ABSTRACT
House dust mite (HDM) allergy represents a highly prevalent inhalant allergy, and exposure to HDM allergens results in allergic rhinitis with persistent symptoms that may not be adequately controlled with available allergy pharmacotherapy. Allergy immunotherapy constitutes a complementary treatment option targeting the underlying immunological mechanisms of allergic disease and represents the only treatment with a potential for disease modification and long-term efficacy. As traditional allergy immunotherapy delivered by subcutaneous injection of specific HDM allergens involves a time-consuming treatment regimen and a risk of systemic adverse reactions, sublingually administered allergy immunotherapy (SLIT) has been investigated as a more convenient treatment option with similar levels of efficacy and an improved safety profile that allows for at-home daily administration.
In this Drug Profile, we provide a review of the clinical data behind the SQ HDM SLIT-tablet, which was recently approved for the treatment of HDM-induced allergic rhinitis by regulatory authorities in Europe and Japan.
Introduction
House dust mite (HDM)-induced respiratory allergic disease
Allergic rhinitis (AR) is a chronic respiratory allergic disease causing major illness and substantial economic burden worldwide [Citation1]. AR affects an estimated 23% of the population across Europe [Citation2] and is associated with symptoms with significant negative impact on patients’ quality of life [Citation1,Citation3]. The house dust mite (HDM) is a major source of indoor allergens and a significant factor in the development of allergic disease, including AR and allergic asthma [Citation4]. Sensitization to HDM allergen has been identified as one of the most prevalent sensitizations among inhalant allergens [Citation5] and global prevalence estimates suggest that HDM allergy may affect 1–2% of the world’s population [Citation6]. In Europe, HDM sensitization was detected in 49% of adult subjects with a clinical diagnosis of AR [Citation2] and almost all HDM allergic adults suffer from HDM-induced AR. In addition, allergic asthma represents a frequent comorbidity of HDM-induced AR, as evidenced by the finding that approximately half of patients with HDM-induced AR also displayed symptoms of asthma [Citation7].
HDM allergens are ubiquitous in human habitats and for many allergic individuals exposure may result in HDM-induced AR with persistent symptoms that may vary in intensity throughout the year [Citation1,Citation8]. Upon allergen exposure, allergic individuals experience IgE-mediated inflammatory responses characterized by symptoms such as rhinorrhea, nasal obstruction, nasal itching and sneezing [Citation1,Citation9,Citation10]. While allergen avoidance has been investigated as a way to reduce HDM allergen levels and symptoms, the majority of interventions have failed to provide clinical benefit [Citation11–Citation13]. Thus, current treatment guidelines for HDM-induced AR focus on symptomatic allergy pharmacotherapy, such as nasal and oral antihistamines and nasal corticosteroids, and allergy immunotherapy (AIT) [Citation14].
In cases where the use of allergy pharmacotherapy fails to provide adequate symptom relief [Citation15], AIT constitutes an additional treatment option. AIT involves repeated administration of allergen to allergic subjects in order to induce immunological tolerance to the specific allergen and ameliorate symptoms associated with subsequent allergen exposure [Citation1].
Mechanism of action of AIT
The mechanism behind AIT-induced tolerance appears to involve a shift in the balance between Th2 and Treg responses, accompanied by increased numbers of Treg cells and suppression of effector T cell function [Citation16,Citation17]. As important regulators of immunological tolerance, Treg cells mediate suppression of allergen-specific immune responses in several ways. Through suppression of dendritic cells supporting effector T cell generation and by direct suppression of Th2 effector cells, Treg cells modulate B cell function and induce allergen-specific antibody responses characterized by strong IgG4 secretion and suppressed IgE secretion. In addition, Treg cells suppress effector T cell migration to the airway mucosa and mediate direct and indirect suppression of mast cells, basophils and eosinophils. Collectively, this modulation of T and B cell responses and inhibition of mast cell, basophil and eosinophil migration and mediator release represent a mechanistic foundation that is key for successful AIT. Thus, unlike symptomatic allergy pharmacotherapy, AIT provides allergen-specific modulation of the underlying immunological mechanisms of allergic disease and represents the only treatment option with a potential for long-term efficacy and modification of the natural course of allergic disease [Citation1,Citation18].
AIT routes of administration
Traditionally, AIT has been administered as subcutaneous injections with specific allergen extracts [Citation19] and the clinical efficacy of subcutaneously administered AIT (SCIT) is well established for HDM-induced AR [Citation20–Citation22]. Current guidelines recommend an AIT treatment period of 3 years for inhalant allergens [Citation1], with frequent (at least biweekly) injections during an initial up-dosing period followed by monthly to bimonthly injections of a maintenance dose. Thus, as SCIT injections must be performed under medical supervision, the many doctor’s visits involved in the treatment regimen make SCIT a time-consuming option requiring a high level of patient commitment [Citation23]. Further, the risk of systemic adverse reactions associated with the invasive character of SCIT leaves obvious room for improvement.
