ABSTRACT
Autoimmune bullous diseases (AIBDs) are characterized by autoantibodies against structural proteins of the dermal–epidermal junction (in pemphigoid diseases) and the epidermal/ epithelial desmosomes (in pemphigus diseases). By far, the most common AIBD is bullous pemphigoid, which is immunopathologically characterized by autoantibodies against BP180 (type XVII collagen) and BP230. IgG and, to a lesser extent, IgA autoantibodies are the major autoantibody isotypes in these disorders. IgE autoantibodies are increasingly reported in particular in bullous pemphigoid. The development of specific and sensitive anti-BP180 IgE ELISA systems, the report of two experimental murine models employing IgE autoantibodies against BP180, and the successful treatment of bullous pemphigoid with the anti-IgE antibody omalizumab have raised interest in the role of IgE autoantibodies and the modulation of their production in AIBDs. Here, the relevance of IgE autoantibodies in the diagnosis, pathophysiology, and treatment decisions of AIBDs, with a focus on bullous pemphigoid, is reviewed.
Introduction
Autoimmune bullous diseases (AIBDs) comprise a heterogeneous group of about a dozen entities that can be divided in three subgroups, pemphigus diseases, pemphigoid diseases, and dermatitis herpetiformis.[Citation1] Clinically, all AIBDs present with frank blistering, erosions, erythema on the integument and/or erosions on surface-close mucous membranes. AIBDs are associated with a high morbidity and, most of them, with an increased mortality.
Pemphigus disorders are characterized by autoantibodies against desmosomal proteins, including desmoglein 1 (in pemphigus foliaceus and the mucocutaneous form of pemphigus vulgaris), desmoglein 3 (in pemphigus vulgaris), and envoplakin (in paraneoplastic pemphigus).[Citation2] In pemphigoid diseases, autoantibodies are directed against structural proteins of the dermal–epidermal junction, leading to a mainly Fc-receptor-mediated inflammatory cascade that results in the infiltration of inflammatory cells in the upper dermis and the release of reactive oxygen species and distinct proteases, which finally induce dermal–epidermal splitting.[Citation3] The target antigens of pemphigoid diseases include BP180 (type XVII collagen) and, less frequently, the intracellular BP230 (in bullous pemphigoid, mucous membrane pemphigoid, pemphigoid gestationis, and linear IgA disease), laminin 332 and α6β4 integrin (in mucous membrane pemphigoid), laminin γ1 (in anti-p200 pemphigoid), and type VII collagen (in epidermolysis bullosa acquisita).[Citation3] In dermatitis herpetiformis, two enzymes, transglutaminase 3 and 2, are recognized by autoantibodies.[Citation4]
Bullous pemphigoid
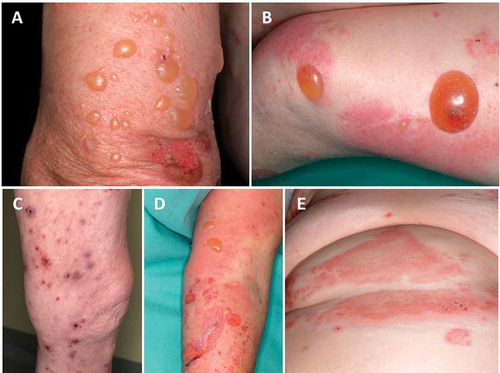
By far, the most frequent AIBD is bullous pemphigoid (BP), with incidences ranging from 13.4 to 66 new cases per 1 million per year [Citation5–Citation7] and in fact, most data about IgE autoreactivity have been raised in this disease. BP mainly affects elderly patients with a mean age at disease onset of 75–80 years and a dramatic rise of incidence to 150–330 new cases per 1 million per year in a population aged above 80 years.[Citation5–Citation8] The 1-year mortality ranges between 15% and 40% and is 2–4-fold higher compared to age- and sex-matched controls.[Citation9] Only a few cases have been reported in infants and adolescents.[Citation10,Citation11] BP patients present with tense blisters and erythema predominantly in flexures of limbs and abdomen, often in conjunction with urticarial plaques. By mechanical friction, blisters may turn into erosions and finally crusts before healing. In about 20% of BP patients, atypical lesions arise, such as prurigo-like nodules, intertrigo-like or dyshidrosiform pemphigoid as well as localized forms and, in 10–20% of BP patients, mild oral erosions occur.[Citation12,Citation13] Blister development is usually preceded by a prodromal phase of weeks or months with pruritus, excoriations, and eczematous lesions, and some patients never develop blisters [Citation12,Citation13] ().
Figure 1. Clinical presentations of bullous pemphigoid. Tense blisters on normal skin (A), tense blisters on erythematous and normal skin (B+D), prurigo-like bullous pemphigoid (C), and urticarial lesions (E).
