Abstract
Non-melanoma skin cancer (NMSC) represents the most common cutaneous neoplasms and, in the past decade, nonsurgical treatment modalities have been established as part of the management of NMSC. Recently, novel noninvasive diagnostic modalities have been developed and, of these, reflectance confocal microscopy (RCM) offers imaging of the skin in vivo with cellular resolution. NMSCs, including basal cell carcinoma, actinic keratosis and squamous cell carcinoma, have been evaluated by RCM, and diagnostic criteria were defined. By correlation with routine histology sections and in comparison with normal skin, RCM showed high sensitivity and specificity values. RCM allows the noninvasive evaluation of a variety of skin conditions, including NMSC. RCM may aid in the diagnosis and differential diagnosis of NMSC, as well as in monitoring of treatment response to topical treatment modalities. Therefore, RCM appears to be a promising diagnostic tool with many possible applications in dermatology.
Information from Citation[27].
![Figure 1. Simplified optical principle of reflectance confocal microscopy illustrating the way of the light from the point light source to the skin and onto the detector.Information from Citation[27].](/cms/asset/6476b741-7c78-414d-8d5e-f2c754b81bb9/ierg_a_11208268_f0001_b.jpg)
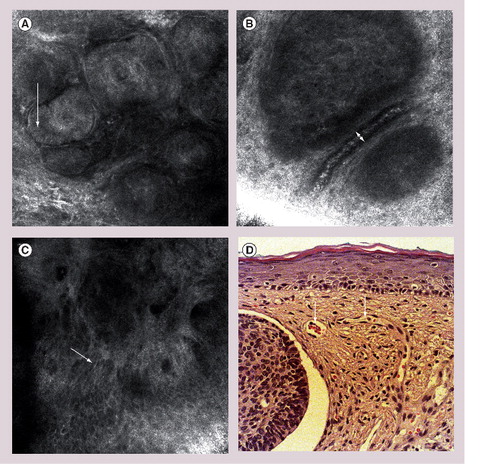
(A) Nests of tumor cells in the dermis, elongated shape of cells and nuclei (arrow), dark spaces in between tumor nodules and surrounding collagen correspond to clefts on histopathology. (B) Large, dilated blood vessels adjacent to dermal tumor nest (arrow) with highly reflective white cells in the center of the vessel correspond to erythrocytes. (C) Polarization of cells (arrow). (D) Representative hematoxylin and eosin histology of basal cell carcinoma with nests of basaloid tumor cells, clefting and blood vessel dilatation (arrow).
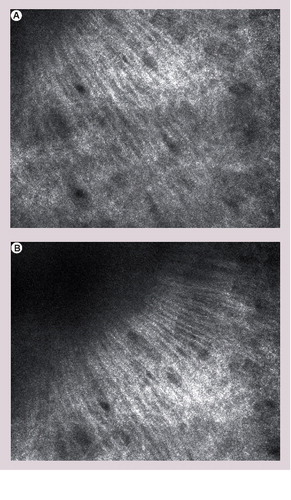
The formation of the collagen along the same axis resembles the arrangement of tumour cells in basal cell carcinoma (streaming).
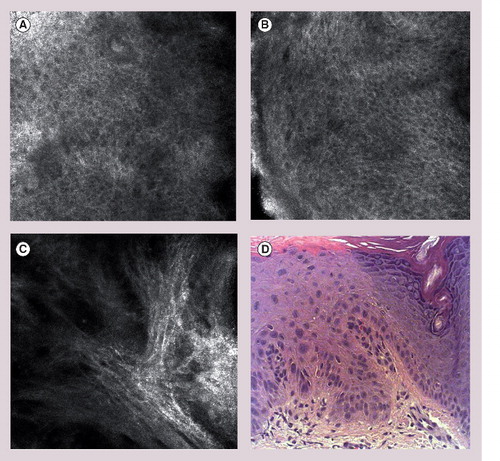
(A & B) Loss of normal epidermal honeycomb pattern, architectural diarray, cellular atypia and pleomorphism. (C) Marked solar elastosis in the upper dermis. (D) Representative hematoxylin and eosin histology of actinic keratoses with proliferation of atypical keratinocytes (‘crowding’).
Medscape: Continuing Medical Education Online
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.com/cme/expertreviews; (4) view/print certificate.
Credit Hours – 0.75
Learning objectives
Upon completion of this activity, participants should be able to:
• Identify the gold standard for the diagnosis of nonmelanoma skin cancer in dermatologic practice
• Identify noninvasive diagnostic techniques for skin cancer
• Describe the prevalence of basal cell carcinoma
• Describe the association between actinic keratosis and squamous cell carcinoma
• List the advantages of reflectance confocal microscopy compared with biopsy
The differential diagnosis of inflammatory and neoplastic skin disorders may often be obtained based on clinical examination, since abnormal skin findings are readily amenable to visual inspection. However, the differential diagnosis may be broad and biopsy with subsequent histology is often performed for confirmation. Therefore, histology remains the current diagnostic ‘gold standard’ in dermatology. Skin cancer biopsies are routinely performed for accurate diagnostic classification and optimized tumor management.
