Table 1. Diseases and conditions to be distinguished from aromatase excess syndrome.
Clinical diagnosis
Four criteria for the clinical diagnosis of AEXS are as follows: bilateral gynecomastia (≥ stage 2), pre- or peripubertal onset of gynecomastia (≥5 and ≤14 years old), exclusion of other well-known causes of gynecomastia (symptomatic gynecomastia, ) and pubertal gynecomastia, and having a genetic trait (autosomal dominant). The first three criteria are indispensable for clinical diagnosis. The fourth criterion, a genetic trait, is not obligatory, but rather pathognomonic. Detection of a maternal genetic trait may be difficult to discern in a small family. A genetic trait is absent in sporadic cases.
Other useful clues for diagnosis are that a patient (proband) presents AEXS by late puberty and appears healthy, other than features of gynecomastia, even when associated with minor symptoms.
The associated symptoms (small testis, high-pitched voice, sparse facial hair, variably advanced bone age and short adult height) are exclusively related to estrogen activities. The absence of estrogen-unrelated symptoms is useful to distinguish symptomatic gynecomastia that occur secondary to a variety of diseases . In symptomatic gynecomastia, patients exhibit symptoms or signs derived from a primary disease, which is unrelated to estrogen activities and often pathognomonic for a primary disease. For example, patients with partial androgen insensitivity syndrome exhibit variable degrees of undervirilization of the external genitalia and patients with gynecomastia associated with Klinefelter syndrome exhibit a tall stature and hypogonadism. Patients with estrogen-producing tumors show gynecomastia associated with other symptoms of excess estrogen, resembling AEXS, but is differed from AEXS by the detection of the tumor by imaging. Thus, the presence of symptoms unrelated to estrogen may indicate the absence of AEXS.
Pubertal gynecomastia should also be differentiated from mild AEXS Citation[35]. Pubertal gynecomastia is characterized by physiological breast enlargement in an otherwise normal healthy boy, and usually appears by the age of 14–14.5 years and stops enlarging spontaneously within 6 months and resolves in 1–3 years Citation[36]. Plasma estrogen and testosterone levels are normal in these boys, although there exists the possibility of an imbalance in hormonal levels, including the relative amount of estrogen excess to androgen. Accelerated growth and bone maturation rates have not been reported for pubertal gynecomastia Citation[37].
Pubertal gynecomastia may be difficult to distinguish from sporadic cases of AEXS with mild symptoms, because both lack symptoms of underlying specific etiologies. In an indistinguishable case, it may be advisable to observe symptoms and suspend clinical diagnosis for 1 year or more, as long as the symptoms are mild.
Endocrinological abnormalities
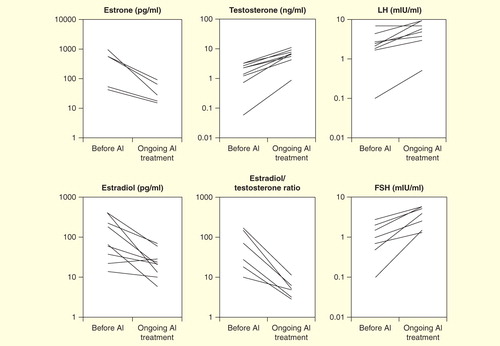
Circulating estrone levels are elevated in 17 out of 18 cases measured, and estradiol levels are elevated in only 13 out of 27 cases and is at normal levels in the remaining 14 cases, which is consistent with the finding that estrone instead of estradiol is the major estrogen produced in male patients with AEXS. Circulating androstenedione and testosterone levels are low or subnormal for chronological age in more than one-half of the patients. The estradiol (pg/ml)/testosterone (ng/ml) ratio, as a reflection of aromatization, becomes high in many cases, but not all: the ratio is >10 in 75% of AEXS cases .
FSH levels are consistently low and response to LH-releasing hormone (LHRH) is poor . LH levels are within low normal limits, and response to LHRH varies from normal to subnormal.