The event leading to dermal–epidermal separation and, as such, blister formation is initiated by binding of autoantibodies against two hemidesmosomal proteins, BP180 and BP230. BP230 is an intracellular plakin-like protein of the hemidesmosomal plaque, while BP180 (type XVII collagen) is a transmembrane glycoprotein with an extracellular C-terminus.[Citation3] The extracellular portion of the 16th non-collagenous domain of BP180 (BP180 NC16A) has been identified as an immunodominant region recognized in about 90% of BP patients.[Citation14] BP230 is targeted in about 50% of patients, with the most immunogenic epitopes located in the globular C-terminal portion.[Citation15,Citation16]
It has been clearly shown in various in vitro and animal models that IgG autoantibodies against BP180 are pathogenic [Citation17–Citation22] and correlate with disease activity [Citation23–Citation26] in BP patients. Most effects of anti-BP180 IgG are Fc receptor-mediated.[Citation27,Citation28]
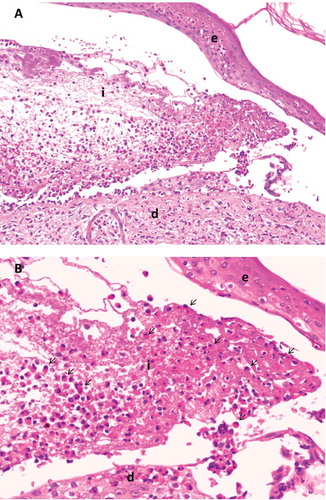
Diagnosis of BP is based on a combination of linear deposits of IgG and/or C3 at the dermal–epidermal junction by direct immunofluorescence (IF) microscopy and serological studies, including indirect IF microscopy on monkey esophagus and salt-split human skin as well as ELISA for BP180 and BP230.[Citation29,Citation30] Lesional histopathology reveals subepidermal splitting and an eosinophil-rich inflammatory infiltrate in the upper dermis (). Furthermore, a link between eosinophils and coagulation has been identified via tissue factor. Coagulation factors such as D-dimers and prothrombin fragments paralleled both eosinophilia and disease severity while fibrinolysis was found to be inhibited in BP. These changes were reversible by corticosteroid treatment.[Citation31,Citation32] Treatment options are mainly oral and/or topical superpotent corticosteroids frequently combined with immunomodulants such as dapsone and tetracyclines or immunosuppressant agents like azathioprine and methotrexate.[Citation33,Citation34] Recent reports about the successful treatment of individual BP patients with the anti-IgE antibody omalizumab [Citation35–Citation40] suggest a pathogenic relevance of IgE autoantibodies.
Figure 2. Lesional histolopathology in bullous pemphigoid. 20x magnification (A). 40x magnification (B). e, epidermis; d, dermis; i, inflammatory infiltrate with lymphocytes and numerous eosinophils (arrows).
Elevated total serum IgE levels
Elevated total serum IgE levels have been described in multiple reports in 70–85% of BP sera and blister fluids [Citation41–Citation50] and have also been found in children with BP [Citation11] and linear IgA disease.[Citation51] Interestingly, in a case report, a mother with pemphigoid gestationis, but not the newborn, had elevated total IgE levels.[Citation52] In contrast, in endemic pemphigus foliaceus but not in pemphigus vulgaris, elevated total IgE serum levels were described.[Citation53–Citation55]
In BP, a positive correlation between total IgE serum levels and anti-basal membrane zone (BMZ) antibody levels has been reported.[Citation42,Citation56] In line with this, total IgE serum levels were found to decline after exacerbation of disease had resolved [Citation42,Citation57] and correlate with disease activity as well as anti-BMZ antibodies.[Citation49,Citation58] In a recent study, we found a correlation of total serum IgE levels with the extent of urticarial/erythematous lesions but not with blisters/erosions.[Citation59] In contrast, Cozzani et al. reported total IgE serum levels to be rather unrelated to disease severity, while mean total serum IgE values were higher in patients with bullae than in patients with urticarial lesions.[Citation60]
Studies on sCD23, the soluble form of a low-affinity FcγRII that is involved in B-cell growth and differentiation, pointed toward a clinical relevance of IgE, since elevated sCD23 levels were detected in BP sera compared to normal controls and sCD23 serum levels correlated with both disease activity and total serum IgE levels in BP.[Citation61,Citation62] Levels of sCD23 were also significantly increased in BP blisters compared to levels in suction blisters.[Citation63] Conformingly, expression of CD23 on peripheral blood B cells was shown to be higher in BP patients than in controls but, however, it did not correlate with sCD23 levels; instead CD23 expression paralleled total serum IgE levels, which were significantly higher in severe compared to moderate BP.[Citation64]
Other inflammatory mediators have also been reported to be involved in this context, e.g. IL-5, the thymus and activation regulated chemokine (TARC), and eosinophil cationic protein levels. IL-5 and eosinophil cationic protein levels in blister fluid of BP patients were reported to correlate with total serum IgE levels,[Citation65] and TARC levels in both sera and blister fluid were elevated in BP sera, and serum TARC levels correlated with total serum IgE levels.[Citation66,Citation67] These data in combination with the significantly higher serum levels of IL-4 and sCD30 [Citation68] point toward a strong T-helpercell type 2 (Th2) response in BP.
Eosinophilia
An inflammatory infiltrate rich of eosinophils is, apart from subepidermal splitting, the key histologic feature of BP [Citation69,Citation70] (). In this line, blood eosinophilia has been reported in 50–60% of BP patients.[Citation48,Citation58,Citation71] We recently observed a correlation of the number of blood eosinophils with the classical phenotype, i.e. a presentation with blisters and erosions, but not the extent of urticae and erythema in a large cohort of well-defined BP patients.[Citation59] In a case report, the extent of blood eosinophilia also correlated with disease severity.[Citation58] The eosinophil chemotactic activity in BP patients is derived from the eosinophil chemotactic factor of anaphylaxis, which participates in the accumulation of eosinophils in BP lesions.[Citation69,Citation71,Citation72] However, levels of eosinophil chemotactic factor of anaphylaxis did not correlate with total serum IgE levels nor with serum eosinophilia in BP.[Citation72] On the other hand, Czech et al. reported elevated levels of total serum IgE, eosinophil cationic protein, and neutrophil-derived myeloperoxidase in blister fluid and serum in BP but not in pemphigus vulgaris patients.[Citation73] Tedeschi et al. found a significant correlation of the strikingly elevated levels of eosinophil cationic protein in blister fluid with the coagulation markers D-Dimer and F1 + 2.[Citation74] In addition to the low-affinity FcεRII, the high-affinity FcεRI is expressed not only on mast cells and basophils,[Citation75] but also on eosinophils.[Citation76] In this line, Messingham et al. recently identified FcεRI on peripheral blood and skin eosinophils.[Citation77] Tanaka et al. found FcεRI expression in 70% of the eosinophils in lesional BP skin.[Citation76] Binding of anti-BP180 NC16A IgE on these eosinophils might result in eosinophil degranulation or may trigger mast cell degranulation, which has been suggested to be in a piecemeal degranulation pattern [Citation78,Citation79] and therefore might contribute to blister development. In addition, gelatinase derived from eosinophils has been reported to cleave the extracellular domain of BP180.[Citation80] In accordance with an IgE-mediated process, activated and degranulated mast cells are present in the dermis of early BP lesions.[Citation79,Citation81]
Skin-bound IgE anti-BMZ autoantibodies
In addition to the elevated total serum IgE levels and eosinophilia in about 70–85% and 50–60% of BP patients, respectively, IgE deposition along the BMZ has been detected by direct IF microscopy of perilesional patients’ skin.[Citation82,Citation83] Provost and Tomasi first described weak linear IgE deposition along the BMZ in 25% of patients, [Citation82] which was recapitulated by others.[Citation47,Citation69,Citation84,Citation85] Yayli et al. detected labeling of IgE along the BMZ in 41% of 44 BP patients, including two patients without IgG and C3 reactivity.[Citation83] In the same study, an association of skin-bound IgE with a certain clinical phenotype, i.e. the presence of pruritic urticarial papules and plaques was reported.[Citation83] In contrast, Bird et al. could not detect anti-BMZ IgE in the skin of 24 BP patients.[Citation86] Accordingly, we were unable to visualize IgE reactivity against the BMZ by direct IF microscopy using three different fluorescein isothiocyanate-labeled anti-IgE antibodies in 20 BP patients (unpublished data).
