Abstract
Anti-drug antibodies against biologic drugs affect efficacy and safety; therefore, it is important to use appropriate assays for immunogenicity testing in clinical studies. This review describes the electrochemiluminescent (ECL) immunoassay, ELISA, radioimmunoassay, and homogeneous mobility shift assay. The characteristics of ECL, used to assess immunogenicity in comparison trials of CT-P13 (Remsima®, Inflectra®) versus its reference medicinal product, infliximab (Remicade®), are also compared with the other assays, based on published literature. These comparisons show that ECL is more sensitive than ELISA, radioimmunoassay, and homogeneous mobility shift assay, and less affected by drug interference than ELISA. Similar immunogenicity was observed for CT-P13 and reference medicinal product using ECL, demonstrating the reliability of this method to assess immunogenicity in comparative studies.
Repeated injections of biologic drugs (also known as biopharmaceuticals) can result in the development of anti-drug antibodies (ADAs), which can cause adverse events such as infusion-related reactions, hypersensitivity, and serum sickness Citation[1,2]. The formation of ADAs can also impact on the pharmacokinetics (PK) and efficacy of biologics, and so assessment of a given drug’s immunogenicity is an important component of its development and regulatory approval process Citation[3,4]. Therefore, use of accurate measurement methods for evaluating serum drug levels and ADAs – which can facilitate interpretation of potential changes in therapeutic effects – is pivotal in the comprehensive quality assessment of biologic drugs Citation[5].
Anti-drug immune responses can result in ADAs with a wide range of affinities and avidities. ADAs in patients receiving biologic drugs are typically low in titer, affinity, and avidity in the initial phases of treatment before developing into stronger-binding immunoglobulins of higher titer Citation[6]. Consequently, both low- and high-affinity ADAs must be detectable by immunogenicity assays used in clinical practice.
In recent years, there has been increasing focus on selection of the most appropriate immunoassay methods for immunogenicity testing in clinical studies Citation[7]. However, accurate detection and quantitative measurement of ADAs have been fraught with difficulty due to technical problems affecting differential assay sensitivity for low- and high-affinity ADAs, and drug molecule interference in various assays Citation[6,8]. Consequently, to improve reliability and optimize the value of data generated from immunogenicity testing, the methods used for immunoassays need to be carefully developed and validated for use in clinical studies Citation[7].
Patent expiry of biologic drugs has created the opportunity for manufacturers to develop biosimilar drugs that are similar in terms of quality, safety, and efficacy to an already licensed reference medicinal product (RMP) Citation[9]. During the development of biosimilars, reliable assay methods are vitally important to compare the immunogenicity of the biosimilar with its RMP Citation[10]. This is because, unlike small-molecule generic drugs that have exactly the same chemical structures as the original products, biosimilars are manufactured using complex processes, which might theoretically lead to differences in immunogenicity reactions when compared with the RMP Citation[3,4].
CT-P13 (Remsima®, Inflectra®) is the first biosimilar monoclonal antibody (mAb) to be approved for use in the EU Citation[11]. Clinical data that contributed to this approval were generated in the Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in AS patients (PLANETAS) and Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in RA patients (PLANETRA) studies Citation[12,13]. These pivotal randomized controlled trials directly evaluated CT-P13 and the infliximab RMP in patients with ankylosing spondylitis (AS) and rheumatoid arthritis (RA), respectively. The studies demonstrated that the two agents are highly comparable in terms of PK, efficacy, and safety. The two agents were also shown to have comparable immunogenicity profiles, and ADAs were measured in the studies in human serum using an electrochemiluminescent (ECL) immunoassay Citation[12,13]. The ECL technique has also been used to assess ADAs to other tumor necrosis factor antagonists and has been reported to have good sensitivity Citation[14].