In this context, sublingually administered AIT (SLIT) meets an unmet medical need. Local administration of specific allergen extracts as sublingual tablets or drops appears to modify similar immunological mechanisms as when extracts are delivered subcutaneously [Citation19,Citation24] and for AR, numerous double-blind, placebo-controlled trials have confirmed that SLIT-tablets are effective in providing clinical benefit [Citation25]. Importantly, based on the observed tolerability profile, SLIT-tablets are approved for at-home administration (provided the initial dose is administered under medical supervision for at least 30 min) and most treatment regimens have been designed for daily dosing [Citation26,Citation27]. Thus, for patients with HDM-induced AR where allergy pharmacotherapy fails to provide adequate symptomatic relief and who expresses reluctance toward the commitment required for SCIT, SLIT-tablets represent a more convenient avenue to the benefits of AIT.
HDM SLIT-tablet treatment of HDM-induced AR
Market overview
Current treatment guidelines for HDM-induced AR include allergy pharmacotherapy and AIT [Citation14]. For HDM-induced AR patients who do not experience adequate symptom relief using available allergy pharmacotherapy, AIT represents a complementary treatment option. Whereas several products for SCIT treatment of HDM-induced AR are commercially available around the world, the practical challenges involved in successful SCIT generate an unmet medical need for a more convenient and easily accessed AIT treatment. With similar levels of efficacy and a superior tolerability profile [Citation28], SLIT appears to meet this unmet medical need and several SLIT products are marketed for pollen-induced AR. Whereas treatment in the form of sublingual drops has been available in multiple countries on a named-patient product basis for decades, SLIT-tablets backed by large amounts of data from clinical development programs have only recently obtained regulatory approval.
For HDM-induced AR, a number of SLIT-tablets are in clinical development. Specifically, the SQ HDM SLIT-tablet (MK-8237; ALK/Merck/Torii), which is the topic of the present Drug Profile, was approved by European regulatory authorities for treatment of HDM-induced AR as well as HDM-induced allergic asthma in August 2015 and approved by regulatory authorities in Japan for treatment of HDM-induced AR in September 2015. In addition, a HDM SLIT-tablet developed by Shionogi and Stallergenes (STG320) was approved by Japanese regulatory authorities in March 2015. Finally, a sublingual allergoid tablet containing HDM allergens modified by carbamylation is available in a number of countries on a named-patient product basis and is undergoing further clinical study in order to support a future marketing authorization application. Thus, evidently, substantial efforts aim at developing SLIT-tablet treatment options and meeting the unmet medical need of HDM-induced AR patients worldwide.
Introduction to the SQ HDM SLIT-tablet
Chemistry
The SQ HDM SLIT-tablet (MK-8237; ALK/Merck/Torii) is a fast-dissolving freeze-dried tablet containing a 1:1 mixture of allergen extracts from the HDM species Dermatophagoides pteronyssinus and Dermatophagoides farinae. Thus, the tablet contains all D. pteronyssinus and D. farinae allergens. A highly standardized production process ensures a 1:1:1:1 ratio of the major allergens Der p 1, Der f 1, Der p 2 and Der f 2 in the SQ HDM SLIT-tablet. D. pteronyssinus and D. farinae represent the two most commonly occurring HDM species worldwide, and the derived group 1 and group 2 allergens are among the most frequently recognized clinically relevant HDM allergens [Citation8].
The SQ HDM SLIT-tablet is formulated as a fast-dissolving oral lyophilisate for daily, at-home sublingual administration, utilizing a well-established formulation technology (Zydis®). Manufactured by a freeze-drying process, this dosage form consists of the drug substance physically entrapped or dissolved within the matrix of a fast-dissolving carrier material designed to disperse rapidly in the mouth [Citation29,Citation30]. Administration of the SQ HDM SLIT-tablet involves placing the freeze-dried tablet under the tongue from where it will dissolve leaving no residues.
Pharmacokinetics and metabolism
As traditional pharmacokinetic studies are not possible with AIT [Citation31], no clinical studies have been conducted to investigate the pharmacokinetic profile and metabolism of the SQ HDM SLIT-tablet. This reflects the finding that for SLIT products, no passive absorption of allergen through the oral mucosa occurs to any relevant extent and during AIT, plasma levels of intact allergen remain below detection limits [Citation31–Citation33]. Upon sublingual administration, allergen is taken up by dendritic cells, particularly Langerhans cells, of the oral mucosa and presented to other cells of the immune system [Citation16,Citation34,Citation35]. As the active components of allergen extracts are composed of polypeptides and proteins, allergen molecules not taken up by antigen-presenting cells are believed to undergo enzymatic hydrolysis during passage through the gastrointestinal tract. Accordingly, there is no evidence to suggest that intact allergen enters the vascular system to any relevant extent after sublingual administration and no reports of SLIT affecting renal or hepatic function have been published.
Pharmacodynamics
Whereas formal pharmacodynamic studies are not possible for allergen products, evaluation of AIT impact on specific immune parameters, including allergen-specific immunoglobulin (Ig) levels, is recommended during development [Citation31]. The process of tolerance induction during AIT involves changes in T cell responses as well as antibody responses to the specific allergen. The current understanding of the mechanism of AIT involves an antigen-presenting cell mediated shift in the balance between Th2 and Treg responses [Citation17]. As AR symptoms caused by mast cell degranulation result from Th2 cell induced B cell secretion of IgE, a shift toward Treg mediated Th2 suppression influences B cell Ig secretion. For instance, serum levels of allergen-specific IgE are known to transiently increase after AIT initiation and then gradually decrease over months or years. However, while these fluctuations in IgE levels indicate AIT modulation of underlying immunological mechanisms, IgE levels represent a poor predictor of clinical improvement during AIT [Citation36–Citation38]. In contrast, increased serum levels of allergen-specific IgG, and particularly IgG4, are consistently observed with AIT [Citation39,Citation40], and IgG is believed to inhibit IgE binding to allergen in a competitive manner [Citation19,Citation41]. Thus, the inhibitory activity against allergen-specific IgE has been suggested as a relevant measure relating treatment-induced changes in levels of Ig secretion to clinically relevant effects of AIT in study populations [Citation17,Citation42].