However, skin biopsies may be associated with significant disadvantages as the procedure itself is invasive, thereby leading to pain and scar formation. Histological processing and staining induce irreversible tissue alterations and may ultimately result in artefacts. A histological evaluation per-definition precludes an in vivo examination and does not permit repeated evaluations over time. Following initial diagnosis by biopsy, a second surgical procedure is often required for complete removal of the lesion.
In the past decades, a number of adjunct diagnostic techniques have been introduced in dermatology, including, among others, dermoscopy, high-frequency ultrasound and optical coherence tomography. These modalities allow the noninvasive evaluation of skin with differences in penetration depth and resolution, but do not visualize cellular details. Recently, reflectance confocal microscopy (RCM) has been evaluated for a variety of inflammatory and neoplastic skin conditions. In contrast to other noninvasive imaging techniques, RCM allows the assessment of cellular details and microstructures of the skin with a resolution comparable to routine histology sections. Herein, we present an overview of RCM with special regard to non-melanoma skin cancer (NMSC).
NMSC: recent developments in epidemiology & management
Non-melanoma skin cancer is the most common cancer in humans and the incidence is increasing continuously. The most important risk factor in the pathogenesis of NMSC is chronic ultraviolet radiation. The past decades have shown a significant change of sun-exposure habits in the general population, and the increase of vacational and recreational sun exposure has contributed to the current epidemiologic developments. Furthermore, a higher rate of skin cancer incidence in young patients has been reported, whereby the majority of cases develop multiple tumors.
Histologically, two major forms of NMSC have to be distinguished: basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).
Basal cell carcinoma
This is the most frequent invasive skin cancer in humans and its incidence is rising continuously worldwide. Recent data from Great Britain report an annual incidence of up 153.9 per 100,000 person-years, with BCC being more common in males Citation[1]. The most important risk factor is ultraviolet radiation, and up to 80% of cases develop on the head and neck area. However, BCC may also occur on other locations, such as the trunk and the extremities, and is always limited to areas with a high density of pilosebaceous units. Three major variants of BCC can be distinguished clinically and histologically, namely, nodular BCC, superficial BCC and morpheaform BCC. Nodular BCC clinically presents as skin-colored-to-erythematous papules or nodules with a pearly or waxy appearance that may develop central ulceration. Superficial BCC is characterized by erythematous-to-brownish plaques with slightly elevated nodular border and potential crusting. The clinical diagnosis of sclerodermiform BCC may be difficult as it often resembles scars. For decades, excisional surgery has been the gold standard of treatment of BCC and still remains the treatment of choice for BCC in facial location and nodular-type BCC. However, recently noninvasive treatment modalities have been developed and shown to be effective for BCC. These include topical imiquimod 5% cream and photodynamic therapy Citation[2–4].
Squamous cell carcinoma
Squamous cell carcinoma is defined as malignant neoplasia of epidermal keratinocytes. In contrast to BCC, which usually develops de novo, squamous cell neoplasia represents a continuous process from SCC in situ, namely, actinic keratoses (AKs) to invasive SCC. Histopathologic grading of AKs has been described, referring to the development of SCC from atypical epidermal keratinocytes. Therefore, a reclassification has been proposed that defines AK as SCC in situCitation[5,6]. Recently, the terms early in situ SCC type AK I, II and III have been suggested for improved description of the lesions, in an effort to optimize the therapeutic management of AKs based on their degree of atypia Citation[7].
Clinically, AKs present as erythematous-to-brownish plaques with overlying hyperkeratosis on sun-exposed skin sites. Multiple AKs usually occur on actinically damaged skin, a phenomenon that has been described by the term ‘field cancerization’ Citation[8]. In these areas, multiple AKs of different grades are present and invasive SCC may develop. Following long-standing sun exposure, substantial atypia of keratinocytes may also occur in clinically normal skin, suggesting the presence of subclinical AK. This finding has further been corroborated by the appearance of ‘subclinical lesions’ in areas of treatment with imiquimod cream 5% for AK in serial clinical studies.
Without treatment, AK may progress into invasive SCC in approximately 5–10% of cases Citation[9], with a higher risk of invasive growth in patients on chronic immunosuppression. At the present time, it is not possible to predict which lesion will progress and at which time point this may happen, either by clinical or histological examination. Therefore, treatment of all AKs is recommended Citation[10]. Clinically, SCC generally appears as a hyperkeratotic, infiltrative plaque or nodule that may ulcerate or have a history of frequent irritation or bleeding. Clinical differentiation of SCC from AK may be difficult, and biopsy with histological examination is required when in doubt.
Diagnostic standards for NMSC
Clinical examination
The first step in evaluating a lesion suspicious for NMSC is the clinical examination. However, the diagnostic accuracy is difficult to assess and depends on the educational level and training of the observer. Reported sensitivity of clinical diagnosis varies from 56 to 90% and specificity from 75 to 90% Citation[11].