Endocrinological abnormalities become apparent in patients with severe gynecomastia. Conversely, it is subtle in patients with mild gynecomastia; thus endocrinological data are not useful for diagnosis. Notably, normal levels of circulating estrogen, especially estradiol, are not sufficient to exclude the possibility of AEXS.
Cytogenetic analysis
Cytogenetic analysis to identify a CYP19A1 mutation is essential for a definitive clinical diagnosis of patients with suspected AEXS. There is no case report in the literature in which karyotyping has detected any abnormality. A high-resolution comparative genomic hybridization using oligonucleotide probes for the long arm of chromosome 15 is useful for the detection of gene amplification and deletion mutations, and precise break points, which may be individually identified by trials of long-range polymerase chain reaction followed by sequencing Citation[8]. A recombination event can be visualized by fluorescent in situ hybridization Citation[1]. It is possible to estimate cryptic promoter sequences based on the structure of the identified recombination, and their function is confirmed by mRNA analysis using 5′-rapid amplification of cDNA ends (5′-RACE). Comparative genomic hybridization cannot detect a simple inversion. Instead, 5′-RACE is used to detect cryptic promoters and associated exons. Breast tissue and skin fibroblasts are the most suitable for study, but mononuclear cells isolated from patient blood samples may be used instead.
Higher levels of aromatase mRNA as well as activity have been reported for fibroblasts isolated from skin biopsy specimens Citation[1,6,8,30]. Immunohistochemistry of the excised breast tissues confirmed aromatase expression of epithelial cells lining the glandular ducts Citation[6]. Examinations of breast tissues specimens may help in the diagnosis if appropriate controls are available; however, it may be indecisive like the serum estradiol level in the diagnosis of AEXS, particularly for AEXS cases with relatively low aromatase activity.
Medical treatment
Aromatase inhibitors, developed for treatment for breast cancer, have been used in 10 male cases of AEXS Citation[1,6,9,21]. The third generation selective inhibitor anastrozole (1 mg/day) has been shown to consistently reduce serum estrone and estradiol levels and increase serum testosterone levels, thereby resulting in significant reduction in the estradiol/testosterone ratio as an index of aromatizing activity and increase in gonadotropin levels .
Use of aromatase inhibitors can alleviate gynecomastia within 6 months and are possibly useful to prevent recurrence of gynecomastia after reduction mastoplasty. It also promotes virilization and increased testicular volume Citation[21,30,38].
Control of premature bone closure is another target of AEXS treatment. Aromatase inhibitors are currently prescribed off-label to increase adult height in boys with short stature due to other causes Citation[38]. Although prescription of aromatase inhibitors has been reported in several AEXS cases, there is insufficient information regarding final height outcomes. In most cases, an aromatase inhibitor was administered after the onset of gynecomastia and at least several years after the onset of growth spurt. Shihara et al. Citation[10] reported a case of a boy who experienced a premature growth spurt, which began at approximately 5 years, achieved maximum velocity in bone growth at 6–7 years, and ended by the age of 8–9 years. His accelerated bone growth started as early as 4 years before the onset of gynecomastia (9 years). His brother also exhibited AEXS with similar growth features. This case raises the concern that treatment initiated after the development of gynecomastia may be too late to prevent premature epiphyseal closure. In this context, genetic diagnosis before the onset of gynecomastia and early intervention is warranted for prophylaxis of short adult stature. The severity of short stature varies among families. Therefore, early intervention should be considered if there exists a family member(s) with short statures.