For the SQ HDM SLIT-tablet, several clinical trials have included measurement of allergen-specific IgE, IgG4 and the inhibitory activity against IgE, as assessed by IgE-blocking factor (IgE-BF). Two phase I trials investigated tolerability and acceptable dose range of the SQ HDM SLIT-tablet in adults and children, respectively, during 28 days of treatment [Citation43]. Both studies reported significant dose-dependent increases in IgE-BF against D. pteronyssinus as well as D. farinae, with doses higher than 4 SQ-HDM resulting in significantly increased levels of IgE-BF after 28 days of treatment. Similarly, levels of specific IgE toward both HDM species showed a significant dose-dependent increase in all active groups, as expected, whereas no changes were observed in the placebo groups [Citation43]. In addition, data from the two studies indicated comparable immunological responses to the SQ HDM SLIT-tablet of an adult versus pediatric population [Citation43].
A 24-week phase II trial assessing dose-related efficacy of the SQ HDM SLIT-tablet using an environmental exposure chamber reported comparable immunological responses [Citation44]. Levels of specific IgE and IgG4 increased in all active groups during the initial 8 weeks of treatment, reaching levels statistically significant from those of the placebo group. Further, at week 24, levels of both immunological parameters demonstrated a pronounced dose-dependence. Importantly, the onset and magnitude of immunological changes correlated with the observed clinical effect of the SQ HDM SLIT-tablet treatment [Citation44].
Collectively, dose-dependence was observed for all assessed immune parameters, regarding onset as well as magnitude of the immunological changes. Thus, responses induced by the SQ HDM SLIT-tablet correspond well with the mechanistic understanding of AIT [Citation19].
Clinical efficacy
Phase I studies
Dose selection for subsequent efficacy trials was based on results from two phase I trials investigating tolerability and the acceptable dose range for treatment with the SQ HDM SLIT-tablet in HDM allergic adults and children, respectively [Citation43]. Testing doses ranging from 1 SQ-HDM to 32 SQ-HDM, these trials identified 16 SQ-HDM as the maximum tolerable dose. However, as this dose involved a higher frequency of treatment-related adverse events (AEs) compared to the lower doses, 12 SQ-HDM was concluded to represent a suitable dose for further clinical investigation in adults and children with HDM-induced respiratory allergic disease [Citation43]. Thus, no subsequent trials included doses beyond 12 SQ-HDM.
Phase II and phase III studies
The clinical efficacy of the SQ HDM SLIT-tablet for HDM-induced AR has been evaluated in three randomized, double-blind, placebo-controlled trials [Citation44–Citation46], as outlined in . In addition to the listed trials based in Europe, the SQ HDM SLIT-tablet has been evaluated in one phase III trial in North America and two phase III trials in Japan.
Table 1. Summary of efficacy results relating to HDM-induced AR.
In MT-02 (EudraCT no. 2006–001795-20), a large phase II trial investigating the efficacy and safety of the SQ HDM SLIT-tablet in adults and adolescents with HDM-induced respiratory allergic disease, impact on AR was evaluated by a combined rhinitis symptom and medication score (total combined rhinitis score, TCRS) [Citation45]. The primary end point in this trial related to HDM-induced allergic asthma [Citation47] and the trial population was selected based on mild to moderate HDM-induced allergic asthma as well as a medical history of HDM-induced AR. However, a post hoc subgroup analysis evaluated the effect on AR in patients reporting a TCRS >0 during the baseline period (i.e. patients with nasal symptoms or medication use at baseline). As shown in , treatment with 6 SQ-HDM for 1 year resulted in a statistically significant reduction in TCRS, corresponding to a relative difference from placebo of 28.8% (). In contrast, despite inducing relative differences from placebo of 17.4 and 26.0%, respectively, the TCRS values observed for the two lower doses included in the trial (1 and 3 SQ-HDM) failed to reach statistical significance (). The clinical effect of 6 SQ-HDM was supported by a statistically significant improvement in quality of life, as assessed by the rhinitis quality of life questionnaire with standardized activities [RQLQ(S)] [Citation45]. Among the individual components of the RQLQ(S) instrument, the greatest improvement was observed for sleep, with 6 SQ-HDM reducing sleep impairment scores by approximately 50%. Thus, collectively, data from the MT-02 trial suggest that 1 year treatment with 6 SQ-HDM modulates the established link between AR, impaired sleep and decreased quality of life [Citation48], resulting in improved AR control, improved sleep and improved quality of life.
