Immunomics combines the understanding of molecular biology and cellular immune function with the application of laboratory tools to enable a detailed analysis of the immune system and its components of health and disease. It is the ability of immunomics to explore and manipulate the immune system’s cellular microenvironment that makes it not only useful at the bench, but also clinically relevant at the bedside. The development and future clinical applications of immunomics can be best understood by first exploring the evolution of its precursors: genomics and proteomics. In its infancy, genomics was defined as the study of the organization of all genes Citation[1]. With the unveiling of the draft sequence of the human genome in 2001, the meaning of genomics evolved from a study of DNA structure to a study of gene function Citation[2Citation,3]. This has been further enhanced by the advent of microarray technology, where the identification of gene expression patterns has been related to disease outcome and drug resistance Citation[4Citation,5]. The natural successor to genomics, proteomics, explores the structure, localization and interactions of proteins encoded by the human genome Citation[6].
In contrast to its predecessors, immunomics faces the challenge of not only understanding and manipulating the genes and proteins involved in immunologic function, but also the complex microenvironment of these cellular systems. The infancy of immunomics has focused on the development of research tools that model the immune system (e.g., algorithms for determining T-cell epitopes and immunologic synapse imaging) and monitor immune function (e.g., tetramers, artificial antigen-presenting cells and immunologic databases) (Box 1). The future of immunomics resides in the ability to use these technologies to shorten the course in the development of new clinical therapies for diseases such as cancer, infections or autoimmune disorders.
One of the great advantages of immunomics over traditional methods is the ability to measure immune function in the development of therapies. Rather than waiting for the clinical manifestations of toxicity, as has been customary in trials for broadly immunosuppressive agents in autoimmune diseases and cancer, the tools of immunomics allow for the detection of subclinical immunologic changes, such as decreased number or function of a specific cell line. By increasing the detectability of potential toxicity, the number of subjects required in a trial decreases.
In contrast to previous immunosuppressive agents, antigen-specific therapy provides an opportunity to develop immunomodulatory therapies that only regulate disease-related inflammation, in turn preserving otherwise normal immune function. In combating infections, it is clear that the host’s ability to mount a proper immune response to an infectious agent is even more important than antimicrobials. Targeted immunotherapy provides the opportunity to reintroduce antigen-specific cells to a host that would otherwise be too immunosuppressed to mount the correct innate or adaptive immune response.
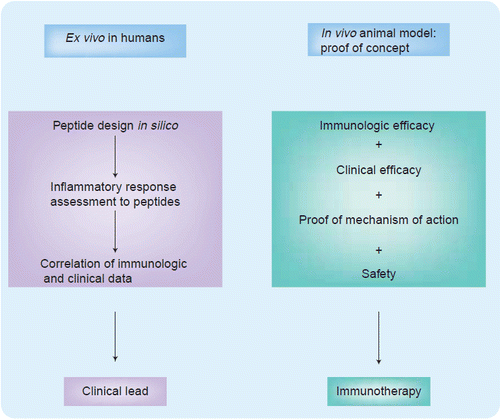
The pathway from development to the clinical application of immunomics entails a stepwise ex vivo progression from peptide design to assessing the inflammatory response of peptides with biomarkers to mining a database to find correlations between the immunologic and clinical data that can determine clinical applicability (Figure 1). Furthermore, the process then continues in vivo where it is crucial to prove that the immuno-therapy is both immunologically and clinically efficacious and safe, in addition to using samples from patients to demonstrate how the immune system has been changed by the therapy to prove the mechanism of action. The ability to validate results in both the preclinical and clinical process is crucial to the development of such immunotherapy. The ways in which immunologic and clinical efficacy, as well as safety, have been addressed to allow for a swift transition from the laboratory into clinical trials are explored throughout this discussion.