Long-term aromatase inhibitor use is necessary to prevent the recurrence of gynecomastia. Moreover, untreated hyperestrogenemia may facilitate gynecomastia progression into a more severe stage and may increase the risk of breast cancer. In this context, prolonged use of aromatase inhibitor(s) may be warranted because the use of aromatase inhibitor(s) in an adjuvant setting of breast cancer prevents the development of contralateral breast cancer. Although selective aromatase inhibitors are relatively safe, side effects, such as arthralgia and bone fracture, have been reported following long-term use in women with breast cancer Citation[39]. Male and female bone may respond differently to sex steroid therapy, and it is unpredictable whether side effects that occur in females also affect males with testosterone levels elevated by the use of aromatase inhibitors. No detrimental effects on bone metabolism have yet been reported in males due to long-term use of aromatase inhibitors Citation[40]. Side effects of aromatase inhibitor use in males have not yet been determined Citation[41].
Expert commentary
Reconfirmation of the physiological role of estrogen
AEXS reconfirms the important physiological roles of estrogen on bone physiology and gonadotropin regulation revealed by the discovery of aromatase deficiency, another disease caused by CYP19A1 mutation.
Males affected by aromatase deficiency show progressive linear growth into adulthood caused by the absence of epiphyseal closure and severe osteopenia, as well as have elevated serum LH and FSH levels. Estrogen replacement therapy to achieve serum estradiol levels in the low-to-normal range can improve these symptoms, suggesting the importance of estrogen in males, for which even testosterone cannot substitute.
AEXS symptoms of accelerated bone maturation and suppressed gonadotropins mirror those presented in aromatase deficiency, again supporting the importance of estrogen in males. Moreover, AEXS has expanded our knowledge of gonadotropin regulation by estrogen Citation[32]. In males with AEXS, baseline FSH and LHRH-stimulated levels are uniformly suppressed, irrespective of disease severity, whereas LH suppression is milder and responds to LHRH to a significant extent. This indicates that estrogen suppresses gonadotropin secretion mainly at the pituitary level, instead of the hypothalamus, and FSH is more sensitive to the negative feedback regulation of estrogen than LH Citation[42–45].
Phenotypes of CYP19A1 mutations support the importance of the roles of estrogen on bone growth and gonadotropin regulation. This does not necessarily, however, diminish the contribution of testosterone because estrogen-induced amelioration of symptoms of aromatase deficiency was observed in the presence of endogenous testosterone, although not with testosterone alone. In other words, symptom amelioration could be a result of cooperation of both steroid hormones. Therefore, further studies, for example, a model of 17α hydroxylase deficiency lacking both androgen and estrogen, are needed to determine the contribution of testosterone.
Role of in situ estrogen
Another noteworthy observation in AEXS is that gynecomastia can occur in patients with estradiol levels within the normal range. Also, Shihara et al. Citation[10] demonstrated that a similar pre-pubertal growth spurt occurred without a detectable elevation in serum estradiol levels. One explanation for this is in situ estrogen production. Cryptic promoters of CYP19A1 mutants may be expressed preferentially in breast tissues or bone so that adrenal androgen is effectively converted to estrone in situ Citation[1,8], which is, in turn, converted in situ into biologically active estradiol by locally expressed 17β-hydroxysteroid dehydrogenase. A similar pathological role of in situ estrogen has been established in breast cancer and suggested for other pathologies, including those of the bone. It would be beneficial to elucidate the role of in situ estrogen production in terms of physiological maturation of male bone. The phenotype of bone in AEXS cases, in which the circulating estradiol levels are within the normal range, will offer insight into the significance of in situ estradiol production.
Five-year view
Here, we reviewed reports of genomic CYP19A1 recombination events, including duplication, deletion and inversion, as well as combinations of these mutations. There are at least two other families in which the use of a cryptic promoter was demonstrated, but no corresponding genomic mutation was defined Citation[5,6]. Furthermore, we came across additional cases/families in which AEXS was clinically suspected, but no causative mutation was identified. Current cutting-edge technologies, especially second-generation (or ‘next-generation’) DNA sequencing technologies employing paired-end mapping or split-read analysis, could reveal hitherto unrecognized recombination events as they have sufficient power to detect even inversions, which are not detectable by currently available arrays, comparative genome hybridization, single-nucleotide polymorphism genotyping assays or read-depth analysis using a next-generation sequence analyzer Citation[46]. Next-generation technologies may prove useful to identify novel gene mutations, other than those to CYP19A1, as causative mutations of AEXS, if any others exist.