Figure 1. Efficacy scores relating to HDM-induced AR from the MT-02, P003, and MT-06 clinical trials. (a) End-of-trial total combined rhinitis scores (TCRS) of the MT-02 trial subpopulation with TCRS>0 at baseline; n=95 (placebo), n=117 (6 SQ-HDM) at end-of-trial assessment. (b) Total nasal symptom scores (TNSS) from each allergen exposure challenge session during the P003 trial; n=34 (placebo), n=36 (6 SQ-HDM), n=36 (12 SQ-HDM) at end-of-trial assessment. (c) Total combined rhinitis scores (TCRS) over time during the MT-06 trial; n=298 (placebo), n=297 (6SQ-HDM), n=284 (12 SQ-HDM) at end-of-trial assessment. Error bars represent 95% confidence intervals; asterisks denote statistically significant differences from placebo. *, P<0.05.
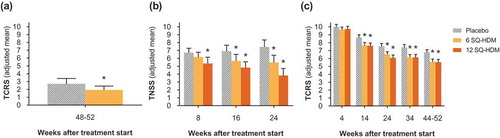
To investigate onset and dose-related efficacy of SQ HDM SLIT-tablet treatment, the P003 trial (ClinicalTrials.gov identifier NCT01644617) was designed to evaluate AR symptoms in a controlled allergen exposure environment during three exposure challenge sessions throughout a total treatment period of 24 weeks [Citation44]. The trial population comprised adults with HDM-induced AR (with or without conjunctivitis; with or without asthma) and HDM allergen challenges were performed as 6-h sessions in the Vienna Challenge Chamber, with subjects scoring symptoms every 15 min. As presented in , after only 8 weeks of treatment with the SQ HDM SLIT-tablet, subjects in the 12 SQ-HDM group reported statistically significantly lower total nasal symptom scores (TNSS) during the challenge session compared with the placebo group. For 6 SQ-HDM, a statistically significant difference from placebo was observed after 16 weeks. Clear dose-dependence was observed during all three challenge sessions and after 24 weeks of treatment, subjects receiving the 12 SQ-HDM dose reported a 48.6% reduction in TNSS during challenge as compared with subjects in the placebo group (). Thus, findings from the P003 trial demonstrate higher efficacy and faster onset of effect of the 12 SQ-HDM dose as compared with 6 SQ-HDM. The observed treatment effect of 12 SQ-HDM was supported by a statistically significant improvement in quality of life after 24 weeks of treatment, as assessed by the RQLQ(S) instrument and rhinoconjunctivitis symptom assessment using a visual analog scale (VAS) prior to the challenge session. As prechallenge RQLQ(S) and VAS evaluation assessed symptoms experienced in the week prior to the challenge session (i.e. during real-life allergen exposure), these findings may support a clinically relevant treatment effect outside of the controlled HDM chamber exposure.
In MT-06 (EudraCT no. 2011–002277-38), a phase III trial evaluating the clinical efficacy of the SQ HDM SLIT-tablet under conditions of real-life exposure to HDM allergens, a combined rhinitis symptom and medication score (i.e. TCRS) was evaluated at several points during a treatment period of approximately 1 year [Citation46]. The trial population included 992 adults with moderate to severe HDM-induced AR despite the use of allergy pharmacotherapy and subjects were randomized to receive daily treatment with placebo, 6 SQ-HDM, or 12 SQ-HDM (). As displayed in , after 14 weeks of treatment, TCRS values reported by subjects in both active treatment groups were significantly lower than values reported by subjects receiving placebo. Notably, both doses led to statistically significant differences from placebo in terms of reduced AR medication scores, and the 12 SQ-HDM dose displayed efficacy on all four individual symptoms included in the AR symptom score, that is, statistically significant score reductions for blocked nose, itchy nose, runny nose and sneezing compared with placebo [Citation46].
To evaluate whether the observed symptom score reductions were large enough to clearly reduce allergy-related morbidity and represent a clinically relevant effect, in MT-06, a pre-specified criterion for clinical relevance defined a numerical difference in TCRS of 1 or greater between actively treated and placebo groups as the minimal clinically relevant effect [Citation46]. The numerical magnitude of this criterion for clinical relevance (TCRS≥1) was selected based on examples of what a TCRS reduction of 1 could mean to an individual patient in terms of reduced symptom severity or reduced need for symptomatic allergy pharmacotherapy. Further, efficacy results for other SLIT-tablets approved for treatment of pollen-induced AR support the notion that a TCRS reduction of 1 constitutes a clinically relevant treatment effect [Citation49–Citation55]. Whereas these trials did not include TCRS as an end point (most report a combined rhinoconjunctivitis symptom and medication score (TCS) as the primary end point), the reported rhinitis symptom and medication scores have allowed for post hoc calculation of TCRS. The post hoc TCRS results correlate the observed clinical effect with a treatment-induced TCRS reduction of approximately 1 and thus formed the basis for the clinical relevance criterion in MT-06 (TCRS≥1). In MT-06, the criterion for clinical relevance was met for both actively treated groups after 14 weeks of treatment and TCRS differences >1 between actively treated and placebo groups were observed for all subsequent assessment points, demonstrating maintained efficacy throughout the remaining approximately 9 months of the study (). In addition, the clinical effect of 12 SQ-HDM was supported by a statistically significant improvement in quality of life, as assessed by the RQLQ(S) instrument. Specifically, whereas differences observed for 6 SQ-HDM did not reach statistical significance, 1 year of treatment with 12 SQ-HDM resulted in significantly lower RQLQ(S) scores compared with placebo within 4 of 7 individual RQLQ(S) domains: nasal symptoms, non-nose/non-eye symptoms, practical problems and sleep impairment [Citation46]. Thus, collectively, data from the MT-06 trial indicate that for patients displaying moderate to severe AR symptoms despite the use of symptom-relieving allergy pharmacotherapy, 1 year of treatment with 12 SQ-HDM is efficacious in providing benefit of significant clinical relevance to patients.