Over the years, a range of methods have been used to measure levels of ADAs to infliximab RMP, including ECL immunoassay, ELISA Citation[15], radioimmunoassay (RIA) Citation[16], and homogeneous mobility shift assay (HMSA) Citation[16,17]. Some of these available technology platforms are based on the bridging immunogenicity assay formats, which have been successfully used for detection and quantification of ADAs in serum or plasma samples, with ELISA and ECL immunoassay formats among the most popular methods using this approach.
In this review article, we describe the ECL immunoassay (which was used in PLANETAS and PLANETRA) and other methods used in studies reported in the literature to detect ADAs (ELISA, RIA, and HMSA) with infliximab RMP, in order to provide an overview of the important aspects of each method. To determine the suitability of the ECL immunoassay for evaluating the immunogenicity of infliximab biosimilars, its characteristics (sensitivity, drug tolerance, and ADA rates) were compared with the other ADA detection methods. Finally, the results of clinical studies investigating the immunogenicity of CT-P13 and RMP using ECL and ELISA or RIA are compared.
Overview of the assay methods used to detect ADAs with CT-P13 & infliximab RMP
The overall methods used for ECL, ELISA, RIA, and HMSA immunoassay techniques are illustrated schematically in . An overview of each technique, together with their relative advantages and disadvantages are described below and summarized in .
Figure 1. Schematic of methods to detect anti-drug antibodies. (A) In bridging ECL, samples are acidified then neutralized with ruthenylated RMP and biotinylated RMP. During incubation, ADAs are bound to both sulfo-tagged and biotinylated RMP to form an antibody–complex bridge, and samples are then loaded onto a streptavidin-coated electrode plate. After washing, only samples that contain ADAs bound to both sulfo-tagged and biotinylated RMP generate an ECL signal. (B) A bridging ELISA is based on the double-antigen format. RMP is used both during the solid-phase to capture antibodies against RMP and during the biotinylated detection phase with neutravidin–horseradish peroxidase. (C) In RIA, any radioactive complex extracted by anti-human lambda light chain antibodies is presumed to be RMP bound to ADA. This is because RMP is an antibody constructed solely of kappa light chains. (D) In HMSA, samples are incubated with florescent-labeled RMP. Resulting immune complexes are separated from the free label by SE-HPLC and the amount of ADA in the samples calculated from the resolved peak areas.
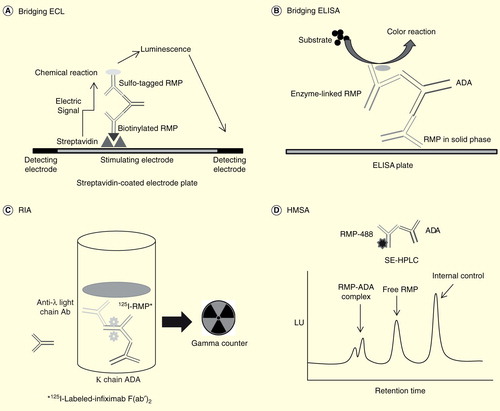
Table 1. An overview of the advantages and disadvantages of ECL, ELISA, RIA, and HMSA methods.
Electrochemiluminescent immunoassay
The ECL immunoassay used in the comparability exercise for CT-P13 and infliximab RMP utilized technology from Meso Scale Discovery (MSD; Rockville, Maryland, USA), in which detection of ADAs is based on the bivalent characteristics of the antibodies Citation[12,13]. To perform this assay, blood samples are acidified and then neutralized with ruthenylated infliximab and biotinylated infliximab. During the incubation, ADAs are bound to both the sulfo-tagged and biotinylated infliximab to form an antibody–complex bridge. Samples are then loaded onto a streptavidin-coated MSD assay plate. After a washing step, only the samples that contain ADAs bound to both the biotinylated and sulfo-tagged infliximab generate an ECL signal. In the presence of tripropylamine, ruthenium produces a chemiluminescent signal that is triggered when voltage is applied.