Anti-BMZ IgE serum antibodies
By indirect IF microscopy on 1 M NaCl-split skin, binding of IgE was described in 33% (six of nine sera), 53% (9 of 17 sera), 7% (1 of 15 sera), 50% (two of four sera), and none of 15 and 30 BP patients.[Citation41,Citation47,Citation69,Citation87–Citation90] In some but not all studies, serum anti-BMZ IgE reactivity was associated with elevated total serum IgE levels.[Citation87,Citation89,Citation90] In two patients lacking IgG and C3 deposition by direct IF microscopy, strong IgE deposition on eosinophils adjacent to the subepidermal splitting was observed.[Citation91] In dogs with BP, IgG1, IgG4, and IgE were found to be the predominant autoantibody isotypes detected by indirect IF microscopy on salt-split canine skin.[Citation92]
BP180-specific IgE autoantibodies
In BP, autoimmune reactivity by direct and indirect IF microscopy was allocated to BP180 and, to a lesser extent, to BP230. Besides IgG autoantibodies, IgE anti-BP180 autoantibodies of the IgA and IgE isotypes are found.[Citation45,Citation93–Citation95] Like IgG, IgA and IgE autoantibodies target mainly the NC16A domain.[Citation25,Citation45,Citation95–Citation97] Epitopes outside the NC16A domain have also been described to be recognized by IgE.[Citation46,Citation97,Citation98] BP180 peptides were detected on mast cells and basophils from BP patients with IgE autoantibodies against NC16A and both cell types degranulated upon exposure to recombinant NC16A.[Citation45] Furthermore, IgE-coated mast cells were detected in perilesional skin of BP patients.[Citation79]
The percentage of BP patients with IgE anti-BP180 NC16A autoantibodies varied substantially in the different reports.[Citation46,Citation83,Citation95,Citation96,Citation99–Citation106] In the initial report, we identified anti-BP180 NC16A IgE reactivity in 55% of 18 BP sera.[Citation96] Subsequent studies found IgE anti-NC16A in 100% of 10, 83% of 18, 22% of 37, 77% of 43, 30% of 67, 61% of 31, and 71% of 56 BP patients.[Citation46,Citation95,Citation99,Citation100,Citation103,Citation104,Citation106] The large variety of anti-BP180 IgE reactivity in these studies may be in part explained by the different test systems, i.e. ELISA or immunoblotting, and the use of diluted or undiluted patient sera. We have recently determined the prevalence of IgE against BP180 NC16A to about 40% in two large independent cohorts of BP patients taking into account the total IgE serum levels (unpublished data).
Several reports also addressed the relation of IgE anti-BP180 antibodies and the clinical phenotype of the corresponding patient. Some studies proposed an association with a more severe form of BP.[Citation102,Citation106] In line, serum levels of anti-NC16A IgE significantly correlated with disease severity in individual BP patients.[Citation96,Citation99,Citation106,Citation107] These data are further supported by Delaporte et al. and Kalowska et al., who found the disappearance of IgE anti-BP180 autoantibodies to be associated with clinical remission.[Citation101,Citation102] Interestingly, in two infants with BP, no IgE reactivity against the NC16A domain was detectable.[Citation10]
Bowszyc-Dmochowska et al. pointed out that the stimulation of Th2 cells may be responsible for the production of IgG4 and IgE autoantibodies to BP180 and BP230.[Citation108] In this context, the specific T cell response after challenge with NC16A, as measured by the release of IL-4 and IgE response, was significantly stronger in BP patients compared to controls, pointing to an important contribution by Th2 cytokines to pathogenesis.[Citation109] Interestingly, two reports related BP to sensitization with specific allergens, hen’s egg allergen and penicillins, as demonstrated by specific serum IgE, patch testing, and basophil histamine release test.[Citation103,Citation110]
BP230-specific IgE autoantibodies
Several studies have found IgE autoantibodies against the intracellular BP230. Engineer et al. reported circulating anti-BP230 IgE in all six sera while no BP180-specific IgE autoantibodies were detected in sera and blister fluid of these BP patients.[Citation70] In contrast, other studies revealed IgE anti-BP230 and anti-BP180 antibodies in 67% of 67 sera and 50% of 32 sera, respectively.[Citation100,Citation111] IgE anti-BP230 reactivity was associated with local eosinophil accumulation.[Citation100] In a report of two BP patients, IgE, but not IgG, reactivity against a recombinant form of the C-terminal region of BP230 was related to disease activity.[Citation112] In 32 other BP patients, IgE antibodies against BP230 preferentially recognized the C-terminus and paralleled total IgE serum levels.[Citation89,Citation111]
Pathogenic relevance of IgE anti-BP180 antibodies
The clinical observations that anti-BP180 IgE serum levels are associated with disease activity and that the anti-IgE antibody omalizumab was effective in several BP patients clearly suggest a pathogenic role of IgE autoantibodies in BP. This hypothesis is supported by three experimental approaches. In the first approach, the effect of anti-BP180 IgE was investigated in vitro. Incubation of cultured human keratinocytes with IgE isolated from BP sera resulted in the release of IL-6 and IL-8,[Citation113,Citation114] two cytokines that may mediate disease development in BP, e.g. by the attraction of inflammatory cells.[Citation20,Citation115–Citation117] The secretion from cultured keratinocytes was also seen in response to incubation with a monoclonal antihuman BP180 NC16A IgE.[Citation114] Furthermore, incubation of keratinocytes with anti-BP180 IgE led to internalization of BP180.[Citation118] Both effects have previously been shown for IgG-anti BP180 antibodies.[Citation20,Citation119] Additionally, incubation of basophils with the monoclonal IgE anti-BP180 antibody led to a BP180-specific histamine release. In line with this, incubation with recombinant NC16A induced histamine release from basophils of BP patients by crosslinking NC16A-specific IgE on the surface of these cells.[Citation45]