Recent progress in high-throughput DNA technology has also shown that genomic rearrangement causing submicroscopic (<5 Mb) copy number variations (CNVs) is far more common in the human genome than previously suspected and can cause hereditary diseases because of a Mendelian or more complex trait as seen in neurogenic disorders and autism. Even if CNVs are identified, it is often difficult to determine a precise genetic mechanism conveying each phenotype because disease phenotypes are complex and the CNV region may harbor multiple genes that function in the progression of disease.
In this context, AEXS provides a unique model to study how these structural variations confer new functions to the human genome. The phenotype highly specific to CYP19A1 function (estrogen excess) is relatively simple; thus the phenotype–genotype correlation is direct and easy to analyze. This is probably because of the coincidence that estrogen, as a gene product, acts powerfully and specifically, and there exists no neighboring genes that show haplo-insufficiency. Moreover, the alternative promoter structure of CYP19A1 features novel genetic mechanisms of gain-of-function, namely deletion- and inversion-based adoption of cryptic promoters.
A future 5-year study should be designed to address questions raised by the AEXS study. For example, the inversion-based mechanism of gain-of-function has never been reported except for AEXS; therefore, it would be interesting to determine whether this mechanism is actually exclusive to AEXS, or whether this mechanism is more commonly used for other diseases not previously identified because of technical limitations. Previous studies of mutations in AEXS have revealed that the 15q21 region, especially of upstream of CYP19A1, is unstable, suggesting that the number and types of mutations may be more frequent than previously thought. There is considerable diversity in the severity of pubertal gynecomastia, as some cases are phenotypically indistinguishable from mild AEXS. Therefore, it is important to determine whether there exist structural variations or polymorphisms relevant to pubertal gynecomastia. Given that CYP19A1recombinations occur as a replication error, somatic cells may also be affected and cause pathologies relevant to excessive estrogen, such as breast cancer and polycystic ovary syndrome; however, such a mutation has not been identified till date Citation[9]. Mammalian CYP19A1 has evolved through the sequential acquisition of promoters. Thus, it would also be beneficial to determine whether any recombination events found in AEXS are relevant to such evolutionary potential and the history of such mutations in particular cases of familial AEXS Citation[47].
Key issues
Aromatase excess syndrome, formerly known as familial gynecomastia, is an autosomal dominant disorder caused by gain-of-function mutations in the aromatase gene (CYP19A1).
Gynecomastia develops during the peri- or pubertal period and continues for life.
The long bones exhibit accelerated growth at early puberty and then premature epiphyseal closure results in short adult stature.
Patients appear healthy, except for manifestations of gynecomastia, which may be associated with minor symptoms exclusively related to estrogen excess.
Serum estradiol levels are elevated in 80% of patients, but are normal in 20%; therefore, a normal serum estradiol level does not exclude a diagnosis of aromatase excess syndrome.
Use of aromatase inhibitors ameliorates gynecomastia.
CYP19A1 mutations serve as fascinating examples to understand how submicroscopic DNA recombination events give rise to gain-of-function mutations.