Using T-cell epitopes as an example, the first step in clinically applying immuno-mics is selecting peptides that are suitable for therapeutic use. While the sequenced human genome provides the opportunity to find the genes associated with a disease and identify potential vaccine epitopes, several algorithms based on naturally occurring ligands have been developed to predict the binding capability of a peptide in order for it to be highly immunogenic Citation[7,8]. Commonly, a variety of these algorithms are compared prior to selecting a peptide candidate. In the last 10 years, with the discovery that CD4+ and CD8+ T-cells have antitumor activity, researchers have been able to focus on enhancing antitumor immunity, such as prolonging antigen presentation of dendritic cells to T-cells to increase immunogenicity Citation[9]. As for the safety of epitope-specific vaccines, several Phase I clinical trials - including one for melanoma, have demonstrated that this form of therapy is well tolerated Citation[10Citation,11].
To further understand disease pathogenesis, a key to immunomics is knowing what occurs at the immunologic synapse when the antigen-presenting cell (APC) interacts with a T-cell. Quantitative imaging of the immunologic synapse has provided the opportunity to model more clearly what occurs at the cellular level, a critical step as our immunologic tools work toward
| • | Epitope-specific antigens (tumor antigens) | ||||
| • | Algorithms for determining T-cell epitopes | ||||
| • | Immunologic synapse imaging | ||||
| • | Tetramers | ||||
| • | Artificial antigen-presenting cells | ||||
| • | Immunologic database | ||||
While the techniques described above are crucial to developing immunotherapies, they are not clinically applicable unless it is possible to reasonably predict responders and nonresponders to a therapy. To this end, measurable surrogates known as biomarkers have come to the forefront in the clinical application of immunomics Citation[15]. Antibody profiles such as the specific immunoglobulin G subclass response in patients with chronic hepatitis B have been shown to be different from those who cleared their infection Citation[16]. With such a correlation between immunologic information and clinical outcome, monitoring of the specific immunoglobulin G subclasses may help predict those who will progress toward chronic hepatitis. Just as antibody profiles have been shown to serve as biomarkers, so too can cytokine profiles. The clinical applications of immunomics with cytokine biomarkers has been further demonstrated in cancer immunotherapy, where ex vivo peripheral blood CD4+ T-cell responses to a vaccine containing melanoma antigens correlated with survival after initiation of immunotherapy in metastatic melanoma Citation[17]. Such a correlation between immunologic data and clinical outcome provides further validation for the use of biomarkers in deciding who should receive a specific therapy and whether the benefits outweigh the risks of a given treatment.
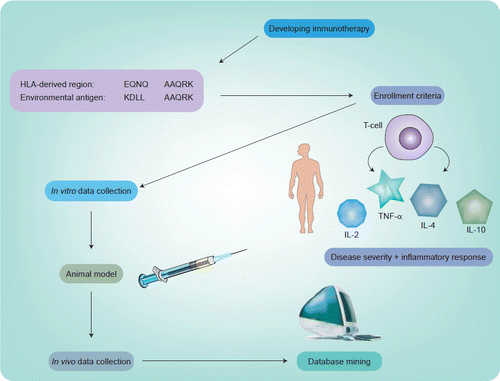
In the authors’ experience, the ability to use the tools of immunomics in a clinical trial has provided a unique opportunity to move forward in a precise and expeditious manner. As illustrated in Figure 1, the first step involved in designing an immunogenic peptide involved the tools of immunomics. Having chosen a candidate for mucosal peptide-specific immunotherapy from a bacterial heat shock protein dnaJ (dnaJP1) that shares homology with rheumatoid arthritis-associated human leukocyte antigen alleles, the next challenge was to decide on who should be enrolled. As noted in Figure 2, since the goal was to follow these patients clinically and immunologically throughout the trial, it was a logical conclusion to base enrollment on both clinically active disease and proinflammtory cytokines that would be most specific to autoimmune disorders. With recent data supporting the role of tumor necrosis factor (TNF)-α antagonists as a form of treatment in rheumatic diseases, the authors decided that sufficient validation existed to support following TNF-α levels as a potentially disease-specific cytokine Citation[18]. The ability to demonstrate tolerability, biologic safety and efficacy of this epitope-specific immunotherapy by evaluating the proinflammatory and anti-inflammatory cytokine responses of peptide-specific T-cells captured by aAPCs, allowed prompt completion of a concomitant Phase I/IIa trial Citation[19]. By applying the tools of immunomics to this clinical trial the authors have been able to learn about pathogenesis as well as shorten the time scale for clinical designs while drawing associations between immunologic and clinical outcomes that are critical to the go/no-go decision process based on predicted responders to a given immunotherapy.