While most data in animal models of BP are generated by the use/induction of IgG anti-BP180 autoantibodies, [Citation18,Citation21,Citation120,Citation121] two other approaches also explored the in vivo effect of anti-BP180 IgE. By the injection of IgE isolated from BP patients into human skin grafted onto the backs of immunodeficient nu/nu mice, some characteristic features of the human disease could be replicated.[Citation93] In particular, the infiltration of eosinophils and the degranulation of mast cells in the upper dermis were seen as well as microscopic subepidermal splitting. Clinically, erythema and pruritus were present but no frank blistering. In a similar model, severe combined immunodeficiency (SCID) mice (which lack T and B lymphocytes) grafted with human skin onto their backs were observed for up to 21 days after the injection of anti-BP180 IgE-producing hybridoma cells below the grafts.[Citation122] Again, erythema but no macroscopic blistering was seen, while histopathology revealed subepidermal split formation.[Citation122] Both in vivo models were not entirely mirroring the patient situation. While one model used IgE isolated from BP sera but only included an observation period of 24 hours and, thus, only addressed the very early events,[Citation93] the other model included a considerably longer observation period but applied a monoclonal IgE anti-BP180 antibody that may not have targeted a major pathogenic epitope(s).[Citation122] In addition, in both models, the effector cells express the murine FcεRs, which have a lower affinity to human IgE.
Interestingly, mice with a genetic deletion of the immunodominant NC14A domain of BP180 (corresponding to the NC16A domain in humans) show elevated total IgE serum levels compared to wild-type animals and start scratching at weeks 10–16 of life without developing blisters or erosions.[Citation123] Further analyses are required to explore the complex interplay of elevated total IgE levels and IgE and IgG autoantibodies against BP180.
Other pemphigoid diseases
In linear IgA disease and pemphigoid gestationis, elevated total IgE levels have been described in individual patients.[Citation51,Citation52] In pemphigoid gestationis, elevated total IgE serum levels were only observed in the mother but not in the child with transient skin lesions.[Citation52] In a patient with epidermolysis bullosa acquisita, high total IgE serum levels correlated with disease activity.[Citation124]
No IgE reactivity against the ectodomain of BP180 was found in sera of 10 patients with linear IgA disease and nine patients with mucous membrane pemphigoid.[Citation95] In contrast, one of four anti-laminin 332 mucous membrane pemphigoid patients showed IgE reactivity against the γ2 chain.[Citation125] Zhou et al. reported serum anti-BMZ IgE by indirect IF microscopy on salt-split skin in sera from one patient with mucous membrane pemphigoid, two patients with linear IgA disease but none of two pemphigoid gestationis patients.[Citation88] In contrast, in 7.7% of 299 pregnant women, anti-BP180 IgE was detected and was shown to label both the cutaneous BMZ and the placental amnion by indirect IF microscopy as well as cultured cytothrophoblasts.[Citation126] IgE binding along the BMZ in the patients’ skin was found in 69% of 13 mucous membrane pemphigoid patients [Citation83] but in none of 2 and 12 patients with pemphigoid gestationis.[Citation127,Citation128]
Pemphigus
Elevated total IgE serum levels were found in patients with endemic pemphigus foliaceus but rarely in pemphigus vulgaris.[Citation49,Citation53–Citation55] Accordingly, in endemic pemphigus foliaceus, anti-desmoglein 1 IgE was found significantly more often and IgE serum levels against LJM11, a major immunogenic component of sand fly salivary gland antigens, were significantly higher compared to controls.[129,130] In contrast to BP, no increased serum levels of total IgE, eosinophil cationic protein, and neutrophil-derived myeloperoxidase were measured in pemphigus vulgaris patients.[Citation73] Intercellular IgE deposits were detected in the epidermis of 37 patients with acute-onset pemphigus vulgaris.[Citation131] In the same study, IgE-anti-desmoglein 3 was detected more frequently in the sera of patients with acute onset compared to chronic active pemphigus vulgaris.[Citation131,Citation132] In summary, IgE reactivity seems to be more relevant in the endemic form of pemphigus foliaceus than in pemphigus vulgaris.
Expert commentary
IgE autoantibodies are present in a high number of BP patients while much less data are available for other autoimmune blistering diseases. Despite various attempts to clarify the pathogenic contribution of IgE autoantibodies in BP, the relevance of IgE in these diseases is not yet fully understood. At present, it is unclear whether IgE autoantibodies may add on to the IgG-mediated processes or rather exert distinct effects. Similar to IgG, much more evidence has been accumulated for the relevance of IgE antibodies against BP180 compared to IgE reactivity against BP230. See for a summary of some of the important references highlighted in this manuscript.
