Abstract
Despite availability of new treatments for patients with Type 2 diabetes mellitus (T2DM), optimal management of glycemic control remains challenging. Treatment with basal insulin can improve HbA1c, but may not be sufficient to control postprandial plasma glucose (PPG) levels. Both fasting plasma glucose (FPG) and PPG levels contribute to overall glycemic control. In patients with moderate hyperglycemia, PPG excursions have a greater contribution to overall hyperglycemia, with this contribution being greatest when HbA1c is approximately 7–8% Citation. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been designed to restore and maintain GLP-1 levels and attenuate PPG excursions. GLP-1RAs that predominantly affect PPG may complement the FPG lowering provided by basal insulin, possibly improving overall glycemic control without additional weight gain and with limited incidence of hypoglycemia. Lixisenatide as an add-on to basal insulin lowers PPG levels, improves HbA1c control and has a beneficial effect on weight in T2DM patients.
Type 2 diabetes mellitus (T2DM) has reached epidemic proportions, with the number of patients with T2DM expected to increase by 50% over the next 20 years Citation[101]. Despite the availability of new treatments for the management of T2DM, optimal management of glycemic control remains a challenge Citation[2,3]. When oral antihyperglycemic agents and lifestyle modifications are no longer capable of maintaining glycemic control, the addition of basal insulin can restore glycated hemoglobin (HbA1c) to 7.0% for 50–60% of patients with T2DM Citation[2,4–6]. However, addition of basal insulin may not always achieve glycemic targets, when postprandial hyperglycemia is an issue Citation[4,7], and is commonly associated with weight gain and a higher incidence of hypoglycemia.
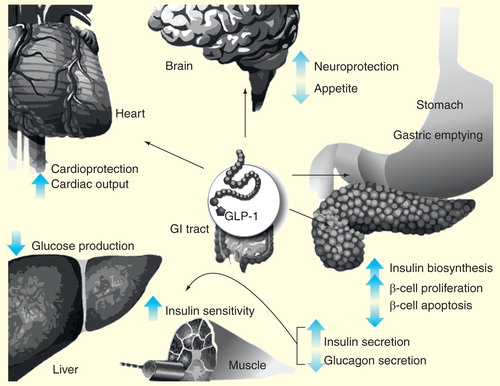
The incretin hormone glucagon-like peptide-1 (GLP-1) has been found to enhance insulin secretion in β-cells . GLP-1 receptor agonists (GLP-1RAs) have been designed to restore and maintain incretin GLP-1 hormone levels and attenuate postprandial plasma glucose (PPG) excursions, allowing an extended duration of action and consequently improving glucose tolerance with low risk of hypoglycemia Citation[8,9]. In addition, they have the ability to support β-cell number and function and control PPG levels; thus, GLP-1RAs, when used in combination with basal insulin, may help to improve overall glycemic control without additional weight gain and with a lower incidence of hypoglycemia than other therapeutic options Citation[9–11].
An important consideration in the overall management of T2DM is the control and prevention of hyperglycemia as it is believed to increase the risk of both microvascular complications and macrovascular disease, such as coronary heart disease Citation[12]. The addition of a GLP-1RA to basal insulin treatment results in a lowering of both fasting plasma glucose (FPG) and PPG levels, which may reduce the risk of hyperglycemia and hence cardiovascular (CV) risk. Furthermore, several studies demonstrate that PPG is an independent risk factor for CV events, and may be a stronger predictor of CV disease and all-cause mortality than FPG Citation[13]. Therefore, PPG is now recognized as an important therapeutic target for improving glycemic control in patients with T2DM Citation[14].
Overview of the clinical landscape
Patients with T2DM are initially treated with oral antihyperglycemic medications and lifestyle changes to achieve glycemic control. When glycemic control maintenance fails, patients receive basal insulin and, often, prandial insulin. This treatment intensification brings with it an increased risk of hypoglycemia and weight gain.
An alternative treatment strategy is the addition of a short-acting prandial GLP-1RA. GLP-1RAs release insulin only in the context of hyperglycemia and hence have a low propensity to cause hypoglycemia. Through their action in delaying gastric emptying and increasing satiety, GLP-1RAs may induce weight loss, and they have been shown to increase insulin secretion and decrease glucagon secretion Citation[15].