Dermoscopy
In order to improve diagnostic accuracy, several noninvasive technologies have been introduced and evaluated. Dermoscopy is well established for the diagnosis of pigmented skin lesions and has been shown to improve diagnostic accuracy for malignant melanoma. Dermoscopy allows the noninvasive evaluation of the skin surface with 10–100-times magnification. The use for NMSC has only recently been investigated and mainly focused on BCC with special regard to the differentiation from malignant melanoma. Diagnostic criteria for dermoscopic evaluation of pigmented BCC have been defined by Menzies and include the absence of pigmented network and the presence of one of the following criteria: mapleleaf-like areas, spoke-wheel areas, large blue–gray ovoid nests, large blue–gray globules, arborizing teleangiectasia and ulceration. Using this model, a sensitivity of 93% for BCC and a specificity of 89% has been reported for BCC when differentiating it from malignant melanoma Citation[12]. A subsequent study evaluated the interobserver agreement of these features and confirmed the reproducibility of the method Citation[13].
Whereas the value of dermoscopy for pigmented BCC has been extensively studied, the evaluation of nonpigmented BCC has only been the subject of recent studies. These have mainly focused on the evaluation of vascular patterns and have described arborizing vessels as the most prominent feature of nonpigmented nodular BCC. Argenziano et al. reported arborizing vessels in 82% of BCC with a positive predictive value of 94% Citation[14]. A study evaluating the dermoscopy findings of 24 superficial BCCs revealed that the majority of theses tumors do not show arborizing vessels, but short, fine teleangietasias in 91.7% of cases Citation[15].
Dermoscopic criteria of SCC have been described in preliminary studies, including glomerular vessels and scaly surface. However, future investigations must be performed in order to define the sensitivity and specificity of these findings.
Histological examination
The evaluation of a sample obtained by skin biopsy is considered as the current gold standard in the diagnosis of NMSC. Biopsy may be performed in different techniques, such as punch, shave or excisional biopsy. Local anesthesia is necessary and scar formation results. Side effects include pain, bleeding or wound infection. The ability of correct diagnosis by skin biopsy and histology is dependent on the physician that aquires the biopsy, as well as on the ability of the dermatopathologist. Studies have mainly focused on the evaluation of interobserver differences, and concordance rates of 0.90 intraclass correlation coefficient (ICC) have been reported for the histological diagnosis of AKs and SCC Citation[16].
Novel diagnostic techniques: reflectance confocal microscopy
In the past decade, a number of adjunct diagnostic techniques have been developed and, to a different extent, evaluated for their applicability in NMSC diagnosis. These include, among others, optical coherence tomography, bispectral fluorescence imaging, raman microscopy, fluorescence imaging, multiphoton imaging, fluorescence confocal microscopy and RCM Citation[17–25]. Among them, fluorescence and RCM are the only techniques that allow an evaluation of cellular details at a near histologic resolution Citation[25–27]. Fluorescence-mode confocal microscopy requires the injection of a fluorescent dye prior to examination, thus making the examination minimally invasive with limited examination time owing to depletion of fluorescent dye. The principle of RCM is comparable to ultrasound but, instead of ultrasound waves, the system is based on the optical reflectivity. A point light source is used to illuminate a small spot within the tissue. The light is reflected from the tissue and conducted through a small pinhole onto a detector (the optical principle of RCM is shown in ). For visualization of a larger horizontal plane within the tissue, the point light source is scanned across the tissue under investigation. The resolution of RCM is dependent on the wavelength of the light source and the numerical aperture of the objective lens. The commercially available confocal microscope (Vivascope® 1500, Mavig GmbH Munich Germany; Lucid Inc., Rochester, NY, USA) uses a 830-nm diode laser and a 30× objective lens with a numerical aperture of 0.9. The maximum laser power of the system is 40 mW. With an axial resolution of 3–5 µm and a lateral resolution of approximately 1 µm, RCM images are comparable to routine histology sections. The penetration depth reaches up to 300 µm, which corresponds to the level of the upper reticular dermis. The use of a laser with a longer wavelength would enhance the penetration, but also result in an impaired resolution. Unlike the vertical sections of routine histology, the images obtained by RCM are horizontal (en face). Furthermore, RCM images are grayscale. The contrast provided by RCM is based on the different refractive indices of cutaneous chromophores: melanin, hemoglobin and cellular microstructures provide the contrast that is necessary for the morphologic description of the different skin layers. Normal skin shows characteristic findings on RCM examination with arrangement of keratinocytes in a regular, honeycomb-like architecture being the most striking feature. The cells vary in size from 10 to 30 µm in the stratum corneum, 20 to 25 µm in the stratum granulosum and a smaller cell size of 15–25 µm in the stratum spinosum. Other morphological structures of the skin, such as dermal papillae, adnexal structures (hair follicles, sebaceous glands and eccrine ducts), collagen and blood vessels, may also be visualized by RCM and correlated to routine histology sections Citation[27–29]. Recently, the RCM device has been equipped with a dermoscopic tool, which further enhances the diagnostic property of the device and allows the direct RCM examination of areas suspicious on dermoscopy.