Summary of efficacy trial results
Collectively, data from the presented efficacy trials demonstrate that the SQ HDM SLIT-tablet provides statistically significant clinical improvement in HDM-induced AR (). Whereas 1 and 3 SQ-HDM were below the clinically effective dose range, 6 SQ-HDM and 12 SQ-HDM both induced significantly reduced rhinitis symptoms and reduced allergy pharmacotherapy use for patients with HDM-induced AR. However, data indicate a more robust effect as well as an earlier onset of effect for the 12 SQ-HDM dose compared with the 6 SQ-HDM dose.
Safety and tolerability
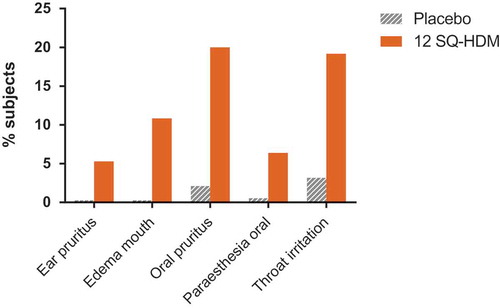
Combined clinical safety data from the reviewed trials indicate that the SQ HDM SLIT-tablet was well tolerated, and the observed safety and tolerability profile of the SQ HDM SLIT-tablet corresponds well with the observed profile for other SLIT-tablets [Citation53,Citation56–Citation58]. outlines the nature of treatment-related AEs reported by subjects receiving 12 SQ-HDM or placebo (based on pooled safety data from trials where the 12 SQ-HDM dose was included, i.e. P003 and MT-06). Importantly, safety data from a phase III trial not included in this Drug Profile, in which the primary end point related to HDM-induced allergic asthma, support the safety and tolerability profile of the SQ HDM SLIT-tablet observed in the reviewed trials [Citation59,Citation60]. The proportion of subjects experiencing AEs was higher among subjects receiving 12 SQ-HDM compared with the placebo group [Citation60]. The majority of treatment-related AEs observed during the clinical development program of the SQ HDM SLIT-tablet were mild, local allergic reactions, most of which occurred within the first few days of SLIT-tablet administration and subsided with continued treatment. Specifically, as presented in , the most commonly observed treatment-related AEs included oral pruritus, throat irritation and mouth edema (reported by 20, 19 and 11% of subjects receiving 12 SQ-HDM, respectively).
Table 2. Treatment-related AEs.
Figure 2. Frequency of treatment-related AEs occurring in ?5% of subjects receiving SQ HDM SLIT-tablet treatment. Combined safety data from trials where the 12 SQ-HDM dose was included, i.e. P003 and MT-06.
During clinical development of the SQ HDM SLIT-tablet, a limited number of treatment-related serious adverse events (SAEs) were observed. A case of severe migraine in a subject receiving 1 SQ-HDM (on day 245 of administration) and a case of severe dizziness in a subject receiving 3 SQ-HDM (on day 3 of administration) were reported during MT-02 and both cases were classified as SAEs because of hospitalization of the involved subjects [Citation47]. During MT-06, idiopathic thrombocytopenic purpura was reported for a subject receiving 6 SQ-HDM and classified as a SAE upon finalization of the trial [Citation60]. In the same trial, a case reported by the investigator as ‘very mild laryngeal edema, no vital risk’ was treated with adrenaline [Citation46,Citation60]. This AE developed upon initial administration of 12 SQ-HDM, performed under medical supervision as per protocol instructions. Upon recovery, the subject continued and completed the trial without any further AEs beyond mild oral pruritus [Citation46,Citation60]. No deaths or cases of anaphylactic shock and no events reported as systemic allergic reactions were reported in any of the trials. No AEs involved local allergic swelling that compromised the airways. This corresponds well with the expected tolerability profile for SLIT products and the observed tolerability profile represents an advantage over the risk of systemic adverse reactions associated with the invasive nature of SCIT.
Collectively, the safety and tolerability profile of the SQ HDM SLIT-tablet supports at-home sublingual administration of doses up to 12 SQ-HDM, provided that initial administration is performed under medical supervision.
