Response to: Hussein MRA. Atypical lymphoid proliferations: the pathologist’s viewpoint. Expert Rev Hematol 2013;6(2):139-53.
Dear Editor,
As Dr. Hussein had argued in his paper, sometimes lymphoid proliferation cannot be easily attributed to either a benign or a malignant class of conditions Citation[1]. In his opinion, a descriptive term like ‘atypical lymphoid proliferation’ can be introduced in the routine practice to report this kind of findings, but only after a complete histopathological assessment, including immunohistochemistry (IHC) and molecular testing, with a comprehensive integration of morphological data with clinical information. We would like to report our experience in this context, describing a single case of Toxoplasma infection. An immunocompetent, 55-year-old, Caucasian man referred to our hospital for axillary and latero-cervical lymphadenopathies. He was otherwise asymptomatic and, careful clinical assessment gave no useful clue for the identification of possible causes of his node enlargement. After ultrasound, Computed Tomography and Computed Tomography-Positron Emission Tomography examinations, an excision (3 cm diameter) of the most significant axillary lymph node was performed. Fresh tissue was sent for pathological examination; a liquid preparation for flow cytometry analysis was made by gently disaggregating the biopsy with the help of tweezers Citation[2]. Cells were analyzed for the following antigens: CD19, CD20, CD45, CD3, CD4, CD5, CD8, CD10, k and λ immunoglobulin light chains Citation[3]. This test revealed the presence of a dim CD20/CD19/CD5 positive, κ light-chain restricted B cells from the suspension, suggesting a clonal process.
Histological examination showed that the architecture of the lymph node was preserved with hyperplastic cortical follicles with germinal centers showing ragged, ‘moth-eaten’ margins and tingible body macrophages (Hematoxylin and Eosin stain, ). On IHC, a consistent number of small T-lymphocytes (CD3 positive, polyclonal; Dako, Glostrup, Denmark) were present in the paracortical zone, accompanied by a parasinusoidal component of B-lymphocytes (CD20 positive, L26; Dako) with morphological features compatible with monocytoid B cells . An additional staining for CD5 antigen (4C7; Dako) demonstrated intense positivity in the entire paracortical zone, targeting both T and B (CD23 negative, DAK; Dako) small lymphocytes . Moreover, clusters of epithelioid histiocytes (CD68 positive, PG-M1; Dako) were present, completing the histological triad of Piringer-Kuchinka lymphadenitis Citation[4]. At high magnification, Hematoxylin–Eosin staining revealed parasitic cysts suggestive of Toxoplasma amastigotes .
Figure 1. Piringer-Kuchinka lymphadenitis features. (A) Cortical follicles with zonal hyperplastic features and germinal centers showing ragged, ‘moth-eaten’ margins with tingible body macrophages, HE, 4×. (B) Clusters of epithelioid hystiocytes forming granulomatous aggregates, Giemsa, 4×. (C) Parasinusoidal monocytoid B-lymphocytes, characteristic hallmark of toxoplasma lymphadenitis, HE, 20×. (D) Parasitic cyst with multiple amastigotes, confirming the infectious etiology, HE, 100×. (E, F) Double positivity to CD20/CD5 markers in paracortical B-cells is an unusual finding in reactive condition, and may lead to a more careful investigation of the process, IHC, 4×. (G) Clonality assay: PCR amplification fragments indicative of patient-specific clonal IGH FR1, IGH FR2, IGH FR3 and IGK VK gene rearrangements which are indicated with an asterisk.
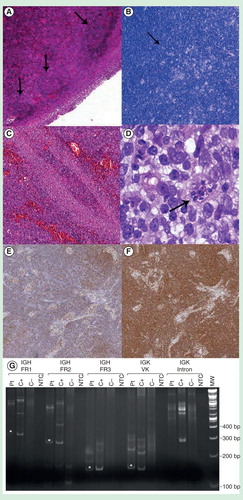
The diagnosis was finally sustained by the positivity of patient’s serum for Toxoplasma IgM antibodies. Moreover, the IHC did not reveal light chain restriction, previously determined by flow cytometry, showing mature polytypic plasma cells in the sinuses. Multiple clonal immunoglobulin heavy (IGH) chain and kappa light (IGK) chain gene rearrangements (IGH FR1, IGH FR2, IGH FR3 and IGK VK) were identified by the ‘IGH + IGK B-Cell Clonality Assay’ (Invivoscribe, San Diego, CA, USA) . This result is indicative of a clonal cell population, although it is not possible to discriminate between a single clone with multiple rearrangements versus multiple clones. Clinical follow-up revealed no peripheral lymphocytosis (2770/μl); moreover, a bone marrow biopsy confirmed absence of any lymphoproliferative process.