As described, this method is an example of a bridging immunogenicity assay, which also forms the basis of ELISA. Although ECL has not been used historically to detect ADAs to infliximab, the platform has advantages of good free drug tolerance, detection of low-affinity antibodies, sensitivity, throughput, and dynamic range Citation[6,8,18]. Furthermore, it has a high serum tolerance, and its detection capability may be improved by increasing the percentage of serum used in the assay Citation[6]. Although a hook effect has been observed with ECL (i.e., false negatives resulting from too high a concentration of ADAs), this method can still detect ADA concentrations of > 1 mg/ml, thus minimizing concerns over missing high-titer ADA responses Citation[6].
Enzyme-linked immunosorbent assay
The most common method that has historically been used for detection of ADAs to infliximab is a bridging ELISA. This method is based on the double-antigen format, in which infliximab is used both during the solid-phase to capture antibodies against infliximab and during the biotinylated detection phase with neutravidin–horseradish peroxidase Citation[19]. When ELISA is used, the results for ADAs are reported as microgram per milliliter on the basis of calibrations made with affinity-purified polyclonal rabbit antimouse IgG F(ab’). However, this assay cannot determine ADAs below certain cut-off levels because infliximab interferes with the assay and the technique is also less reliable for detection of low-affinity ADAs than high-affinity ADAs because of the repeated washing involved Citation[6,8]. False-positive findings caused by non-specific binding of other immunoglobulins or rheumatoid factors are another problem with solid-phase ELISA Citation[20]. Other ELISA methods using anti-human λ chain-conjugated antibody as the detector antibody, instead of labeled infliximab, have also been used to measure ADAs in serum samples from patients with inflammatory bowel disease Citation[21,22].
Radioimmunoassay
The RIA method, as illustrated in , is based on the principle that infliximab is an antibody constructed solely of kappa light chains and that any radioactive complex extracted by anti-human lambda light chain antibodies would be infliximab bound to antibody (irrespective of the heavy chain isotype), and not free infliximab. Another RIA method captures serum ADAs with a sepharose-bound reagent such as protein A, protein G, or antibodies to human immunoglobulin, followed by incubation with 125I-labeled-infliximab F(ab’)2 Citation[23–25]. A major advantage of this method is that the infliximab is in solution and does not suffer from the denaturing effect of coating as seen in some other methods. Thus, it can avoid the risk of false-positive results seen in bridging ELISAs due to binding of rheumatoid factors or non-specific antibody binding Citation[23]. Disadvantages of the RIA method are associated with the complexity of the test, the prolonged incubation time, and safety concerns related to the handling of radioactive material in many clinical laboratories.
Homogeneous mobility shift assay
The HMSA technique is based on the incubation of serum samples with fluorescent-labeled infliximab to detect ADA levels Citation[17]. The immune complexes formed in the incubation mixture are separated from the free label by size-exclusion high-performance liquid chromatography, with the amount of ADA in the samples calculated from the resolved peak areas. The HMSA method was developed after the PLANETAS and PLANETRA studies were initiated. HMSA has better sensitivity than the ELISA method and has the advantage of being able to measure ADAs when infliximab is present in the serum Citation[17].
Comparisons of sensitivity & drug tolerance levels among the assay methods used to evaluate ADAs with CT-P13 & RMP
Comparisons of sensitivity and drug tolerance levels among the assay methods of interest are summarized in and are described below.
Table 2. Comparison of sensitivity and drug tolerance level among the different assay methods.
ECL
For the studies comparing CT-P13 with RMP Citation[12,13], the ECL method was used in a 2-tiered ADA assay: in the first step, ADAs are determined in a screening assay that utilizes a bridging ELISA principle, while solid phase-bound biotinylated drug captures ADA. Bound ADAs are developed with a sulfo-tagged drug molecule (thus utilizing the bivalency of immunoglobulin molecules). In a second step, ADA is either confirmed true-positive or false-positive utilizing the same assay principle by a competitive ligand-binding principle, which is the confirmation assay.