Table 1. Important references.
Five-year view
Studies with a high number of well-defined patient and control cohorts are needed to fully unravel the exact percentage of BP patients with anti-BP180 IgE reactivity and to clarify its association with clinical and laboratory characteristics of BP. Furthermore, the results of an ongoing placebo-controlled prospective trial with an anti-IgE antibody in BP may further elucidate the clinical relevance of elevated total serum IgE and IgE autoantibodies in this disease. Future mouse models may employ strains that express the human high-affinity FcεRI to allow a more realistic reflection of the effects exerted by IgE autoantibodies in autoimmune blistering diseases. Distinguishing between IgG- and IgE-mediated effects will be helpful to choose the appropriate therapeutic regimens.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Key issues
Total IgE levels and the soluble FcεRII are elevated in BP.
Total IgE levels correlate with disease activity, levels of IL-5, and eosinophilic cationic protein.
Eosinophilia is a typical feature of BP with elevated levels of eosinophil chemotactic factor and myeloperoxidase.
Deposits of IgE in human skin have been found along the BMZ in patients with BP and mucous membrane pemphigoid.
BP180-specific IgE autoantibodies are mainly directed against the NC16A domain.
BP180 NC16A-specific IgE autoantibodies correlate with disease severity in BP
BP230-specific IgE autoantibodies are variably found in BP.
The successful use of the anti-IgE antibody omalizumab in individual patients with BP points toward a pathogenic role of IgE autoantibodies in this disease.
Several in vitro approaches and two mouse models suggest a pathogenically relevant role of anti-BP180 IgE in the early phase of BP.
References
- Schmidt E, Zillikens D. The diagnosis and treatment of autoimmune blistering skin diseases. Dtsch Ärztebl Int. 2011;108(23):399–405.
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355(17):1800–1810.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332.
- Sárdy M, Kárpáti S, Merkl B, et al. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195(6):747–757.
- Bertram F, Bröcker E-B, Zillikens D, et al. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. JDDG J Dtsch Dermatol Ges. 2009;7(5):434–439.
- Langan SM, Smeeth L, Hubbard R, et al. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: population based cohort study. Bmj. 2008;337(7662):160–163.
- Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132(8):1998–2004.
- Jung M, Kippes W, Messer G, et al. Increased risk of bullous pemphigoid in male and very old patients: A population-based study on incidence. J Am Acad Dermatol. 1999;41(2 Pt 1):266–268.
- Schmidt E, Borradori L, Joly P. Epidemiology of autoimmune bullous diseases. In: Murrell D, Ed. Blistering diseases. Heidelberg: Springer; 2015. p. 251–264.
- Chimanovitch I, Hamm H, Georgi M, et al. Bullous pemphigoid of childhood: Autoantibodies target the same epitopes within the nc16a domain of bp180 as autoantibodies in bullous pemphigoid of adulthood. Arch Dermatol. 2000;136(4):527–532.
- Robinson JW, Odom RB. Bullous pemphigoid in children. Arch Dermatol. 1978;114(6):899–902.
- Della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167(5):1111–1117.
- Schmidt E, Della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Dermatol Clin. 2011;29(3):427–438, viii–ix.
- Zillikens D, Rose PA, Balding SD, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109(4):573–579.
- Blöcker IM, Dähnrich C, Probst C, et al. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br J Dermatol. 2012;166(5):964–970.
- Skaria M, Jaunin F, Hunziker T, et al. IgG autoantibodies from bullous pemphigoid patients recognize multiple antigenic reactive sites located predominantly within the B and C subdomains of the COOH-terminus of BP230. J Invest Dermatol. 2000;114(5):998–1004.
- Liu Z, Diaz LA, Swartz SJ, et al. Molecular mapping of a pathogenically relevant BP180 epitope associated with experimentally induced murine bullous pemphigoid. J Immunol. 1995;155(11):5449–5454.
- Ujiie H, Shibaki A, Nishie W, et al. A novel active mouse model for bullous pemphigoid targeting humanized pathogenic antigen. J Immunol. 2010;184(4):2166–2174.
- Nishie W, Sawamura D, Goto M, et al. Humanization of autoantigen. Nat Med. 2007;13(3):378–383.
- Schmidt E, Reimer S, Kruse N, et al. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115(5):842–848.
- Schulze FS, Beckmann T, Nimmerjahn F, et al. Fcγ receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am J Pathol. 2014;184(8):2185–2196.
- Sitaru C, Schmidt E, Petermann S, et al. Autoantibodies to bullous pemphigoid antigen 180 induce dermal–epidermal separation in cryosections of human kin. J Invest Dermatol. 2002;118(4):664–671.
- Schmidt E, Obe K, Bröcker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136(2):174–178.
- Amo Y, Ohkawa T, Tatsuta M, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2001;26(1):14–18.
- Hofmann S, Thoma-Uszynski S, Hunziker T, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002;119(5):1065–1073.
- Tsuji-Abe Y, Akiyama M, Yamanaka Y, et al. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005;37(3):145–149.
- Bieber K, Sun S, Ishii N, et al. Animal models for autoimmune bullous dermatoses. Exp Dermatol. 2010;19(1):2–11.
- Yu X, Holdorf K, Kasper B, et al. FcγRIIA and FcγRIIIB are required for autoantibody-induced tissue damage in experimental human models of bullous pemphigoid. J Invest Dermatol. 2010;130(12):2841–2844.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10(2):84–89.
- Schmidt E, Goebeler M, Hertl M, et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges JDDG. 2015;13(7):713–727.
- Marzano AV, Tedeschi A, Fanoni D, et al. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160(2):266–272.