Evaluation with RCM may be performed in vivo & ex vivo
With regard to NMSC ex vivo RCM has mainly been used in the setting of Mohs micrographic surgery. Respective clinical studies have shown the applicability of RCM to detect characteristic features of BCC and SCC in ex vivo sections Citation[30–32]. Limitations included the detection of small tumor islands and the detection of sclerosing and infiltrative BCC Citation[30,33]. A recent publication described the rapid detection of BCC in the setting of Mohs micrographic surgery by using confocal mosaics Citation[33]. Ex vivo RCM may, therefore, be a potential guide for micrographic surgery and may accelerate the surgical procedure by avoiding the need for frozen histology sections.
The in vivo application is of special interest for the diagnosis of skin cancer, since diagnosis may be obtained noninvasively and immediately at the time of the examination without tissue removal. Furthermore, serial evaluations of the same lesion may be performed over a period of time, whereby therapeutic effects may be documented.
As the penetration depth is limited to a maximum of approximately 300 µm, cutaneous disorders that affect the epidermal and the upper dermal compartment are especially amenable for RCM evaluation. Similar to other noninvasive diagnostic techniques, the majority of performed studies have focused on the evaluation of pigmented lesions. However, NMSC has been the subject of recent studies, whereby diagnostic RCM parameters have been established and sensitivity and specificity analyses for both BCC and AK were performed.
Basal cell carcinoma
In a recent analysis, five major criteria for the RCM diagnosis of BCC have been defined, including elongated monomorphic basaloid nuclei, polarization of these nuclei along the same axis, prominent inflammatory infiltrate, increased dermal vasculature with tortuosity of the tumor vessels and pleomorphism of the overlying epidermis with loss of normal honeycomb pattern (Box 1). The presence of polarized nuclei showed the highest sensitivity and specificity of 91.6 and 97%, respectively, with the presence of four or more criteria by RCM the diagnosis of BCC had a sensitivity of 82.9% and a specificity of 95.7% Citation[34]. In this study, minor variations were described regarding the different subtypes of BCC (nodular, superficial and infiltrative BCC) Citation[19]. The variations of RCM features with regard to BCC subtypes are now addressed.
Nodular BCC
Besides the aforementioned criteria, nodular BCC additionally presents nests of aggregated tumor cells in the upper dermis, which are often adjacent to large and dilated blood vessels. By applying the horizontal mapping function on the level of the upper dermis, the revealing mosaics show characteristic morphology with multiple tumor nodules or digitiform structures, which are separated from the dermal collagen by fibrosis. The tumor aggregates themselves are characterized by the formation of crowded cells with variable refractivity. The cells in the periphery of the tumor nodule usually show peripheral palisading, as well as elongated nuclei. Peritumoral clefting can be visualized by confocal microscopy as dark spaces surrounding the tumor nodules, which correspond to those clefts seen on routine histology. Variable inflammation defined by the presence of small, highly refractive cells in the dermis can be seen. The RCM features of nodular BCC are presented in .
Superficial BCC
In superficial BCC, the pathology is concentrated on the lower part of the epidermis and the superficial dermis. Aggregation of polarized cells with elongated nuclei with orientation along the same axis can be visualized in the basal cell layer and the superficial dermis. Other features, including increased dilatation and tortuosity of the vasculature and variable degree of inflammatory cells, may also be seen.
Infiltrative BCC
Imaging of infiltrative BCC by RCM shows aggregation of monomorphic cells with elongated nuclei in the upper dermis, surrounded by a dense and cell-rich stroma. Peripheral palisading is usually absent and the borders between tumor cell aggregates and stroma are poorly defined Citation[30,34]. The diagnosis of infiltrative BCC remains more challenging when compared with nodular and superficial BCC as the features are often less pronounced Citation[30].
Pigmented BCC
Pigmented variants of BCC may be seen in all previously described subtypes. On RCM imaging, dendritic highly refractive cells in the upper dermis can be visualized, corresponding to melanocytes on histopathologic exam. Furthermore, bright oval-to-stellate structures with indistinct borders can be imaged by RCM, correlating to melanophages on routine histology Citation[35,36]. The presence of highly refractive cells may, initially, be misleading by suggesting that the lesion be melanocytic, but the presence of specific BCC features (e.g., monomorphic cells with elongated nuclei or polarization) confirm the diagnosis of BCC.
Residual or recurrent BCC
When using RCM in clinical practice, the investigator may be faced with the question of residual or recurrent BCC after previous excision. To date, no studies have been performed regarding this question. According to our experience, it might be very challenging to detect residual or recurrent BCC in surrounding scar tissue. The formation of the collagen bundles within the scar may resemble polarization along the same axis or peritumoral fibrosis .