Currently, several GLP-1RAs are available or in late-stage development. These agents may be classified by their pharmacokinetic properties as either short-acting or long-acting GLP-1RAs, which may provide other non-glycemic-related advantages. GLP-1RAs are also associated with delayed gastric emptying, inhibiting postprandial glucagon secretion and appetite suppression Citation[16]. Through the stimulation of insulin secretion, GLP-1RAs reduce PPG levels and lower the risk of hypoglycemia, as well as having a beneficial effect on patient weight stability Citation[10]. In addition, some GLP-1RAs may have a prandial effect on glucose excursions.
Long-acting GLP-1RAs
Long-acting GLP-1RAs – such as liraglutide, albiglutide, dulaglutide and exenatide long-acting release (LAR) – lower blood glucose levels through stimulation of insulin secretion and reduction of glucagon levels. They have an average half-life of 12 h to several days Citation[15]. Exenatide LAR is available in some markets and is based on a formulation that encapsulates exenatide in microspheres made of poly (D,L-lactic-co-glycolic acid), and administered as a once weekly injection Citation[17]. Liraglutide is a GLP-1RA with a half-life of about 12 h given as a once daily (q.d.) injection Citation[18].
Long-acting GLP-1RAs stimulate fasting insulin secretion, and predominantly reduce FPG levels, glucagon secretion and body weight Citation[15]. Modest reductions in postprandial hyperglycemia have been reported with long-acting GLP-1 RAs, but because of their non-prandial properties they have minimal impact on gastric emptying over long-term use, due to tachyphylaxis Citation[15,19]. The impact of all action of GLP-1RAs on gastric emptying appears to be determined by repeated dosing. As such, even long-acting GLP-1RAs have a sustained effect on slowing the gastric emptying, but the magnitude of this effect diminishes with time Citation[19,20].
Short-acting GLP-1RAs
Currently available short-acting prandial GLP-1RAs, such as exenatide and lixisenatide, predominantly lower PPG levels and insulin concentrations via retardation of gastric emptying, which depends on the baseline rate of gastric emptying. The resistance of prandial GLP-1RAs to cleavage by dipeptidyl peptidase-4 (DPP-4) confers a plasma half-life of 2–4 h Citation[15]. Owing to their short half-life, the effects of prandial GLP-1RAs on FPG are less pronounced than those of long-acting agents. Therefore, prandial GLP-1RAs lead to a substantial delay of gastric emptying, and a blunting of PPG excursions, consequently acting as an effective prandial therapy. The delayed gastric emptying effect makes prandial GLP-1RAs compatible for use with basal insulin Citation[21]. Exenatide is available in some markets, has a half-life of 2 h and is administered as a twice daily (b.i.d.) injection Citation[3]. Lixisenatide will be discussed in detail in this article.
GLP-1RAs & CV variables
Glycemic control and the prevention of CV comorbidities remain key unmet needs in the treatment of T2DM. Patients with T2DM have a greater incidence of CV disease than the general population Citation[22]. Clinical evidence suggests that GLP-1 may improve lipid profiles and other biomarkers of CV disease Citation[12]. Studies have shown that prandial and long-acting GLP-1RAs may have differing CV influence: short-acting GLP-1RAs have shown either no or a small effect on heart rate (0–2 bpm), whereas long-acting GLP-1RAs produced a moderate increase (2–5 bpm) Citation[15,23,24]. Both long- and short-acting GLP-1RAs reduce blood pressure. A recent study showed that treatment with GLP-1RAs exenatide and liraglutide reduced systolic blood pressure and diastolic blood pressure by 1–5 mmHg compared with insulin, glimepiride and placebo in patients with T2DM Citation[25].
Introduction to the drug
GLP-1RAs have become an established therapeutic option in the treatment of T2DM and are now recommended in combination as part of two-drug and three-drug diabetes treatment options.
Lixisenatide is one of four GLP-1RAs that are clinically available, and is a q.d. prandial GLP-1RA for the treatment of T2DM. Lixisenatide as monotherapy, in combination with one or two OADs and in combination with basal insulin therapy, produced significant improvements in overall glycemia, with a particularly prominent effect on PPG levels Citation[26–28].