The assay sensitivity was 50.8 ng/ml using affinity-purified monoclonal human anti-infliximab antibody (Data on file, Celltrion). A floating cut point with a 5% false-positive rate was used to determine positivity in a screening assay of samples. The floating cut point is calculated by the method reported by Shankar et al. (multiplying a normalization factor, determined during assay validation to the background data obtained during sample analysis) Citation[26]. Approximate concentrations corresponding to this cut point were similar to the level of relative assay sensitivity. Regarding drug tolerance, 150 ng/ml ADAs could be measured in the presence of >5 μg/ml infliximab in the serum, while >50 μg/ml of the drug was needed in order to completely mask the presence of ADAs (86–97% reduction) (Data on file, Celltrion). The assay precision for the positive control samples at three concentrations (150, 575, and 1000 ng/ml in 100% human serum) of ADAs over several runs of analysis was ≤17.8%. The assay precision for the pooled negative control over several runs of analysis was 20.2% (Data on file, Celltrion).
ELISA
ECL is sensitive and shows good free drug, serum tolerance, and detection of low-affinity ADAs compared with ELISA Citation[6,8]. Many published clinical studies have used the ELISA method to evaluate ADAs to infliximab in patient serum Citation[19,27–35]. In one study, involving patients treated with infliximab for Crohn’s disease (CD), the mean cut-off point used was 1.69 μg/ml in serum samples from 40 patients who had never been treated with infliximab Citation[19]. A negative ADA result was defined as a concentration lower than 1.69 μg/ml, with a serum infliximab concentration lower than 1.40 μg/ml; an indeterminate result as lower than 1.69 and 1.40 μg/ml or higher, respectively. The result was defined as positive when the ADA concentration was higher than 1.69 μg/ml and the infliximab concentration was lower than 1.40 μg/ml Citation[19].
RIA
ECL is more sensitive than RIA in detecting ADAs . RIA has been used to quantify ADAs in studies of RMP in patients with RA Citation[24,25]. When RIA methods are used to measure ADA, results are expressed as arbitrary units (AU) per milliliter, where 1 AU/ml equals approximately 10 μg/l Citation[24,25]. The lower limit of quantification with RIA is 12 AU/ml Citation[36]. It has been shown that infliximab levels as low as 2 μg/ml reduced the binding of 125I-infliximab by a median of 40% (range 0–76%) in samples of antibody positive sera used for specific competition experiments, and >200 μg/ml of the drug was needed to completely mask the presence of antibodies in this assay (80–100% reduction) Citation[24].
HMSA
ECL is more sensitive than HMSA in detecting ADAs . The HMSA method has been developed and validated for the measurement of infliximab and ADAs to infliximab Citation[17] and used in a post-hoc analysis of samples from patients with CD who failed treatment with RMP Citation[16]. The lower limit of quantification and upper limit of quantification for HMSA were shown to be 0.011 and 0.54 μg/ml, respectively Citation[17], with the effective serum concentrations corresponding to the lower limit of quantification and upper limit of quantification being 0.56 and 27 μg/ml, respectively. The cut-off point for ADA was 1.19 μg/ml, with 3% false-positive rate Citation[17].
Immunogenicity results from clinical studies in patients with rheumatic disease with CT-P13 or infliximab RMP using different immunoassay methods
A summary of published ADA results using a range of immunoassay techniques is presented in . The HMSA method has not been used to assess the immunogenicity of RMP in clinical studies of patients with rheumatic diseases, and so data are not summarized for this assay.
Table 3. Historical ADA rates reported with CT-P13 or infliximab RMP using ECL, ELISA, and RIA assay methods†.
During validation of the assay used during clinical studies of CT-P13, immunogenicity testing was performed separately with both CT-P13 tag and RMP tag. Results obtained with the two tags were similar (data not shown); therefore, ADAs to RMP were used in the final assay. In the PLANETRA study, ADAs were detected in 122/302 RA patients (48%) in the CT-P13 arm and 122/300 RA patients (48%) in the RMP arm at week 30. No marked differences were observed between the two treatment arms in terms of development of ADAs Citation[13]. In the PLANETAS study, ADAs were detected in 32/128 AS patients (27%) in the CT-P13 arm and 25/122 AS patients (23%) in the infliximab RMP arm, again at week 30 Citation[12].