Actinic keratosis
Actinic keratosis histologically represents a SCC in situ and shows similar cellular and morphological changes. Invasive SCC may only be distinguished from AK by infiltration through the basement membrane into the dermis. Histological criteria of AK include parakeratosis, keratinocyte atypia, loss of normal maturation and architecture of the epidermis and pleomorphism of cells and nuclei. A preliminary study by Aghassi et al. analyzed and described RCM criteria of AK, which corresponded well to histological findings Citation[37]. Recent studies have evaluated the sensitivity and specificity, and values greater than 80% were reported in two independent studies with the highest rates for epidermal pleomorphism, architectural disruption/loss of normal honeycomb pattern and cellular atypia. On RCM examination, disruption of the stratum corneum is visualized by detached highly refractive cells with polygonal shape. The presence of dark round areas in the center of the corneocytes corresponds to parakeratosis. On the level of the stratum granulosum and spinosum atypia, pleomorphism of the cells can be detected by variation of shape, size and arrangement of the keratinocytes Citation[38–40]. Depending on the grade of dysplasia (AK grade I, II or III), the atypia involves the basal cell layer and stratum spinosum in mild-to-moderate (AK grade I and II) or all three layers, including the stratum granulosum (AK III). In the dermis, prominent bundles of collagen and lace-like amorphous material corresponding to solar elastosis can be visualized besides dilated blood vessels and variable inflammatory infiltrate. Representative RCM images of AK and corresponding hematoxylin and eosin histology are shown in & Box 1.
Differences have been observed regarding the reliability of correct diagnosis depending on the level of RCM training; high concordance rates were achieved in experienced RCM observers Citation[38–40].
Invasive SCC
The difficulty of distinguishing early invasive SCC from AK has already been the subject of the preliminary study published by Aghassi et al.Citation[37]. Different factors contribute to the failure of detecting early squamous cell invasion: impaired resolution in the upper dermis, hyperkeratosis (which additionally reduces resolution) and the fact that the images obtained by RCM are horizontal. However, there are some features indicating invasion. Any lesion with full-thickness atypia of the whole epidermis with parakeratosis present in the area of the acrosyringia (hair follicles) should be paid special attention and requires biopsy or excision. Future studies must prove if these features might be sensitive and specific in the differentiation of AK and early invasive SCC.
In cases of fully developed SCC, the diagnosis is not as challenging. Besides epidermal changes, atypical aggregates of keratinocytes may be visualized in the dermis Citation[41].
Discussion
Novel diagnostic imaging tools have been introduced and evaluated for a variety of cutaneous diseases, including NMSC. RCM offers the evaluation of microstructures of the skin in cellular resolution and the images obtained are comparable to routine histology sections. RCM offers several advantages for the dermatologist as the imaging is noninvasive, painless, in vivo and allows for the evaluation of the skin without processing artefacts. Therefore, monitoring of the same lesion over a period of time can be performed and changes can be visualized. In this regard, treatment effects become visible and morphological evaluation of a therapeutic healing process may be seen. Treatment of imiquimod 5% for BCC has been evaluated successfully and clearance has been confirmed by RCM Citation[42,43]. Recently, studies have described the features of trichoepithelioma as a differential of BCC and disseminated superficial actinic porokeratosis as a differential of AK Citation[44,45].
However, there are a number of technical and optical limitations that remain to be addressed. Among them is the restricted penetration depth of approximately 300 µm, which does not allow the visualization of cutaneous structures or pathologic changes of deeper parts of the dermis. Furthermore, the horizontal images impede the evaluation of vertical invasion and tumor depth. In hyperkeratotic lesions, such as AK, invasive SCC or keratoacanthoma, the penetration is limited owing to absorption and scattering of light. In these cases, careful curettage of the hyperkeratotic scale may clearly improve visualization of the lesion. The current contact device of the commercially available system also has some limitations. The diameter of 3 cm does not allow for the examination of nonflat surfaces, such as the lateral parts of the nose or parts of the ear. Smaller skin contact ring devices are currently being tested for clinical applicability. In addition, a new handheld device has recently been introduced, which may help to overcome these problems in the future.
Reflectance confocal microscopy is an emerging technique and, although specific features of AK, BCC, melanoma and many other cutaneous diseases have been described, image interpretation may sometimes be difficult. For example, cells of dendritic appearance may either represent melanocytes, melanophages or Langerhans’ cells. Similar to the majority of novel techniques, training is needed for the performance of the procedure and interpretation of the images.
So far, RCM has been performed mainly in academic medical centers and research settings. However, the option of noninvasive and in vivo evaluation of the skin may represent a powerful adjunct diagnostic tool for use in everyday clinical practice. Clinical applications include a variety of situations. The features of many inflammatory and neoplastic skin conditions have already been described and may be differentiated by RCM. Therapeutic effects may be identified and monitored, and efficacy can be assessed by RCM imaging. Furthermore, RCM may aid in identifying the most representative site for biopsy within a selected lesion, thereby avoiding sampling errors or false-negative test results. In this regard, the differential diagnosis of melanoma from benign nevi currently represents the field of greatest interest. With regard to NMSC, further applications are possible and reasonable. The differentiation of BCC subtypes may be performed and treatment decisions may be influenced by RCM to either surgical (nodular and sclerosing type) or topical treatment (superficial type). Pigmented BCC may be differentiated from malignant melanoma or other pigmented lesions and ease treatment planning. Diagnostic biopsy may be avoided in selected patients, allowing the adequate surgical procedure to be performed without further delay. In AKs, the extent of actinic damage may be evaluated beyond clinically visible AKs and the identification of subclinical lesions may determine the treatment area. By monitoring treatment response, RCM may help to adjust treatment frequency and duration, thereby maximizing therapeutic outcome and potentially reducing treatment-related expenses.