Regulatory status
In August 2015, the SQ HDM SLIT-tablet was approved by regulatory authorities in 11 European countries (Austria, Czech Republic, Denmark, Finland, France, Germany, Italy, Norway, Poland, Slovakia and Sweden). Specifically, treatment with the SQ HDM SLIT-tablet is indicated in adult patients (18–65 years) diagnosed by clinical history and a positive test of HDM sensitization (skin prick test and/or specific IgE) with at least one of the following conditions:
Persistent moderate to severe HDM-induced AR despite the use of symptom-relieving medication
HDM-induced allergic asthma not well controlled by inhaled corticosteroids and associated with mild to severe HDM-induced AR and where patients’ asthma status has been carefully evaluated before the initiation of treatment
In Japan, regulatory authorities approved the SQ HDM SLIT-tablet in September 2015 for treatment of HDM-induced AR based on positive results from a large phase III trial conducted in Japan. In the U.S., a large phase III trial investigating the efficacy of SQ HDM SLIT-tablet treatment (12 SQ-HDM vs. placebo) in adults and adolescents with HDM-induced AR (ClinicalTrials.gov identifier NCT01700192) is aimed at providing further documentation to support a similar application for regulatory approval. Thus, large numbers of HDM allergic patients worldwide will get access to treatment with the SQ HDM SLIT-tablet within the near future.
Conclusion
The clinical data reviewed in this Drug Profile demonstrate that treatment with the SQ HDM SLIT-tablet provides significant improvement in HDM-induced AR. SQ HDM SLIT-tablet treatment results in improved disease control and specifically, HDM allergic patients report lower symptom scores, decreased allergy pharmacotherapy use and an improved quality of life. Additionally, the reviewed safety data indicate a favorable safety profile, which constitutes an important advantage over available SCIT treatment options. Doses up to 12 SQ-HDM display a safety and tolerability profile that supports at-home sublingual administration once the first tablet is tolerated (when administered under medical supervision). In conclusion, treatment with 12 SQ-HDM delivers robust efficacy as well as a favorable safety and tolerability profile and importantly, the SQ HDM SLIT-tablet represents a treatment option that meets an unmet medical need of patients with persistent moderate to severe HDM-induced AR not adequately controlled by symptom-relieving allergy pharmacotherapy.
Expert commentary and five-year view
The clinical development program behind the SQ HDM SLIT-tablet represents the largest development program for an HDM AIT product to date and a clear ambition of developing an evidence-based SLIT-tablet with documented clinical benefits for patients suffering from HDM-induced respiratory allergic disease. Specifically, the SQ HDM SLIT-tablet was developed as an improved AIT treatment option, enhancing access to AIT and meeting the unmet medical need of patients with HDM-induced AR experiencing inadequate symptomatic relief with available allergy pharmacotherapy. For HDM-induced AR patients presented with AIT as a complementary treatment option, traditionally, subcutaneous delivery of allergen extracts represented the go-to route of administration. However, because of the practical challenges involved and the risk of systemic adverse reactions associated with the invasive character of SCIT, sublingually administered allergen extracts have been investigated as a more convenient AIT treatment option. In the context of AIT for HDM-induced AR, the data presented in this Drug Profile demonstrate that treatment with the SQ HDM SLIT-tablet delivers robust efficacy and a favorable tolerability profile. As this allows for at-home sublingual administration, the SQ HDM SLIT-tablet represents a highly convenient AIT treatment option for patients, as opposed to the more time-consuming treatment regimen involved for SCIT.
Although head-to-head comparative trials of SCIT versus SLIT in terms of efficacy and safety are scarce, and none have evaluated the SQ HDM SLIT-tablet against SCIT formulations, the two routes of administration modulate similar mechanisms resulting in similar levels of efficacy [Citation61,Citation62]. In terms of safety, all evidence points toward a more favorable safety profile of SLIT products compared with SCIT [Citation63]. Thus, for the SQ HDM SLIT-tablet, the observed robust efficacy and favorable safety profile appear to offer significant clinical benefits over existing SCIT treatment options. As clinical experience with the SQ HDM SLIT-tablet accumulates, new data from real-life studies or further randomized controlled trials are likely to shed light on several as of yet unanswered questions, including the clinical benefit of SQ HDM SLIT-tablet treatment of pediatric populations, the potential for combination SLIT-tablet therapy for polysensitized patients and the long-term efficacy beyond SQ HDM SLIT-tablet treatment cessation.
Information resources
In the context of AR, the Allergic Rhinitis and its Impact on Asthma (ARIA) initiative aims to educate and implement evidence-based management of AR in conjunction with asthma worldwide and the ARIA website (www.whiar.org) represents a relevant resource providing comprehensive reports and guideline documents. Specifically, the 2008 update of the ARIA Report reviews scientific evidence on the definition and classification of AR, risk factors, mechanisms, diagnosis, and treatment [Citation1], and the ARIA 2010 Revision provides additional perspective on the various AR treatment options by answering key clinical questions based on objective analysis according to the WHO GRADE methodology [Citation14].
Current knowledge on the mechanisms of AIT, clinical use of AIT in the management of AR worldwide, and unmet needs and ongoing developments in the AIT field has been reviewed in a recent consensus report [Citation19] as part of the PRACTALL initiative, which brings together the American Academy of Allergy, Asthma & Immunology (AAAAI) and the European Academy of Allergy and Clinical Immunology (EAACI). In addition, the World Allergy Organization (WAO) has published two position papers on SLIT to identify indications, contraindications and practical aspects of the treatment [Citation26,Citation61].
Further information about the SQ HDM SLIT-tablet and more details on the clinical trials reviewed in the present Drug Profile are available in publications on individual trials: MT-02 (EudraCT no. 2006–001795-20) [Citation45], P003 (ClinicalTrials.gov identifier NCT01644617) [Citation44] and MT-06 (EudraCT no. 2011–002277-38) [Citation46].