- Marzano AV, Tedeschi A, Polloni I, et al. Prothrombotic state and impaired fibrinolysis in bullous pemphigoid, the most frequent autoimmune blistering disease. Clin Exp Immunol. 2013;171(1):76–81.
- Eming R, Sticherling M, Hofmann SC, et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges JDDG. 2015;13(8):833–844.
- Joly P, Roujeau J-C, Benichou J, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321–327.
- Yu KK, Crew AB, Messingham KAN, et al. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71(3):468–474.
- Dufour C, Souillet AL, Chaneliere C, et al. Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol. 2012;166(5):1140–1142.
- Fairley JA, Baum CL, Brandt DS, et al. Pathogenicity of IgE in autoimmunity: Successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. 2009;123(3):704–705.
- London VA, Kim GH, Fairley JA, et al. Successful treatment of bullous pemphigoid with omalizumab. Arch Dermatol. 2012;148(11):1241–1243.
- Yalcin AD, Genc GE, Celik B, et al. Anti-IgE monoclonal antibody (omalizumab) is effective in treating bullous pemphigoid and its effects on soluble CD200. Clin Lab. 2014;60(3):523–524.
- El-Qutob D. Off-Label Uses of Omalizumab. Clin Rev Allergy Immunol. 2015.
- Nieboer C. Serum IgE levels in patients with bullous pemphigoid. Acta Derm Venereol. 1985;65(3):273–274.
- Asbrink E, Hovmark A. Serum IgE levels in patients with bullous pemphigoid and its correlation to the activity of the disease and anti-basement membrane zone antibodies. Acta Derm Venereol. 1984;64(3):243–246.
- Bernard P, Catanzano G, Vignaud St Florent JD, et al. Bullous pemphigoid with pemphigus type antibodies in vivo. 2 cases. Ann Dermatol Vénéréologie. 1986;113(8):671–676.
- Kippes W, Schmidt E, Roth A, et al. Immunopathologic changes in 115 patients with bullous pemphigoid. Hautarzt. 1999;50(12):866–872.
- Dimson OG, Giudice GJ, Fu CL, et al. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. 2003;120(5):784–788.
- Dresow SK, Sitaru C, Recke A, et al. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol. 2009;160(2):429–432.
- Parodi A, Rebora A. Serum IgE antibodies bind to the epidermal side of the basement membrane zone splits in bullous pemphigoid. Br J Dermatol. 1992;126(5):526–527.
- Bernard P, Venot J, Constant F, et al. Blood eosinophilia as a severity marker for bullous pemphigoid. J Am Acad Dermatol. 1987;16(4):879–881.
- Arbesman CE, Wypych JI, Reisman RE, et al. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch Dermatol. 1974;110(3):378–381.
- Balakirski G, Merk HF, Megahed M. Bullous pemphigoid: a new look at a well-known disease. Hautarzt. 2014;65(12):1013–1016.
- Colombo M, Volpini S, Orini S, et al. Linear IgA bullous dermatosis: the importance of a correct differential diagnosis. Minerva Pediatr. 2008;60(3):351–353.
- Bonifazi E, Meneghini CL. Herpes gestationis with transient bullous lesions in the newborn. Pediatr Dermatol. 1984;1(3):215–218.
- Bruns GR, Ablin RJ, Guinan PD. Serum immunoglobulin E in pemphigus. J Invest Dermatol. 1978;71(3):217–218.
- Bruns GR, Ablin RJ. Levels of serum IgE in patients with Brazilian pemphigus foliaceus and pemphigus vulgaris. Allergol Immunopathol (Madr). 1979;7(6):407–410.
- Abréu Vélez AM, Warfvinge G, Herrera WL, et al. Detection of mercury and other undetermined materials in skin biopsies of endemic pemphigus foliaceus. Am J Dermatopathol. 2003;25(5):384–391.
- Lamb PM, Patton T, Deng J-S. The predominance of IgG4 in prodromal bullous pemphigoid. Int J Dermatol. 2008;47(2):150–153.
- Khan A, Ayyar R, Michaelson JD, et al. Transfer factor treatment in bullous pemphigoid. Impaired T cell function. Dermatologica. 1980;161(4):243–249.
- Mori O, Hachisuka H, Kusuhara M, et al. Bullous pemphigoid in a 19-year-old woman. A case with unusual target antigens. Br J Dermatol. 1994;130(2):241–245.
- Van Beek N, Schwemm N, Schulze F, et al. Abstract FV01/01 Die Serumspiegel von IgE Antikörpern gegen die Immunodominante BP180-NC16A Domäne korrelieren mit der Krankheitsaktivität von Patienten mit bullösem Pemphigoid. Abstract JDDG. 2015;13(S1):69.
- Cozzani E, Parodi A, Rebora A, et al. Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatol Venereol JEADV. 2001;15(4):317–319.
- Furukawa F, Kumagai S, Sakamoto Y, et al. Elevated serum levels of IgE-binding factor/soluble CD23 in bullous pemphigoid. J Dermatol Sci. 1994;7(2):150–154.
- Maekawa N, Hosokawa H, Soh H, et al. Serum levels of soluble CD23 in patients with bullous pemphigoid. J Dermatol. 1995;22(5):310–315.
- Schmidt E, Bröcker E, Zillikens D. HIgh levels of soluble cd23 in blister fluid of patients with bullous pemphigoid. Arch Dermatol. 1995;131(8):966–967.
- Inaoki M, Sato S, Takehara K. Elevated expression of CD23 on peripheral blood B lymphocytes from patients with bullous pemphigoid: correlation with increased serum IgE. J Dermatol Sci. 2004;35(1):53–59.
- D’Auria L, Pietravalle M, Mastroianni A, et al. IL-5 levels in the serum and blister fluid of patients with bullous pemphigoid: correlations with eosinophil cationic protein, RANTES, IgE and disease severity. Arch Dermatol Res. 1998;290(1–2):25–27.