Toxoplasmosis is an infectious disease caused due to Toxoplasma gondii and rarely affects immunocompetent people. In immunocompromised hosts (typically those with HIV infection), the clinical manifestation ranges from an isolated lymphadenopathy (mononucleosis-like) to a more aggressive condition characterized by meningoencephalitis, pneumonitis, myocarditis, hepatitis and retinochoroiditis. Although it seems to be a mere reactive condition, our case was peculiar and could be probably classified as atypical lymphoid proliferation, as suggested by Dr. Hussein. Indeed, the classical morphologic appearance of toxoplasmic lymphadenitis was unusually accompanied by an oligoclonal proliferation of CD5-positive/CD23-negative B-lymphocytes, representing a finding of uncertain interpretation. The most straightforward explanation is that of an opportunistic infection superimposed on a ‘smoldering’ chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). However, many laboratory and clinical findings did not support a truly neoplastic nature of that process, particularly due to the absence of peripheral lymphocytosis and bone marrow involvement. Contini et al. demonstrated a monotypic expression of kappa serum free light chains in immunocompromised HIV patients with toxoplasma encephalitis, suggesting a pathogenetic role related to a possible reactivation of T. gondii Citation[5]. Our case was negative for HIV infection, thus not revealing an apparent cause of immunodeficiency. Chen et al. studied the antigen mimicry phenomenon in mice infected with the protozoan, which showed the presence of anti-murine HSP70 antibodies produced by host’s lymphocytes Citation[6]. They also discovered that a particular strain of B-cells, known as B1 cell line, with characteristic CD20/CD5-positive, CD23-negative immunoprofile, was responsible for the synthesis of these oligoclonal immunoglobulins. Following the hypothesis of a smoldering CLL, toxoplasma lymphadenitis in our case could be the epiphenomenon of an early CLL-related immunosuppression. In the last few years, isolated monoclonal B-cell lymphocytosis (MBL) showed an increasing incidence, probably due to our improved diagnostic skills. MBL, as a kind of pre-CLL ‘status’, has been differently interpreted Citation[7,8]. Gibson reported good prognosis of 36 patients with an extramedullary CLL-like lymphocytosis, suggesting the indolent behavior of these ‘neoplastic-like’ cells and coining the appropriate term ‘CLL of uncertain significance’ Citation[7]. We also added our contribution to the study of these forms describing an incidental osseous MBL, which differed from those reported in the literature in the absence of CD5, probably representing a further manifestation of these particular conditions Citation[9]. Finally, Shanafelt et al. tried to define the biological and clinical features of MBL and they found a low rate of transformation to hematological malignancies Citation[8]. All these recent discoveries demonstrate the possibility that our patient had an incipient neoplastic clone, still of subclinical degree, responsible for the superinfection by Toxoplasma and incidentally found during the diagnostic process. In theory, a close follow-up and a prognostic evaluation would be very relevant to confirm that hypothesis Citation[8]. Alternatively, our case may be interpreted as a reactive toxoplasma lymphadenitis in an immunocompetent person, evoking an exaggerated B1-cell activation with over-production of k chain-restricted immunoglobulin. A well-known paradigm in CLL assumes that B-cell receptor is implicated in the pathogenesis of the disease Citation[10,11]. The induction of lymphoma/leukemia by hyperstimulation of these receptors represents a natural consequence and a suggestive hypothesis, already proposed by researchers Citation[12]. Based on these findings, the B-cell clone could be selected through immune system stimulation by an exogenous agent. Understanding these events is crucial to elucidate the pathogenetic mechanism of this neoplasm and to improve the outcome of patients by a prompt therapeutic approach, allowing an early suppression of the neoplastic clone and, at the same time, improving the course of the inducing/opportunistic infection. In this sense, patients may benefit from new target therapies developed in the last few years, directed against B-cell receptor and other B-cell antigens Citation[13,14], obviously associated with appropriate therapeutic approaches for microbial agents. In conclusion, we agree with Hussein about the need of a descriptive term to define such a condition, essentially due to our current poor diagnostic accuracy. However, when possible, every effort should be made by the pathologist to provide useful information about the nature and the biological behavior of certain lesions, optimizing our investigation equipment. Indeed, this case confirmed how only the complete histopathological analysis (including flow cytometry, morphology, IHC and molecular biology) allows characterization of these ambiguous entities.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
References
- Hussein MRA. Atypical lymphoid proliferations: the pathologist’s viewpoint. Expert Rev Hematol 2013;6(2):139-53
- Chang Q, Hedley D. Emerging applications of flow cytometry in solid tumor biology. Methods San Diego Calif. luglio 2012;57(3):359-67
- Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood 2008;111(8):3941-67
- Stansfeld AG. The histological diagnosis of toxoplasmic lymphadenitis. J Clin Pathol 1961;14(6):565-73
- Contini C, Fainardi E, Cultrera R, et al. Evidence of cerebrospinal fluid free kappa light chains in AIDS patients with Toxoplasma gondii encephalitis. J Neuroimmunol 2000;108(1-2):221-6
- Chen M, Aosai F, Mun HS, et al. Anti-HSP70 autoantibody formation by B-1 cells in Toxoplasma gondii-infected mice. Infect Immun 2000;68(9):4893-9
- Gibson SE, Swerdlow SH, Ferry JA, et al. Reassessment of small lymphocytic lymphoma in the era of monoclonal B-cell lymphocytosis. Haematologica 2011;96(8):1144-52
- Shanafelt TD, Ghia P, Lanasa MC, et al. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia 2010;24(3):512-20
- Pagni F, Zannella S, Valenzise V, et al. Occult monoclonal B-cell disorder of hyoid bone. Hematol Oncol 2014;32(2):107-9
- Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol 2003;21:841-94
- Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood 2004;103(12):4389-95
- Kil LP, Yuvaraj S, Langerak AW, Hendriks RW. The role of B cell receptor stimulation in CLL pathogenesis. Curr Pharm Des 2012;18(23):3335-55
- Badar T, Burger JA, Wierda WG, O’Brien S. Ibrutinib: a paradigm shift in management of CLL. Expert Rev Hematol 2014;7(6):705-17
- Awan FT, Byrd JC. New strategies in chronic lymphocytic leukemia: shifting treatment paradigms. Clin Cancer Res Off J Am Assoc Cancer Res 2014;20(23):5869-74