In summary, RCM represents a promising optical technique in clinical and investigational dermatology and may have a significant impact on the diagnosis and management of melanoma and NMSC in the future. However, further investigations are needed to better assess the values of the previous findings.
Expert commentary
The current diagnostic gold standard in dermatology is a skin biopsy and subsequent routine histopathologic evaluation. An increasing interest in noninvasive diagnostic technologies has been observed in recent years. The ability of imaging the skin without any invasive procedure opens up new perspectives for both physicians and patients. With RCM, cellular details of the skin can be visualized and analyzed. RCM enables the visualization of pathologic changes in the epidermal and upper dermal compartment, both in vivo and noninvasively. RCM may be used for the differential diagnosis of inflammatory and neoplastic skin conditions, including NMSC. The potential to guide biopsy procedures, monitor therapy and detect subclinical lesions illustrate the wide range of applications in clinical and investigational dermatology. Thus, RCM is a promising technique that may, ultimately, impact the diagnostic and therapeutic management of skin disease. However, although the technique is easily learned, standardized training with regard to image interpretation is important and should be performed systematically in practical training courses. Owing to present technical limitations, RCM will not replace routine histopathology, but rather represents an adjunct diagnostic tool. In that regard, RCM may help to reduce the number of diagnostic biopsies, increase diagnostic accuracy, evaluate the lateral tumor margins and assess therapeutic efficacy.
Five-year view
To date, RCM is used mainly in university and research settings. However, owing to the immense potential of the technique, it will most likely find its way into daily clinical practice of hospital and office-based dermatologists. RCM may be used for the differential of NMSC (e.g., BCC and AK) and pigmented lesions (e.g., nevi, melanoma and pigmented BCC), yet the technique has a tremendous potential for the evaluation of other inflammatory and proliferative skin conditions. The further development of the technique will include 3D images of the skin, which may improve diagnosis and offers new insight into cutaneous pathology. RCM may also be applied in a teledermatology setting, whereby images may be evaluated by experts in the field, which may be of great benefit in difficult or rare cases. Future developments may include the development of skin-directed dyes or antibodies, thereby enhancing RCM imaging and diagnostic accuracy.
Box 1. Reflectance confocal microscopy features of actinic keratosis and basal cell carcinoma.
Common reflectance confocal microscopy features of basal cell carcinoma
Stratum corneum
• Parakeratosis may be present
• Superficial disruption in ulcerated basal cell carcinoma
Stratum granulosum and spinosum
• Slight atypia with disruption of honeycomb pattern and pleomorphism may be present
Stratum basal/dermo–epidermal junction
• Monomorphic cells with elongated nuclei
• Polarization of these elongated nuclei along the same axis
• Streaming: all tumor cells are oriented along the same axis
• Palisading: peripheral cells in the tumor nodule are oriented in a parallel manner forming an outer line perpendicular to the stroma
Dermis
• Increased vascularity
• Vessels with large caliber and high blood flow
• Increased tortuosity
• Inflammatory infiltrate composed of small highly refractile around cells
• Monomorphic polarized cells and/or formation of tumor nodules in nodular basal cell carcinoma
Common reflectance confocal microscopy features of actinic keratosis
Stratum corneum
• Parakeratosis
• Superficial disruption with single detached polygonal cells
Stratum granulosum and spinosum
• Keratinocyte atypia with disruption of honeycomb pattern
• Pleomorphism of keratinocytes with variation in size and shape of cells and nuclei
Stratum basal/dermo–epidermal junction
• Atypia/pleomorphism
Dermis
• Increased vascularity
• Mild-to-moderate dilatation of vessel diameter
• Increased tortuosity
• Solar elastosis with amorphous moderately refractile lacy material adjacent to abnormal fibrotic bundles
Key issues
• Non-melanoma skin cancer (NMSC) represents the most common cancer in humans.
• Reflectance confocal microscopy (RCM) is a noninvasive diagnostic technique that offers imaging of the skin with cellular resolution.
• Diagnosis and differential of pathologic skin changes can be obtained by RCM.
• High sensitivity and specificity rates have been reported for the diagnosis of NMSC.
• RCM allows for the evaluation of skin lesions over time (monitoring of treatment response and efficacy).
• In the future, RCM may help to reduce the number of diagnostic biopsies.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Financial & competing interests disclosure
CME AUTHOR
Désirée Lie, MD, MSEd, Clinical Professor, Family Medicine, University of California, Orange; Director, Division of Faculty Development, UCI Medical Center, Orange, California, USA. Disclosure: Désirée Lie, MD, MSEd, has disclosed no relevant financial relationships.
EDITOR: Elisa Manzotti, Editorial Director, Future Science Group. Disclosure: Elisa Manzotti has disclosed no relevant financial relationships
AUTHORS
Martina Ulrich, MD, Department of Dermatology, Venerology, and Allergy, Skin Cancer Center Charité, Charité-Universitätsmedizin, Berlin, Germany. Disclosure: Martina Ulrich, MD, has disclosed no relevant financial relationships.