Chemistry
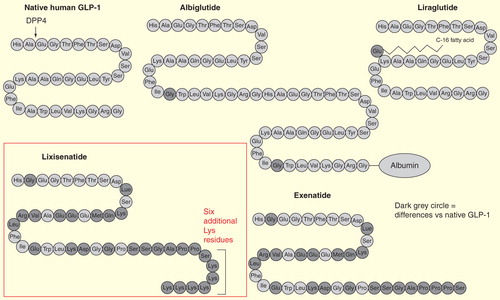
Lixisenatide is a 44-amino-acid molecule (amino-acid sequence: H2N-HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK-CONH2) and is based on exendin-4, which has been modified by the addition of six additional Lys residues at the C-terminal and the deletion of one Pro residue in order to prevent immediate physiological degradation by DPP-4 Citation[8,29].
Pharmacodynamics
Lixisenatide improved first-phase insulin response by sevenfold and second-phase insulin response by threefold when compared with placebo in patients with T2DM Citation[30]. When lixisenatide was given as monotherapy, the mean 2 h PPG and 2 h glucose excursions were significantly improved from baseline (4.5–5.5 mM vs 0.7 mM) compared with placebo Citation[30]. Moreover, lixisenatide as an add-on to metformin reduced levels of 2 h post-meal glucose, glucagon, insulin, C-peptide and pro-insulin compared with placebo Citation[30].
Pharmacokinetics & metabolism
The pharmacokinetic profiles of 5–20 µg lixisenatide q.d. or b.i.d. were assessed in combination with metformin and/or sulfonylurea and showed that the mean apparent clearance (CL/F) was 21–29 l/h, whereas at the 20 µg dose, the median time to maximal concentration (tmax) was 1.3 h for both the q.d. and b.i.d. regimens. The study showed that the mean area under the curve (AUC) and peak plasma concentration (Cmax) increased in a dose-dependent manner (at 20 µg q.d., AUC was 847.8 h·pg/ml and Cmax was 187.2 pg/ml). The half-life (t1/2) varied with the frequency of dosing, with a median t1/2 of 2.8 and 3.2 h with q.d. and b.i.d. administration, respectively, at the 20 µg dose Citation[31]. Other studies showed that proteolytic degradation of lixisenatide takes place in the renal tubules after being filtered by the kidneys Citation[32]. In addition, lixisenatide exhibited dose-dependent pharmacokinetics, with levels reaching peak plasma concentrations within 1.3–2.3 h, suggesting that it may be suitable for q.d. dosing Citation[31].
Clinical efficacy
Overall glycemic control consists of reaching targets in combined parameters of PPG, FPG and HbA1c. Previous GLP-1RAs exhibited good efficacy in lowering HbA1c and controlling FPG levels while reducing body weight Citation[33]. Because of their effect on lowering body weight without increasing hypoglycemia, liraglutide and exenatide LAR are effective in combination with basal insulin Citation[6]. Lixisenatide improves HbA1c levels and enables a greater proportion of patients to achieve HbA1c <7% Citation[34]. In addition, lixisenatide has a beneficial effect on weight, with a limited risk of hypoglycemia and due to the slowing of gastric emptying, markedly reduces PPG levels. Altogether, lixisenatide may be an effective treatment option in combination with basal insulin.
Phase I & Phase II studies Citation[102]
Lixisenatide was evaluated in Phase I and Phase II trials .
Table 1. Summary of lixisenatide in Phase I and Phase II trials.
Phase III studies
The GetGoal clinical trial program was a set of 11 trials that investigated the safety and efficacy of lixisenatide in patients with T2DM , in lixisenatide as monotherapy (GetGoal-Mono), as an add-on therapy to metformin (GetGoal-F1, GetGoal-X), sulfonylureas (GetGoal-S) and pioglitazones (GetGoal-P) Citation[27,35,36]. The program began in 2008 and has enrolled more than 5000 patients globally. Three of the trials from the GetGoal program consisted of 24-week, randomized, placebo-controlled, Phase III trials designed to investigate the efficacy and safety of lixisenatide in combination with basal insulin: GetGoal-L, -L-Asia and -Duo-1 Citation[27,36,37].
