BGA: Brain–gut axis; IBS: Irritable bowel syndrome; MC: Mast cell.
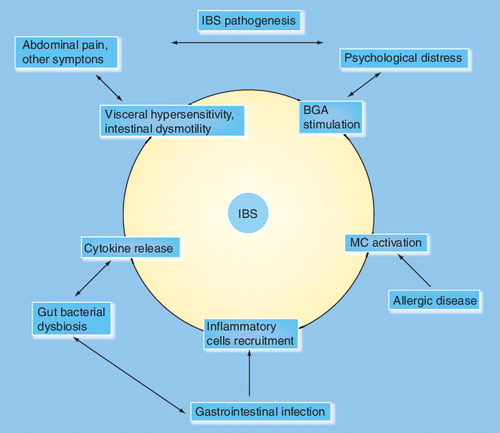
Irritable bowel syndrome (IBS) remains the most common cause of gastrointestinal (GI) complaints and the most frequent reason for consultation in the gastroenterology clinic. IBS is perceived as a benign, nonprogressive condition without significant tissue injury but still causing major morbidity, and is associated with considerable personal and societal burden Citation[1]. In fact, IBS is associated with a significant decrease in quality of life, work productivity and increased utilization of more healthcare resources than those with other organic GI diseases Citation[2]. The lack of successful therapeutic options for IBS patients is the main cause of high morbidity, poor quality of life and loss of productivity. Therefore, a better understanding of the underlying mechanism of this disorder would facilitate more effective therapy and have a major positive impact on IBS patients, their families and employers.
Initial attempts to understand the causes of IBS were hampered by the lack of standardized terminology. The Manning criteria addressed this deficiency by developing a symptom-based classification that has been refined by the Rome I, II and, most recently by the Rome III categorization Citation[3–5]. With a standardized classification system to work with, researchers have now been able to find evidence that strongly suggests that IBS is not one disorder but a diverse group of disorders with different precipitating factors, such as bacterial overgrowth, postinfectious mucosal inflammation, food intolerance/hypersensitivity, stress and changes in diet. For example, acute intestinal infection is an initial trigger for at least a third of IBS patients and is called ‘postinfectious IBS’ Citation[6]. Studies demonstrate the importance of environmental triggers in IBS. Although the concept that environmental factors contribute to or initiate the development of IBS is provocative, current investigations are focused on a wide range of factors and how they affect the interplay between the body and its response to the environment.
Similarly, atopic disorders reflect a broad range of illnesses arising from contact with seemingly innocuous environmental exposures. Atopy is a state of genetically determined immune hypersensitivity, which can manifest in multiple organs simultaneously or individually over a lifespan. Although the GI tract is not considered a common presenting organ in adult atopic disorders, the footprint of allergic disease can be found in this organ if looked for carefully. In an earlier report by our group, we identified a certain group of patients who had undergone intestinal biopsies for evaluation of abdominal complaints that showed increased mucosal mast cells Citation[7]. Clinically, these same patients fulfilled criteria for IBS and appeared to have specific associated non-GI manifestations of atopic disease. We tested the idea of ‘atopic IBS’ in a recent prospective survey of consecutive unselected patients seen in the gastroenterology, allergy and immunology and internal medicine clinics Citation[8]. Our data confirmed that seasonal allergic rhinitis, allergic eczema and atopy (allergic rhinitis, asthma and allergic eczema) were highly correlated and predictive of the presence of IBS. This was particularly seen in diarrhea-predominant IBS, regardless of the reason for appointment or the clinic visited. Food allergy alone was not predictive of IBS. In addition, the number of IBS patients identified during the pollen season in the allergy and immunology clinic was comparable to the number seen in the gastroenterology clinic Citation[8].
The link between IBS and atopy could be explained on the basis of a common immunological origin or the relationship of both disorders to common factors such as stress, allergen exposure or an infection. The case for an immunologic connection is feasible because the mast cell is a key element in the pathogenesis of both disorders. On the one hand, mast cells are a pivotal element in allergic disorders, while, on the other hand, mast cells have an important function in IBS as the final link in the brain–gut axis Citation[9]. The mast cell in this role produces a variety of proinflammatory mediators that result in local end organ dysfunction as well as systemic reactions. The mast cell is activated by IgE-mediated and non-IgE-mediated stimuli that can produce gastrointestinal inflammation accompanied by respiratory and skin manifestations. The inflammation in these tissues resulting from mast cell activation reflects the Th2 cytokine profile Citation[10]. In fact, both inhaled and ingested allergens are capable of causing simultaneous local and systemic responses, sometimes simultaneously Citation[101]. This was illustrated in a study that demonstrated an increase in mast cells and eosinophils in the duodenal biopsies during birch pollen season in patients with seasonal allergic rhinitis Citation[11]. The majority of these patients also complained of IBS-like symptoms and oral allergy syndrome that was associated with the severity of the pollen season. Oral allergy syndrome is a collection of symptoms such as itching of the tongue, palate and throat, sometimes accompanied by swelling of the lips and tongue Citation[12]. The trigger for the syndrome appears to be the proteins that are shared between pollen and food such as fruits, tree nuts and vegetables. Stress also appears to be the modifying factor causing the development of the protein and exaggerates the response to these protein antigens. The pathogenic pathway of the food hypersensitivity reactions in response to these food and inhalant proteins in adults with IBS appear to be Class 2 mast cell IgE-mediated allergy Citation[13] and IgG4-mediated responses Citation[14,15].