Key issues
Patients with HDM-induced AR not adequately controlled on symptom-relieving allergy pharmacotherapy present an unmet medical need.
AIT targets the underlying mechanisms in HDM-induced AR by modifying the immunological response to HDM.
SLIT-tablets represent a highly convenient avenue to AIT.
The SQ HDM SLIT-tablet provides robust efficacy in HDM-induced AR, resulting in fewer symptoms, reduced medication use and improved patient quality of life.
The SQ HDM SLIT-tablet has a favorable safety and tolerability profile; transient, mild local allergic reactions represent the most commonly occurring adverse reactions.
Treatment with the SQ HDM SLIT-tablet provides a clinically relevant benefit and meets an unmet medical need of patients with persistent moderate to severe HDM-induced AR not adequately controlled by allergy pharmacotherapy.
Financial and competing interests disclosure
L Klimek has Board membership with MEDA Pharma, Germany, Bencard Allergie, Germany; has performed consultancy for ALK-Abelló, Denmark, Allergopharma, Deutschland, Bionorica, Germany, Boehringer Ingelheim, Germany, GSK, Great Britain, Lofarma, Italy, Novartis, Switzerland, MEDA Pharma, Germany, MSD, USA, Phadia/Thermofisher, Sweden, Optima, Germany; has grants/grants pending from ALK-Abelló, Denmark, Allergopharma, Germany, Artu-Biologicals, Netherlands, Bencard, Great Britain, Bionorica, Germany, Biomay, Austria, Cytos, Switzerland, HAL, Netherlands, Hartington, Spain, GSK, Great Britain, Leti, Spain, Lofarma, Italy, Novartis, Switzerland, Roxall, Germany; received payment for lectures including service on speakers bureaus from ALK-Abelló, Denmark, Allergopharma, Germany, Bionorica, Germany, Boehringer Ingelheim, Germany, GSK, Great Britain, Lofarma, Italy, Novartis, Switzerland, MEDA Pharma, Germany, MSD, USA, Phadia/Thermofisher, Sweden, Optima, Germany; and has received payment for manuscript preparation from MEDA Pharma, Germany, Dr. Pfleger, Germany, Bionorica, Germany. H Mosbech has acted as consultant for ALK-Abelló, Denmark. P Zieglmayer received lecture fees from ALK-Abelló, Denmark, Allergopharma, Germany, Bencard, Germany, Novartis, Austria, Stallergenes, Austria, Thermo Fisher Scientific, received grants from Allergopharma, Allergy Therapeutics, Biomay, Calistoga, GSK, HAL, MSD, Ono, Oxagen, RespiVert, Stallergenes, VentirX, and is a Sigmapharm, Stallergenes advisory board member. D Rehm and B S Stage are employed by ALK, the manufacturer of the SQ HDM SLIT-tablet. P Demoly has acted as a consultant and speaker for Stallergenes, Circassia, ALK, ThermoFisherScientific and Chiesi as well as speaker for Merck, Astra Zeneca, Allergopharma, Allergy Therapeutics and GlaxoSmithKline. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
ALK, the manufacturer of the SQ HDM SLIT-tablet, provided and funded medical writing assistance. The authors would like to acknowledge Ida Mosbech Smith, ALK, for assistance with medical writing, editorial assistance, and submission assistance.
References
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160.
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764.
- Bousquet J, Bullinger M, Fayol C, et al. Assessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 Health Status Questionnaire. J Allergy Clin Immunol. 1994;94:182–188.
- Gandhi VD, Davidson C, Asaduzzaman M, et al. House dust mite interactions with airway epithelium: role in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13:262–270.
- Bousquet PJ, Chinn S, Janson C, et al. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62:301–309.
- Colloff MJ. Dust mites. Dordrecht: Springer; 2009.
- Linneberg A, Henrik Nielsen N, Frolund L, et al. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57:1048–1052.
- Calderon MA, Linneberg A, Kleine-Tebbe J, et al. Respiratory allergy caused by house dust mites: What do we really know?. J Allergy Clin Immunol. 2015;136:38–48.
- Sibbald B, Rink E. Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax. 1991;46:895–901.
- Ciprandi G, Cirillo I, Klersy C, et al. Nasal obstruction is the key symptom in hay fever patients. Otolaryngol Head Neck Surg. 2005;133:429–435.
- Nurmatov U, Van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–165.
- Terreehorst I, Duivenvoorden HJ, Tempels-Pavlica Z, et al. The effect of encasings on quality of life in adult house dust mite allergic patients with rhinitis, asthma and/or atopic dermatitis. Allergy. 2005;60:888–893.
- Custovic A, Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update (in collaboration with GA(2)LEN). Allergy. 2005;60:1112–1115.
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476.
- Canonica GW, Tarantini F, Complati E, et al. Efficacy of desloratadine in the treatment of allergic rhinitis: a meta-analysis of randomized, double-blind, controlled trials. Allergy. 2007 Apr;62(4):359–366.
- Allam JP, Novak N. Immunological mechanisms of sublingual immunotherapy. Curr Opin Allergy Clin Immunol. 2014;14:564–569.
- Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27.
- Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:586–593.
- Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296.
- Pichler CE, Helbling A, Pichler WJ. Three years of specific immunotherapy with house-dust-mite extracts in patients with rhinitis and asthma: significant improvement of allergen-specific parameters and of nonspecific bronchial hyperreactivity. Allergy. 2001;56:301–306.
- Varney VA, Tabbah K, Mavroleon G, et al. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–1082.
- Mungan D, Misirligil Z, Gurbuz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite-sensitive patients with rhinitis and asthma–a placebo controlled study. Ann Allergy Asthma Immunol. 1999;82:485–490.
- Alvarez-Cuesta E, Bousquet J, Canonica GW, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl 82):1–20.
- Scadding G, Durham S. Mechanisms of sublingual immunotherapy. J Asthma. 2009;46:322–334.
- Calderon MA, Casale TB, Togias A, et al. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127:30–38.
- Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization position paper 2009. World Allergy Organ J. 2009;2:233–281.
- Dretzke J, Meadows A, Novielli N, et al. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–1366.
- Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740–752.
- Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. J Pharm Pharmacol. 1998;50:375–382.
- Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm. 2011;61:117–139.
- EMEA. Committee for Medicinal Products for Human Use (CHMP) and Efficacy Working Party (EWP): Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases; EMEA/CHMP/EWP/18504/2006. 2008.
- Bagnasco M, Altrinetti V, Pesce G, et al. Pharmacokinetics of Der p 2 allergen and derived monomeric allergoid in allergic volunteers. Int Arch Allergy Immunol. 2005;138:197–202.
- Bagnasco M, Mariani G, Passalacqua G, et al. Absorption and distribution kinetics of the major Parietaria judaica allergen (Par j 1) administered by noninjectable routes in healthy human beings. J Allergy Clin Immunol. 1997;100:122–129.
- Novak N, Allam JP. Mucosal dendritic cells in allergy and immunotherapy. Allergy. 2011;66(Suppl 95):22–24.
- Novak N, Bieber T, Allam JP. Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy. 2011;66:733–739.
- Gleich GJ, Zimmermann EM, Henderson LL, et al. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–271.
- Bousquet J, Braquemond P, Feinberg J, et al. Specific IgE response before and after rush immunotherapy with a standardized allergen or allergoid in grass pollen allergy. Ann Allergy. 1986;56:456–459.
- van RR, van Leeuwen WA, Dieges PH, et al. Measurement of IgE antibodies against purified grass pollen allergens (Lol p. 1, 2, 3 and 5) during immunotherapy. Clin Exp Allergy. 1997;27:68–74.
- Flicker S, Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int Arch Allergy Immunol. 2003;132:13–24.
- Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–318.
- Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010 Apr;40(4):598–606.
- Shamji MH, Ljorring C, Francis JN, et al. Functional rather than immunoreactive levels of IgG(4) correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226.
- Corzo JL, Carrillo T, Pedemonte C, et al. Tolerability during double-blinded randomised phase I trials with the house dust mite allergy immunotherapy tablet in adults and children. J Investig Allergol Clin Immunol. 2014;24:154–161.
- Nolte H, Maloney J, Nelson HS, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–1501.
- Mosbech H, Canonica GW, Backer V, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015;114:134–140.
- Demoly P, Emminger W, Rehm D, et al. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2015. doi:10.1016/j.jaci.2015.06.036.
- Mosbech H, Deckelmann R, De Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568–575.
- Koinis-Mitchell D, Craig T, Esteban CA, et al. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130:1275–1281.
- Durham SR, Yang WH, Pedersen MR, et al. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–809.
- Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.
- Nelson HS, Nolte H, Creticos P, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80.
- Blaiss M, Maloney J, Nolte H, et al. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71.
- Maloney J, Bernstein DI, Nelson H, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112:146–153.
- Creticos PS, Maloney J, Bernstein DI, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131:1342–1349.
- Nolte H, Hebert J, Berman G, et al. Randomized controlled trial of ragweed allergy immunotherapy tablet efficacy and safety in North American adults. Ann Allergy Asthma Immunol. 2013;110:450–456.
- Nolte H, Amar N, Bernstein DI, et al. Safety and tolerability of a short ragweed sublingual immunotherapy tablet. Ann Allergy Asthma Immunol. 2014;113:93–100.
- Didier A, Worm M, Horak F, et al. Sustained 3-year efficacy of pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol. 2011;128:559–566.
- Bergmann K, Demoly, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mites allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–1614.
- Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergy immunotherapy tablet in adults with allergic asthma - a randomized clinical trial. Manuscript submitted to JAMA 2015.
- Emminger W, Hernandez D, Cardona V, et al. SQ house dust mite SLIT-tablet is well-tolerated in patients with HDM repiratory allergic disease. Manuscript submitted to Allergy 2015.
- Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6.
- Saporta D. Efficacy of sublingual immunotherapy versus subcutaneous injection immunotherapy in allergic patients. J Environ Public Health. 2012;2012:492405.
- Aasbjerg K, Dalhoff KP, Backer V. Adverse events during immunotherapy against grass pollen-induced allergic rhinitis - differences between subcutaneous and sublingual treatment. Basic Clin Pharmacol Toxicol. 2015;117:73–84.