Financial & competing interests disclosure
This work was supported by Grants for Research on Rare and Intractable Diseases (H23-Nanzi-Ippan-090) from the Ministry of Health, Labor, and Welfare, Japan and partly by the Grant-in-Aid for Scientific Research (A) 25253092 and Grant-in-Aid for challenging Exploratory Research 25670694 from the Japan Society for the Promotion of Science. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Shozu M, Sebastian S, Takayama K, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med 2003;348:1855-65
- Nowakowski H, Lenz W. Genetic aspects in male hypogonadism. Recent Prog Horm Res 1961;17:53-95
- Wallach EE, Garcia CR. Familial gynecomastia without hypogonadism: a report of three cases in one family. J Clin Endocrinol Metab 1962;22:1201-6
- Hemsell DL, Edman CD, Marks JF, et al. Massive extranglandular aromatization of plasma androstenedione resulting in feminization of a prepubertal boy. J Clin Invest 1977;60:455-64
- Berkovitz GD, Guerami A, Brown TR, et al. Familial gynecomastia with increased extraglandular aromatization of plasma carbon19-steroids. J Clin Invest 1985;75:1763-9
- Stratakis CA, Vottero A, Brodie A, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab 1998;83:1348-57
- Demura M, Martin RM, Shozu M, et al. Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum Mol Genet 2007;16:2529-41
- Fukami M, Shozu M, Soneda S, et al. Aromatase excess syndrome: identification of cryptic duplications and deletions leading to gain of function of CYP19A1 and assessment of phenotypic determinants. J Clin Endocrinol Metab 2011;96:E1035-43
- Fukami M, Suzuki J, Nakabayashi K, et al. Lack of genomic rearrangements involving the aromatase gene CYP19A1 in breast cancer. Breast Cancer 2014;21(3):382-5
- Shihara D, Miyado M, Nakabayashi K, et al. Aromatase excess syndrome in a family with upstream deletion of CYP19A1. Clin Endocrinol 2013. [Epub ahead of print]
- Sebastian S, Takayama K, Shozu M, Bulun SE. Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol Endocrinol 2002;16:2243-54
- Bulun SE, Sebastian S, Takayama K, et al. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 2003;86:219-24
- Shozu M, Zhao Y, Bulun SE, Simpson ER. Multiple splicing events involved in regulation of human aromatase expression by a novel promoter, I.6. Endocrinology 1998;139:1610-17
- Bulun SE, Takayama K, Suzuki T, et al. Organization of the human aromatase p450 (CYP19) gene. Semin Reprod Med 2004;22:5-9
- Sumitani H, Shozu M, Segawa T, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 2000;141:3852-61
- Shozu M, Murakami K, Inoue M. Aromatase and leiomyoma of the uterus. Semin Reprod Med 2004;22:51-60
- Shozu M, Sumitani H, Segawa T, et al. Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19). J Clin Endocrinol Metab 2002;87:2540-8
- Kasai T, Shozu M, Murakami K, et al. Increased expression of type I 17beta-hydroxysteroid dehydrogenase enhances in situ production of estradiol in uterine leiomyoma. J Clin Endocrinol Metab 2004;89:5661-8
- Ishikawa H, Reierstad S, Demura M, et al. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab 2009;94:1752-6
- Shozu M, Akasofu K, Harada T, Kubota Y. A new cause of female pseudohermaphroditism: placental aromatase deficiency. J Clin Endocrinol Metab 1991;72:560-6
- Martin RM, Lin CJ, Nishi MY, et al. Familial hyperestrogenism in both sexes: clinical, hormonal, and molecular studies of two siblings. J Clin Endocrinol Metab 2003;88:3027-34
- Tiulpakov A, Kalintchenko N, Semitcheva T, et al. A potential rearrangement between CYP19 and TRPM7 genes on chromosome 15q21.2 as a cause of aromatase excess syndrome. J Clin Endocrinol Metab 2005;90:4184-90
- Sher ES, Migeon CJ, Berkovitz GD. Evaluation of boys with marked breast development at puberty. Clin Pediatr 1998;37:367-71