In summary, immunomics has evolved from the development of tools to understand the innate and adaptive immune response to the clinical applications of these tools. Immunomics provides an opportunity to define more clearly who should be enrolled in a trial based on immunologic response, to follow these immunologic markers throughout a treatment course, and to correlate secondary outcomes such as clinical improvement to enrollment criteria. In turn, the clinical process is shortened providing a unique opportunity to lower both the cost and time spent in developing a therapy. The future of immunomics lies in the continued pursuit for validation of immunologic markers that can be correlated with clinical outcome with the primary focus of developing more directed forms of immunotherapy for the treatment of cancer, infectious diseases and autoimmune disorders.
References
- McKusick VA, Ruddle FH. A new discipline, a new name, a new journal. Genomics 1(1), 1–2 (1987).
- Perspective: genomics: structural and functional studies of genomes. Genomics 45(2), 244–249 (1997).
- Baltimore D. Our genome unveiled. Nature 409, 814–818 (2001).
- Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature 405, 827–836 (2000).
- Wulfkuhle J, Espina V, Liotta L, Petricoin E. Genomic and proteomic technologies for individualisation and improvement of cancer treatment. Eur. J. Cancer 40(17), 2623–2632 (2004).
- Smith KD, Bolouri H. Dissecting innate immune responses with the tools of systems biology. Curr. Opin. Immunol. 17, 49–54 (2005).
- Rammensee H, Bachmann J, Emmerich N, Bacho OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50, 213–219 (1999).
- Peters B, Tong W, Sidney J, Sette A, Weng Z. Examining the independent binding assumption for binding of peptide epitopes to MHC-1 molecules. Bioinformatics 19, 1765–1772 (2003).
- Wang R. Enhancing antitumor immune responses: intracellular peptide delivery and identification of MHC class II-restricted tumor antigens. Immunol. Rev. 188(1), 65–80 (2002).
- Rosenberg SA, Zhai Y, Yang JC et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl Cancer Inst. 90, 1894–1900 (1998).
- Dillman R, Selvan S, Schiltz P et al. Phase I/II trial of melanoma patient-specific vaccine of proliferating autologous tumor cells, dendritic cells, and GM-CSF: planned interim analysis. Cancer Biother. Radiopharm. 19(5), 658–665 (2004).
- Burack WR, Lee KH, Holdorf AD, Dustin ML, Shaw AS. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 169, 2837–2841 (2002).
- Prakken B, Wauben M, Genini D et al. Artificial antigen-presenting cells as a tool to exploit the immune synapse. Nature Med. 6(12), 1406–1410 (2000).
- Hoffmann TK, Donnenberg VS, Friebe-Hoffmann U et al. Competition of peptide-MHC class I tetrameric complexes with antiCD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry 41, 321–328 (2000).
- Cope AP, Feldmann M. Emerging approaches for the therapy of autoimmune and chronic inflammatory disease. Curr. Opin. Immun. 16, 780–786 (2004).
- Gregorek H, Dzierzanowska-Fangrat K, Woynarwoski M et al. Persistence of HBVDNA in children with chronic hepatitis B who seroconverted to antiHBs antibodies after interferon-α therapy: correlation with specific IgG subclass responses to HBsAg. J. Hepatol. 42, 486–490 (2005).
- Hsueh EC, Famatiga E, Shu S, Ye X, Morton DL. Peripheral blood CD4+ T-cell response before postoperative active immunotherapy correlates with clinical outcome in metastatic melanoma. Ann. Surg. Oncol. 11, 892–899 (2004).
- Criscione LG, St Clair EW. Tumor necrosis factor-α antagonists for the treatment of rheumatic diseases. Curr. Opin. Rheumatol. 14, 204–211 (2002).
- Prakken BJ, Samodal R, Le TD et al. Epitope-specific immunotherapy induces immune deviation of pro-inflammatory T-cells in rheumatoid arthritis. Proc. Natl Acad. Sci USA 101(12), 4228–4233 (2004).