The complexity of the pathogenic pathways that underlie the mechanism of food reactions in IBS patients may explain the difficulty in diagnosing atopic IBS and why IgE-based standard food skin and radioallergosorbent tests are not reliable. A more productive approach may be to identify the cross-reacting foods associated with pollen allergy and then use fresh extracts of the food proteins for standard skin and patch tests. Further studies are needed to validate this approach in identifying those atopic IBS patients with food allergy. Another approach is use to IgG4 antibody titers against food antigens advocated by several researchers Citation[16,17]. However, these antibodies are seen in many subjects without symptoms and unless a predictive threshold is identified, could simply represent normal IgG response to food antigens without clinically relevant allergic reactions. Further studies are required to establish the specificity of IgG serology before its use can be recommended in clinical practice.
Additionally, mast cells are a key components of the innate immune system Citation[18]. Mast cells play a role in both IBS and atopy by contributing to the visceral hypersensitivity, intestinal dysmotility and the stress response. Mast cells are important in mucosal barrier function and may have a role in the alteration of gut microbiota demonstrated in both IBS and atopic dermatitis and asthma Citation[19,20]. Thus, it is reasonable to consider therapeutic interventions to control mast cell activation in patients with atopic IBS. Indeed, blocking mast cell mediators, such as histamine, has produced significant symptom reduction in IBS patients Citation[7]. However, a randomized, double-blind placebo-controlled study is required to determine whether mast cell-directed therapy is effective in the treatment of IBS patients with history of atopic disorders (atopic IBS). Examples of these therapies are mast cell stabilizers, inhibitors of mast cell activation and inhibitors of mast cell products such as H1/H2 blockers.
To summarize, recognition of atopic triggers, such as seasonal allergic rhinitis and atopic eczema, in adults with accompanying IBS will offer an opportunity to investigate immune hypersensitivity mediated by mast cells and determine the characteristics of cell–cytokine interactions and profiles. Clinically, it will allow us to determine the importance of Class 2 IgE-mediated food allergy Citation[8,21] and the interplay between inhaled and ingested antigen in producing symptoms. This approach will lead to appropriate food elimination. It will also allow the assessment of treatment with histamine blockers, cromolyn and leukotrienes antagonists which could provide symptom control in at least a subgroup of patients with IBS that we have called atopic IBS.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Horwitz BJ, Fisher RS. The irritable bowel syndrome. N. Engl. J. Med.344(24), 1846–1850 (2001).
- Paré P, Gray J, Lam S et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin. Ther.28(10), 1726–1735 (2006).
- Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br. Med. J.2(6138), 653–654 (1978).
- Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut45(Suppl. 2), II1–II5 (1999).
- Mostafa R. Rome III: The functional gastrointestinal disorders, third edition, 2006. World J. Gastroenterol.14(13), 2124–2125 (2008).
- McKendrick MW, Read NW. Irritable bowel syndrome – post salmonella infection. J. Infection29(1), 1–3 (1994).
- Jakate S, Demeo M, John R, Tobin M, Keshavarzian A. Mastocytic enterocolitis: increased mucosal mast cells in chronic intractable diarrhea. Arch. Pathol. Lab. Med.130(3), 362–367 (2006).
- Tobin MC, Moparty B, Farhadi A, DeMeo MT, Bansal PJ, Keshavarzian A. Atopic irritable bowel syndrome: a novel subgroup of irritable bowel syndrome with allergic manifestations. Ann. Allergy Asthma Immunol.100(1), 49–53 (2008).
- Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J. Gastroenterol.13(22), 3027–3030 (2007).
- Broide DH. Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol.108(2 Suppl.), S65–S71 (2001).
- Magnusson J, Lin XP, Dahlman-Höglund A et al. Seasonal intestinal inflammation in patients with birch pollen allergy. J. Allergy Clin. Immunol.112(1), 45–50 (2003).
- Sloane D, Sheffer A. Oral allergy syndrome. Allergy Asthma Proc.22(5), 321–325 (2001).
- Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J. Allergy Clin. Immunol.103(5 Pt 1), 717–728 (1999).
- Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut53(10), 1459–1464 (2004).
- Zar S, Benson MJ, Kumar D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am. J. Gastroenterol.100(7), 1550–1557 (2005).
- Zuo XL, Li YQ, Li WJ et al. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy37(6), 823–830 (2007).
- Zar S, Mincher L, Benson MJ, Kumar D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand. J. Gastroenterol.40(7), 800–807 (2005).
- Karulin AY, Hesse MD, Yip HC, Lehmann PV. Indirect IL-4 pathway in type 1 immunity. J. Immunol.168(2), 545–553 (2002).
- Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin. Experiment. Allergy30(11), 1604–1610 (2000).
- O’Mahony L, McCarthy J, Kelly P et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology128, 541–551 (2005).
- Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J. Allergy Clin. Immunol.106(1 Pt 1), 27–36 (2000).
Websites
- The Allergy Report. American Academy of Allergy, Asthma, and Immunology. Milwaukee, WI, USA, 1996–2005. www.aaaai.org