Susanne Astner, MD, Department of Dermatology, Venerology, and Allergy, Skin Cancer Center Charité, Charité-Universitätsmedizin, Berlin, Germany. Disclosure: Susanne Astner, MD, has disclosed no relevant financial relationships.
Eggert Stockfleth, PhD, Department of Dermatology, Venerology, and Allergy, Skin Cancer Center Charité, Charité-Universitätsmedizin, Berlin, Germany. Disclosure: Eggert Stockfleth, PhD, has disclosed no relevant financial relationships.
Joachim Röwert-Huber, MD, Department of Dermatology, Venerology, and Allergy, Skin Cancer Center Charité, Charité-Universitätsmedizin, Berlin, Germany. Disclosure: Joachim Röwert-Huber, MD, has disclosed no relevant financial relationships.
References
- Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int. J. Cancer121(9), 2105–2108 (2007).
- Marks R, Gebauer K, Shumack S et al. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results for a mulitcenter 6-week dose-response trial. J. Am. Acad. Dermatol.44, 807–813 (2001).
- Geisse JK, Rich P, Pandya A et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: a double-blind, randomized, vehicle-controlled study. J. Am. Acad. Dermatol.47, 390–398 (2002).
- Morton CA, MacKie RM, Whitehurst C, Moore JV, McColl JH. Photodynamic therapy for basal cell carcinoma: effect of tumor thickness and duration of photosensitizer application on response. Arch. Dermatol.134, 248–249 (1998).
- Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J. Am. Acad. Dermatol.42(1 Pt 2), 11–17 (2000).
- Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st-century perspective. Arch. Dermatol.139, 66–70 (2003).
- Röwert-Huber J, Patel MJ, Forschner T et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br. J. Dermatol.156(Suppl. 3), 8–12 (2007).
- Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res.63 (8), 1727–1730 (2003).
- Green A, Battistutta D. Incidence and determinants of skin cancer in a high-risk Australian population. Int. J. Cancer46, 356– 361 (1990).
- Stockfleth E, Kerl H. Guideline Subcommittee of the European Dermatology Forum. Guidelines for the management of actinic keratoses. Eur. J. Dermatol.16(6), 599–606 (2006).
- Mogensen M, Jemec GB. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: a review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol. Surg.33(10), 1158–1174 (2007).
- Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch. Dermatol.136(8), 1012–1016 (2000).
- Peris K, Altobelli E, Ferrari A et al. Interobserver agreement on dermoscopic features of pigmented basal cell carcinoma. Dermatol. Surg.28(7), 643–645 (2002).
- Argenziano G, Zalaudek I, Corona R et al. Vascular structures in skin tumors: a dermoscopy study. Arch. Dermatol.140(12), 1485–1489 (2004).
- Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol. Surg.31(12), 1710–1713 (2005).
- Davis DA, Donahue JP, Bost JE et al. The diagnostic concordance of actinic keratosis and squamous cell carcinoma. J. Cutan. Pathol.32 (8), 546–551 (2005).
- Ericson MB, Uhre J, Strandeberg C et al. Bispectral fluorescence imaging combined with texture analysis and linear discrimination for correlation with histopathologic extent of basal cell carcinoma. J. Biomed. Opt.10(3), 034009 (2005).
- Stenquist B, Ericson MB, Strandeberg C. Bispectral fluorescence imaging of aggressive basal cell carcinoma combined with histopathological mapping: a preliminary study indicating a possible adjunct to Mohs micrographic surgery. Br. J. Dermatol.154(2), 305–309 (2006).
- Lieber CA, Kanter EM, Mahadevan-Jansen A. Comparison of Raman spectrograph throughput using two commercial systems: transmissive versus reflective. Appl. Spectrosc.62(5), 575–582 (2008).
- Olmedo JM, Warschaw KE, Schmitt JM, Swanson DL. Optical coherence tomography for the characterization of basal cell carcinoma in vivo: a pilot study. J. Am. Acad. Dermatol. (3), 408–412 (2006).
- Galletly NP, McGinty J, Dunsby C et al. Fluorescence lifetime imaging distinguishes basal cell carcinoma from surrounding uninvolved skin. Br. J. Dermatol.159(1), 152–161 (2008).
- Lin SJ, Jee SH, Kuo CJ et al. Discrimination of basal cell carcinoma from normal dermal stroma by quantitative multiphoton imaging. Opt. Lett.15, 31(18), 2756–2758 (2006).
- Nijssen A, Maquelin K, Santos LF et al. Discriminating basal cell carcinoma from perilesional skin using high wave-number Raman spectroscopy. J. Biomed. Opt.12(3), 034004 (2007).
- Yaroslavsky AN, Salomatina EV, Neel V, Anderson R, Flotte T. Fluorescence polarization of tetracycline derivatives as a technique for mapping nonmelanoma skin cancers. J. Biomed. Opt.12(1), 014005 (2007).
- Astner S, Dietterle S, Otberg N, Röwert-Huber HJ, Stockfleth E, Lademann J. Clinical applicability of in vivo fluorescence confocal microscopy for noninvasive diagnosis and therapeutic monitoring of nonmelanoma skin cancer. J. Biomed. Opt.13(1), 014003 (2008).