The percentage of subjects who screened positive for ADAs in these comparative studies of CT-P13 and RMP was somewhat higher than some values previously reported in the literature Citation[12,13,24,25,27–34,37,38]. Most of the historical studies of RMP used the ELISA method to measure ADAs and rates were low in the majority of studies Citation[27,28,31,32,37,38]. For example, one of the earliest studies to use ELISA to study the immunogenicity of infliximab, published in 1998, reported that 17.4% of patients receiving this drug in combination with methotrexate developed ADAs Citation[28]. However, four studies reported rates similar to PLANETRA Citation[27,29,33,34]. ADA rates were 40–50% in two studies that used the RIA method in patients with RA, which are similar results to those generated with ECL in PLANETRA Citation[24,25].
Discussion
The advent of biosimilars in recent years has increased the focus on the validity, sensitivity and reliability of assays used to evaluate comparability of immunogenicity between a biosimilar and its RMP. The results from this comparison of immunoassay methods to detect ADAs in studies of CT-P13 and RMP suggest that ECL has better sensitivity than ELISA, RIA, and HMSA, and shows greater tolerance to free drug than ELISA. However, there are major caveats when comparing across data sets: heterogeneity in patient populations, use of concomitant immunosuppressants, methods, timing of antibody measurements, and outcome measures.
Studies that have directly evaluated and validated results between different immunoassays are generally lacking, with further research in this area warranted. In the few studies that have been performed, a good correlation between ADA to infliximab measurements for the different immunoassay methods has been observed. For example, this was shown in the study by Vande Casteele et al. that compared three different assays (an in-house developed RIA, a bridging ELISA, and a commercially available kit from Biomedical Diagnostics [BMD; Marne La Vallée, France]) for measuring ADA to infliximab in serum samples and spiked controls Citation[36]. Steenholdt et al. published a post-hoc comparison of different ADA assays in patients with CD Citation[16]. Samples from patients enrolled in a randomized controlled trial that assessed the use of assays for ADAs to guide therapy decisions during infliximab therapy were analyzed by three binding assays (RIA, ELISA, and HMSA). Correlations were statistically significant (p < 0.001) in all pairwise comparisons (Pearson r, 0.77–0.96). However, statistical agreement between assays could not be estimated accurately because the different assays reported values on different arbitrary scales. In general, there is a lack of historical data comparing different technologies within the same trial.
Focusing on the results of PLANETRA and PLANETAS, similar immunogenicity results were observed for CT-P13 and infliximab RMP using the ECL immunoassay Citation[12,13]. More recently, an ELISA method developed by Ben-Horin et al. was used to study the cross-immunogenicity of CT-P13 and infliximab RMP Citation[39]. ADAs in patients with inflammatory bowel disease recognized and functionally inhibited CT-P13 and infliximab to a similar degree. This study suggests similar immunogenicity between CT-P13 and RMP with shared immunodominant epitopes in both products Citation[39].
ADA rates reported with infliximab RMP in the literature using ELISA varied between 0% and 50%, with the rates lower than published for the ECL immunoassay in some, but not all, studies.
Conclusions
The ECL immunoassay used to evaluate immunogenicity in the pivotal PLANETRA and PLANETAS studies appears to have better sensitivity than ELISA, RIA, and HMSA, and is more tolerable to free drug than the ELISA method used in clinical studies of the RMP. These characteristics may be responsible for the higher ADA rates observed with ECL compared with ELISA in some studies. Overall, these results support use of the ECL immunoassay as a reliable method to test for ADA when comparing infliximab biosimilars.