- Siiteri PK, MacDonald PC. Role of extraglandular estrogen in human endocrinology. In: Handbook of physiology: endocrinology (volume 2): female reproductive system section 7. American Physiological Society; Washington, DC: 1973. 615-29
- Schweikert HU, Milewich L, Wilson JD. Aromatization of androstenedione by isolated human hairs. J Clin Endocrinol Metab 1975;40:413-17
- Schweikert HU, Milewich L, Wilson JD. Aromatization of androstenedione by cultured human fibroblasts. J Clin Endocrinol Metab 1976;43:785-95
- George FW, Wilson JD. Pathogenesis of the henny feathering trait in the Sebright bantam chicken. Increased conversion of androgen to estrogen in skin. J Clin Invest 1980;66:57-65
- McPhaul MJ, Matsumine H, Herbst MA, Wilson JD. Aromatase expression in extragonadal tissues of the Sebright chicken is controlled by a retroviral promoter. Trans Assoc Am Physicians 1991;104:141-9
- Matsumine H, Herbst MA, Ou SH, et al. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J Biol Chem 1991;266:19900-7
- Binder G, Iliev DI, Dufke A, et al. Dominant transmission of prepubertal gynecomastia due to serum estrone excess: hormonal, biochemical, and genetic analysis in a large kindred. J Clin Endocrinol Metab 2005;90:484-92
- Fukami M, Tsuchiya T, Vollbach H, et al. Genomic basis of aromatase excess syndrome: recombination- and replication-mediated rearrangements leading to CYP19A1 overexpression. J Clin Endocrinol Metab 2013;98:E2013-21
- Fukami M, Shozu M, Ogata T. Molecular bases and phenotypic determinants of aromatase excess syndrome. Int J Endocrinol 2012;2012:584807
- Fukami M, Miyado M, Nagasaki K, et al. Aromatase excess syndrome: a rare autosomal dominant disorder leading to pre- or peri-pubertal onset gynecomastia. Pediatr Endocrinol Rev 2014;11:298-305
- NHLW grants system database. http://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201231069A [Last accessed 14 February 2014]
- Lazala C, Saenger P. Pubertal gynecomastia. J Pediatr Endocrinol Metab 2002;15:553-60
- Moore DC, Schlaepfer LV, Paunier L, Sizonenko PC. Hormonal changes during puberty: V. Transient pubertal gynecomastia: abnormal androgen-estrogen ratios. J Clin Endocrinol Metab 1984;58:492-9
- Einav-Bachar R, Phillip M, Aurbach-Klipper Y, Lazar L. Prepubertal gynaecomastia: etiology, course and outcome. Clin Endocrinol (Oxf) 2004;61:55-60
- Wit JM, Hero M, Nunez SB. Aromatase inhibitors in pediatrics. Nat Rev Endocrinol 2012;8:135-47
- Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22:1401-8
- de Ronde W. Therapeutic uses of aromatase inhibitors in men. Curr Opin Endocrinol Diabetes Obes 2007;14:235-40
- Bradley KL, Tyldesley S, Speers CH, et al. Contemporary systemic therapy for male breast cancer. Clin Breast Cancer 2014;14:31-9
- Huang ES, Miller WL. Effects of estradiol–17 beta on basal and luteinizing hormone releasing hormone-induced secretion of luteinizing hormone and follicle stimulating hormone by ovine pituitary cell culture. Biol Reprod 1980;23:124-34
- Alexander DC, Miller WL. Regulation of ovine follicle-stimulating hormone beta-chain mRNA by 17 beta-estradiol in vivo and in vitro. J Biol Chem 1982;257:2282-6
- Mercer JE, Phillips DJ, Clarke IJ. Short-term regulation of gonadotropin subunit mRNA levels by estrogen: studies in the hypothalamo-pituitary intact and hypothalamo-pituitary disconnected ewe. J Neuroendocrinol 1993;5:591-6
- Pitteloud N, Dwyer AA, DeCruz S, et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 2008;93:784-91
- Zhang Y, Haraksingh R, Grubert F, et al. Child development and structural variation in the human genome. Child Dev 2013;84:34-48
- Guillen Y, Ruiz A. Gene alterations at Drosophila inversion breakpoints provide prima facie evidence for natural selection as an explanation for rapid chromosomal evolution. BMC Genomics 2012;13:53