- Echigo T, Hasegawa M, Shimada Y, et al. Both Th1 and Th2 chemokines are elevated in sera of patients with autoimmune blistering diseases. Arch Dermatol Res. 2006;298(1):38–45.
- Kakinuma T, Wakugawa M, Nakamura K, et al. High level of thymus and activation-regulated chemokine in blister fluid and sera of patients with bullous pemphigoid. Br J Dermatol. 2003;148(2):203–210.
- De Pitá O, Frezzolini A, Cianchini G, et al. T-helper 2 involvement in the pathogenesis of bullous pemphigoid: role of soluble CD30 (sCD30). Arch Dermatol Res. 1997;289(12):667–670.
- Baba T, Sonozaki H, Seki K, et al. An eosinophil chemotactic factor present in blister fluids of bullous pemphigoid patients. J Immunol. 1976;116(1):112–116.
- Engineer L, Bhol K, Kumari S, et al. Bullous pemphigoid: interaction of interleukin 5, anti-basement membrane zone antibodies and eosinophils. A preliminary observation. Cytokine. 2001;13(1):32–38.
- Bushkell LL, Jordon RE. Bullous pemphigoid: a cause of peripheral blood eosinophilia. J Am Acad Dermatol. 1983;8(5):648–651.
- Czarnetzki BM, Kalveram KJ, Dierksmeier U. Serum eosinophil chemotactic factor levels in patients with bullous pemphigoid, drug reactions and atopic eczema. J Invest Dermatol. 1979;73(2):163–165.
- Czech W, Schaller J, Schöpf E, et al. Granulocyte activation in bullous diseases: release of granular proteins in bullous pemphigoid and pemphigus vulgaris. J Am Acad Dermatol. 1993;29(2 Pt 1):210–215.
- Tedeschi A, Marzano AV, Lorini M, et al. Eosinophil cationic protein levels parallel coagulation activation in the blister fluid of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol JEADV. 2015;29(4):813–817.
- Blank M, Gisondi P, Mimouni D, et al. New insights into the autoantibody-mediated mechanisms of autoimmune bullous diseases and urticaria. Clin Exp Rheumatol. 2006;24(1 Suppl 40):S20–25.
- Tanaka Y, Takenaka M, Matsunaga Y, et al. High affinity IgE receptor (Fc epsilon RI) expression on eosinophils infiltrating the lesions and mite patch tested sites in atopic dermatitis. Arch Dermatol Res. 1995;287(8):712–717.
- Messingham KN, Holahan HM, Frydman AS, et al. Human eosinophils express the high affinity IgE receptor, FcεRI, in bullous pemphigoid. PLoS One. 2014;9(9):e107725.
- Kaminer MS, Murphy GF, Zweiman B, et al. Connective tissue mast cells exhibit time-dependent degranulation heterogeneity. Clin Diagn Lab Immunol. 1995;2(3):297–301.
- Dvorak AM, Mihm MC, Osage JE, et al. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J Invest Dermatol. 1982;78(2):91–101.
- Kasahara-Imamura M, Hosokawa H, Maekawa N, et al. Activation of Fc epsilon RI-positive eosinophils in bullous pemphigoid. Int J Mol Med. 2001;7(3):249–253.
- Woodley DT. The role of IgE anti-basement membrane zone autoantibodies in bullous pemphigoid. Arch Dermatol. 2007;143(2):249–250.
- Provost TT, Tomasi TB. Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol. 1974;18(2):193–200.
- Yayli S, Pelivani N, Beltraminelli H, et al. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol. 2011;165(5):1133–1137.
- Nieboer C, Van Leeuwen HE. IgE in the serum and on mast cells in bullous pemphigoid. Arch Dermatol. 1980;116(5):555–556.
- Moriuchi R, Nishie W, Ujiie H, et al. In vivo analysis of IgE autoantibodies in bullous pemphigoid: A study of 100 cases. J Dermatol Sci. 2015;78(1):21–25.
- Bird P, Friedmann PS, Ling N, et al. Subclass distribution of IgG autoantibodies in bullous pemphigoid. J Invest Dermatol. 1986;86(1):21–25.
- Hadi SM, Barnetson RS, Gawkrodger DJ, et al. Clinical, histological and immunological studies in 50 patients with bullous pemphigoid. Dermatologica. 1988;176(1):6–17.
- Zhou S, Wakelin SH, Allen J, et al. Blister fluid for the diagnosis of subepidermal immunobullous diseases: a comparative study of basement membrane zone autoantibodies detected in blister fluid and serum. Br J Dermatol. 1998;139(1):27–32.
- Ghohestani RF, Cozzani E, Delaporte E, et al. IgE antibodies in sera from patients with bullous pemphigoid are autoantibodies preferentially directed against the 230-kDa epidermal antigen (BP230). J Clin Immunol. 1998;18(3):202–209.
- Soh H, Hosokawa H, Asada Y. IgE and its related phenomena in bullous pemphigoid. Br J Dermatol. 1993;128(4):371–377.
- Talanin NY, Shelley WB, Shelley ED. IgE bullous disease. Clin Exp Dermatol. 1997;22(2):82–86.
- Favrot C, Dunston SM, Paradis M, et al. Isotype determination of circulating autoantibodies in canine autoimmune subepidermal blistering dermatoses. Vet Dermatol. 2003;14(1):23–30.
- Fairley JA, Burnett CT, Fu C-L, et al. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. 2007;127(11):2605–2611.
- Csorba K, Schmidt S, Florea F, et al. Development of an ELISA for sensitive and specific detection of IgA autoantibodies against BP180 in pemphigoid diseases. Orphanet J Rare Dis. 2011;6:31.
- Christophoridis S, Büdinger L, Borradori L, et al. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol. 2000;143(2):349–355.
- Döpp R, Schmidt E, Chimanovitch I, et al. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42(4):577–583.
- Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol. 2005;125(3):467–472.