Expert commentary
The assessment of immunogenicity for biologic drugs is an important factor in their development, approval, and use in clinical practice. When selecting techniques to generate data in clinical studies, recognition of the most robust immunoassay method is therefore crucial. Because of the advent of biosimilar versions of originator biologics or RMPs, the results of comparative immunogenicity testing have become even more of a focus for regulators and clinicians considering appropriate treatment selection for their patients. Reliable detection and accurate measurement of ADAs are complicated by technical challenges affecting the available immunoassay methods, including differences in assay sensitivity for low- and high-affinity ADAs, and drug molecule interference in the assay methods. Consequently, to improve reliability and optimize the value of data generated from immunogenicity testing, the methods used for immunoassays need to be carefully developed and validated for use in clinical studies. This review compares the ECL immunoassay, ELISA, RIA, and HMSA methods and provides an overview of outcomes when the techniques were used to measure ADAs in studies of infliximab or its biosimilar CT-P13. Our comparisons show that ECL appears to be more sensitive than ELISA, RIA, and HMSA, and less affected by drug interference than ELISA. Importantly, similar immunogenicity was observed for CT-P13 and RMP using the ECL method in the comparability studies leading to regulatory approval.
Five-year view
As the impetus behind the launch of biosimilars continues, the need to identify reliable and reproducible methods to compare these drugs and their relevant RMPs will continue. In this context, during the next phase of extended availability of effective biologic drugs, the immunoassay methods used in comparison programs will need to be carefully developed and validated for use in clinical studies. So far, there are few studies that have directly compared the results generated by different immunoassay methods and further study in this area is warranted. Evidence available to date, including our comparisons, shows that ECL has better sensitivity, with a lower cut point and greater drug tolerance than the ELISA method that has been used in a large number of studies of infliximab RMP. Overall, these results support continued use of the ECL immunoassay as a reliable method to test for ADA when comparing infliximab biosimilars in the future.
The advent of biosimilars has the potential to reduce the cost of treatment and may increase the number of patients who can receive therapies to improve symptoms and quality of life. However, it is important that a biosimilar is comparable to its reference medicinal product (RMP) in terms of immunogenicity profiles, pharmacokinetics, efficacy, and safety.
CT-P13 is the first biosimilar monoclonal antibody to be authorized for use in the EU. The Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in RA patients (PLANETRA) and Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in AS patients (PLANETAS) clinical trials showed that CT-P13 is highly comparable to its infliximab RMP.
A key concern with infliximab is the development of anti-drug antibodies (ADAs), which can negatively affect efficacy and safety of the drug. Therefore, it is crucial to compare the development of ADAs between CT-P13 and infliximab RMP using an appropriate assay method.
The available assays include electrochemiluminescent (ECL) immunoassay, ELISA, radioimmunoassay, and homogeneous mobility shift assay.
ECL was used in the PLANETRA and PLANETAS clinical trials, and showed similar ADA rates between CT-P13 and RMP (PLANETRA: 48 and 48%, respectively; PLANETAS: 27 and 23%, respectively).
Comparison of literature-reported values appeared to show that ECL is more sensitive than ELISA, radioimmunoassay, and homogeneous mobility shift assay, and less affected by drug interference than ELISA, with the caveat that patient and study heterogeneity limit cross-study comparisons.
ADA rates with infliximab RMP were higher for ECL than ELISA in some, but not all, studies, a finding likely related to the better sensitivity and drug tolerance of ECL. Further research directly comparing ADA detection methods is warranted.
Overall, these results support use of ECL as a reliable ADA assay method to compare infliximab formulations.
Acknowledgements
Editorial support (writing assistance, assembling tables and figures, collating author comments, grammatical editing, and referencing) was provided by Mark O’Connor (Aspire Scientific Limited, Bollington, UK) and was funded by Celltrion Healthcare Co., Ltd (Incheon, Republic of Korea).