- Rajadhyaksha M, Anderson RR, Webb RH. Video-rate confocal scanning laser microscope for imaging human tissues in vivo. Appl. Opt.38, 1–12 (1999).
- Rajadhyaksha M, González S, Zavislan JM, et al.In vivo confocal scanning laser microscopy of human skin II, advances in instrumentation and comparison with histology. J. Invest. Dermatol.13, 293–303 (1999).
- Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J. Invest. Dermatol.104(6), 946–952 (1995).
- Huzaira M, Rius F, Rajadhyaksha M, Anderson RR, González S. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J. Invest. Dermatol.116(6), 846–852 (2001).
- Chung VQ, Dwyer PJ, Nehal KS et al. Use of ex vivo confocal scanning laser microscopy during Mohs surgery for nonmelanoma skin cancers. Dermatol. Surg.30(12 Pt 1), 1470–1478 (2004).
- Horn M, Gerger A, Koller S et al. The use of confocal laser-scanning microscopy in microsurgery for invasive squamous cell carcinoma. Br. J. Dermatol.156(1), 81–84 (2007).
- Gerger A, Horn M, Koller S et al. Confocal examination of untreated fresh specimens from basal cell carcinoma: implications for microscopically guided surgery. Arch. Dermatol.141(10), 1269–1274 (2005).
- Patel YG, Nehal KS, Aranda I, Li Y, Halpern AC, Rajadhyaksha M. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J. Biomed. Opt.12(3), 034027 (2007).
- Nori S, Rius-Díaz F, Cuevas J et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J. Am. Acad. Dermatol.51(6), 923–930 (2004).
- Agero AL, Busam KJ, Benvenuto-Andrade C et al. Reflectance confocal microscopy of pigmented basal cell carcinoma. J. Am. Acad. Dermatol.54(4), 638–643 (2006).
- Segura S, Puig S, Carrera C, Palou J, Malvehy J. Dendritic cells in pigmented basal cell carcinoma: a relevant finding by reflectance-mode confocal microscopy. Arch. Dermatol.143(7), 883–886 (2007).
- Aghassi D, Anderson RR, Gonzalez S. Confocal laser microscopic imaging of actinic keratoses in vivo: a preliminary report. J. Am. Acad.Dermatol.43, 42–48 (2000).
- Ulrich M, Maltusch A, Röwert-Huber J et al. Actinic keratoses: non-invasive diagnosis for field cancerisation. Br. J. Dermatol.156(Suppl. 3), 13–17 (2007).
- Ulrich M, Maltusch A, Rius-Diaz F et al. Clinical applicability of in vivo reflectance confocal microscopy for the diagnosis of actinic keratoses. Dermatol. Surg.34(5), 610–619 (2008).
- Horn M, Gerger A, Ahlgrimm-Siess V et al. Discrimination of actinic keratoses from normal skin with reflectance mode confocal microscopy. Dermatol. Surg.34(5), 620–625 (2008).
- Astner S, Ulrich M, Cuevas J, González S. Actinic Keratosis. Reflectance Confocal of Cutaneous Tumors. González S, Halpern AC, Gill M (Eds). Informa Healthcare, London, UK (2007).
- Goldgeier M, Fox CA, Zavislan JM, Harris D, Gonzalez S. Noninvasive imaging, treatment, and microscopic confirmation of clearance of basal cell carcinoma. Dermatol. Surg.29(3), 205–210 (2003).
- Torres A, Niemeyer A, Berkes B et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol. Surg.30(12 Pt 1), 1462–1469 (2004).
- Ardigo M, Zieff J, Scope A et al. Dermoscopic and reflectance confocal microscopy findings of trichoepithelioma. Dermatology215, 354–358 (2007).
- Ulrich M, Forschner T, Röwert-Huber J et al. Differentiation between actinic keratoses and disseminated superficial actinic porokeratoses with reflectance confocal microscopy. Br. J. Dermatol.156(Suppl. 3), 47–52 (2007).
Noninvasive diagnosis of non-melanoma skin cancer: focus on reflectance confocal microscopy
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.com/cme/expertreviews. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Table 1. Activity Evaluation
1. Which of the following is considered the gold standard for the diagnosis of skin cancer?
□ A Physical examination
□ B Dermoscopy
□ C Histology
□ D None of the above
2. Which of the following is not a noninvasive diagnostic method for detecting skin cancer?
□ A Skin biopsy
□ B Optical coherence tomography
□ C Dermoscopy
□ D High-frequency ultrasound
3. Which of the following does not describe basal cell carcinoma (BCC)?
□ A Prevalence estimated at 150 per 100,000 person-years
□ B More common in females
□ C Majority develop in head and neck area
□ D There are 3 variants
4. What percentage of actinic keratosis lesions is estimated to progress to squamous cell carcinoma if left untreated?
□ A 1% to 2%
□ B 5% to 10%
□ C 11% to 15%
□ D Over 20%
5. Which of the following is not considered an advantage of reflectance confocal microscopy for the diagnosis of nonmelanoma skin cancer?
□ A Noninvasive
□ B Painless
□ C High penetration depth
□ D Can monitor lesion over time