- Fairley JA, Bream M, Fullenkamp C, et al. Missing the target: characterization of bullous pemphigoid patients who are negative using the BP180 enzyme-linked immunosorbant assay. J Am Acad Dermatol. 2013;68(3):395–403.
- Messingham KAN, Noe MH, Chapman MA, et al. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. 2009;346(1–2):18–25.
- Ishiura N, Fujimoto M, Watanabe R, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2008;49(2):153–161.
- Kalowska M, Ciepiela O, Kowalewski C, et al. Enzyme-linked Immunoassay Index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Derm Venereol. 2015.
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157(8):3642–3647.
- Pomponi D, Di Zenzo G, Zennaro D, et al. Detection of IgG and IgE reactivity to BP180 using the ISAC® microarray system. Br J Dermatol. 2013;168(6):1205–1214.
- Liu B, Zuo Y, Zhou X, et al. Establishment of enzyme-linked immunosorbent assay in the detection of BP180NC16A-specific IgE and its significance in bullous pemphigoid. Zhonghua Yi Xue Za Zhi. 2013;93(28):2244–2247.
- Ma L, Wang M, Wang X, et al. Circulating IgE anti-BP180 autoantibody and its correlation to clinical and laboratorial aspects in bullous pemphigoid patients. J Dermatol Sci. 2015;78(1):76–77.
- Iwata Y, Komura K, Kodera M, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. 2008;144(1):41–48.
- Kamiya K, Aoyama Y, Noda K, et al. Possible correlation of IgE autoantibody to BP180 with disease activity in bullous pemphigoid. J Dermatol Sci. 2015;78(1):77–79.
- Bowszyc-Dmochowska M, Dmochowski M. Immediate hypersensitivity phenomena in bullous pemphigoid: critical concepts. J Med. 2002;33(1–4):189–198.
- Pickford WJ, Gudi V, Haggart AM, et al. T cell participation in autoreactivity to NC16a epitopes in bullous pemphigoid. Clin Exp Immunol. 2015;180(2):189–200.
- Borch JE, Andersen KE, Clemmensen O, et al. Drug-induced bullous pemphigoid with positive patch test and in vitro IgE sensitization. Acta Derm Venereol. 2005;85(2):171–172.
- Fania L, Caldarola G, Müller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol Orlando Fla. 2012;143(3):236–245.
- Cozzani E, Micalizzi C, Parodi A, et al. Anti-230 kDa circulating IgE in bullous pemphigoid: relationship with disease activity. Acta Derm Venereol. 1997;77(3):236.
- Messingham KN, Srikantha R, DeGueme AM, et al. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187(1):553–560.
- Messingham KAN, Onoh A, Vanderah EM, et al. Functional characterization of an IgE-class monoclonal antibody specific for the bullous pemphigoid autoantigen, BP180. Hybridoma. 2012;31(2):111–117.
- Inaoki M, Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J Dermatol Sci. 1998;16(2):152–157.
- Schmidt E, Bastian B, Dummer R, et al. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288(7):353–357.
- Liu Z, Giudice GJ, Zhou X, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100(5):1256–1263.
- Hiroyasu S, Ozawa T, Kobayashi H, et al. Bullous pemphigoid IgG induces BP180 internalization via a macropinocytic pathway. Am J Pathol. 2013;182(3):828–840.
- Iwata H, Kamio N, Aoyama Y, et al. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (Type XVII Collagen) and weakens cell attachment. J Invest Dermatol. 2009;129(4):919–926.
- Liu Z, Diaz LA, Troy JL, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92(5):2480–2488.
- Hirose M, Recke A, Beckmann T, et al. Repetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in mice. J Immunol. 2011;187(3):1176–1183.
- Zone JJ, Taylor T, Hull C, et al. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol. 2007;127(5):1167–1174.
- Hurskainen T, Kokkonen N, Sormunen R, et al. Deletion of the major bullous pemphigoid epitope region of collagen XVII induces blistering, autoimmunization, and itching in mice. J Invest Dermatol. 2015;135(5):1303–1310.
- Miyake H, Morishima Y, Komai R, et al. Epidermolysis bullosa acquisita: correlation of IgE levels with disease activity under successful betamethasone/dapsone combination therapy. Acta Derm Venereol. 2001;81(6):429.
- Natsuga K, Nishie W, Shinkuma S, et al. Circulating IgA and IgE autoantibodies in antilaminin-332 mucous membrane pemphigoid. Br J Dermatol. 2010;162(3):513–517.
- Noe MH, Messingham KAN, Brandt DS, et al. Pregnant women have increased incidence of IgE autoantibodies reactive with the skin and placental antigen BP180 (type XVII collagen). J Reprod Immunol. 2010;85(2):198–204.
- Proença NG, Yagima M, De Almeida F, et al. Immunopathological studies in herpes gestationis. Med Cutánea Ibero-Lat-Am. 1977;5(2):121–127.
- Jabłońska S, Chorzelski TP, Maciejowska E, et al. Immunologic phenomena in herpes gestationis. Arch Immunol Ther Exp (Warsz). 1978;26(1–6):769–774.
- Qian Y, Prisayanh P, Andraca E, et al. IgE, IgM and IgG4 anti-desmoglein 1 autoantibody profile in endemic pemphigus foliaceus (Fogo Selvagem). J Invest Dermatol. 2011;131(4):985–987.
- Qian Y, Jeong JS, Abdeladhim M, et al. IgE anti-LJM11 sand fly salivary antigen may herald the onset of Fogo Selvagem in endemic Brazilian regions. J Invest Dermatol. 2015;135(3):913–915.
- Nagel A, Lang A, Engel D, et al. Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol Orlando Fla. 2010;134(3):320–330.
- Spaeth S, Riechers R, Borradori L, et al. IgG, IgA and IgE autoantibodies against the ectodomain of desmoglein 3 in active pemphigus vulgaris. Br J Dermatol. 2001;144(6):1183–1188.