Financial & competing interests disclosure
JS Kim, SH Kim, BO Kwon and SS Hong are employees of Celltrion. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, et al. Immunogenicity of anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol 2010;38:82-9
- Brennan FR, Morton LD, Spindeldreher S, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs 2010;2:233-55
- US Food and Drug Administration. Guidance for industry: Scientific considerations in demonstrating biosimilarity to a reference product. 2015. Available from: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf [Last accessed 25 June 2015]
- European Medicines Agency. Guideline on similar biological medicinal products. 2014. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf [Last accessed 21 July 2015]
- Shankar G, Shores E, Wagner C, et al. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol 2006;24:274-80
- Liang M, Klakamp SL, Funelas C, et al. Detection of high- and low-affinity antibodies against a human monoclonal antibody using various technology platforms. Assay Drug Dev Technol 2007;5:655-62
- Shankar G, Arkin S, Cocea L, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J 2014;16:658-73
- Mikulskis A, Yeung D, Subramanyam M, et al. Solution ELISA as a platform of choice for development of robust, drug tolerant immunogenicity assays in support of drug development. J Immunol Methods 2011;365:38-49
- World Health Organization. Expert committee on biological standardization. geneva, 19 to 23 October 2009. Guidelines on evaluation of similar biotherapeutic products (SBPs). 2009. Available from: www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf [Last accessed 19 February 2015]
- Weise M, Bielsky MC, De Smet K, et al. Biosimilars: what clinicians should know. Blood 2012;120:5111-17
- European Medicines Agency. Committee for medicinal products for human use (CHMP). assessment report: remsima (infliximab). 2013. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf [Last accessed 28 July 2015]
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013;72:1605-12
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613-20
- Bloem K, van Leeuwen A, Verbeek G, et al. Systematic comparison of drug-tolerant assays for anti-drug antibodies in a cohort of adalimumab-treated rheumatoid arthritis patients. J Immunol Methods 2015;418:29-38
- Hernandez-Florez D, Valor L, de la Torre I, et al. Comparison of two ELISA versions for infliximab serum levels in patients diagnosed with ankylosing spondylitis. Rheumatol Int 2015;35:1021-5
- Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014;109:1055-64
- Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods 2012;382:177-88
- Xiao Y, Halford A, Hayes R. Bioanalytical method development and validation of biosimilars: lessons learned. MOJ Immunol 2014;1:00004
- Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601-8
- Degn SE, Andersen SH, Jensen L, et al. Assay interference caused by antibodies reacting with rat kappa light-chain in human sera. J Immunol Methods 2011;372:204-8
- Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014;63:1258-64
- Ben-Horin S, Mazor Y, Yanai H, et al. The decline of anti-drug antibody titres after discontinuation of anti-TNFs: implications for predicting re-induction outcome in IBD. Aliment Pharmacol Ther 2012;35:714-22
- Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr Opin Immunol 2008;20:431-5
- Bendtzen K, Geborek P, Svenson M, et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 2006;54:3782-9
- Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:711-15
- Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 2008;48:1267-81
- Ducourau E, Mulleman D, Paintaud G, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 2011;13:R105
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552-63
- Pascual-Salcedo D, Plasencia C, Ramiro S, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:1445-52
- Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:1233-8
- St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432-43
- Vultaggio A, Matucci A, Nencini F, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010;65:657-61
- Kosmac M, Avcin T, Toplak N, et al. Exploring the binding sites of anti-infliximab antibodies in pediatric patients with rheumatic diseases treated with infliximab. Pediatr Res 2011;69:243-8
- Ruperto N, Lovell DJ, Cuttica R, et al. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis 2010;69:718-22
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932-9
- Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012;36:765-71
- Braun J, Deodhar A, Dijkmans B, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum 2008;59:1270-8
- Xu Z, Seitz K, Fasanmade A, et al. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol 2008;48:681-95
- Ben-Horin S, Yavzori M, Benhar I, et al. Cross-immunogenicity: antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut 2015. [Epub ahead of print]
- European Medicines Agency. Remicade (infliximab). summary of product characteristics. 2009. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf [Last accessed 28 July 2015]
- Ruperto N, Lovell DJ, Cuttica R, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56:3096-106