Table 2. Summary of GetGoal Phase III clinical trial program.
GetGoal-L
In GetGoal-L, the efficacy and safety of lixisenatide 20 µg q.d. as an add-on therapy to basal insulin was investigated in 495 patients inadequately controlled on a combination of basal insulin ± metformin. Lixisenatide significantly reduced HbA1c vs placebo and increased the proportion of patients achieving HbA1c <7% (28.0 vs 12.0% for placebo; p < 0.0001). Significant improvements in 2-h PPG after a standardized breakfast were seen with lixisenatide vs placebo (LS mean difference of −3.8 mmol/l; 95% CI: −4.7 to −2.9; p < 0.0001). Lixisenatide reduced body weight compared with placebo (LS mean difference of −1.3 kg; p < 0.0001). A decrease in insulin dose at study end was seen with lixisenatide compared with placebo (−5.6 U vs −1.9 U; p = 0.012). The incidence of symptomatic hypoglycemia was comparable between groups (27.7% for lixisenatide vs 21.6% for placebo), whereas four cases of severe hypoglycemia occurred in the lixisenatide group (1.2%) compared with none in the placebo group (0.0%) Citation[14,28].
GetGoal-L-Asia
GetGoal-L-Asia evaluated lixisenatide 20 µg q.d. vs placebo in 311 Asian patients not achieving glycemic control with basal insulin with or without a sulfonylurea. Lixisenatide significantly improved HbA1c (LS mean difference vs placebo –0.9%; p < 0.0001) and the proportion of patients achieving HbA1c <7% (35.6 vs 5.2%; p < 0.0001) and HbA1c ≤6.5% (17.8 vs 1.3%; p < 0.0001) was greater compared with placebo. In addition, the 2-h PPG and glucose excursion was reduced with lixisenatide compared with placebo. In this Asian study population, mean changes in body weight were small, but there was a trend to weight decreases with lixisenatide, with no statistically significant differences between lixisenatide and placebo (LS mean change: −0.4 kg vs +0.1 kg, respectively; 95% CI: −0.93 to −0.06; p = 0.0857). Symptomatic hypoglycemia was similar in patients not receiving sulfonylureas, but was more frequent with lixisenatide vs placebo (32.6 vs 28.3%) Citation[37].
GetGoal-Duo-1
GetGoal-Duo-1 assessed 446 patients inadequately controlled on oral antihyperglycemic medications who were treated with lixisenatide 20 µg q.d. In this study, insulin glargine was systemically titrated in patients during a run-in period of 12 weeks. Subsequently, patients who successfully achieved a FPG level of ≤7.8 mmol/l and HbA1c of 7.0–9.0% were randomized to either lixisenatide 20 µg q.d. or placebo for 24 weeks with continuation of basal insulin treatment. Lixisenatide significantly reduced HbA1c vs placebo (LS mean difference: −0.3%; 95% CI: 0.5 to −0.2; p < 0.0001).
The proportion of patients achieving HbA1c <7% was higher compared with placebo (56 vs 39%, respectively). After a standardized breakfast, lixisenatide significantly improved 2-h PPG (LS mean difference: 3.2; 95% CI: −3.95 to −2.37; p < 0.0001) and FPG increased slightly from baseline for both lixisenatide and placebo (LS mean difference: −0.1; 95% CI: −0.5 to 0.2; p = 0.5142). Lixisenatide had a favorable effect on body weight (LS mean difference vs placebo: −0.9 kg; p = 0.0012); after randomization, body weight increased by 1.2 kg in the placebo group and 0.3 kg in the lixisenatide group. A total of 20.2% of patients experienced symptomatic hypoglycemia with lixisenatide vs 11.7% for placebo Citation[38].
Lixisenatide vs other GLP-1RAs
In a Phase III, open-label, non-inferiority trial of lixisenatide vs short-acting exenatide IR in patients inadequately controlled on metformin (GetGoal-X), glycemic control was comparable between lixisenatide and exenatide IR. After 24 weeks, mean FPG and the proportion of subjects achieving HbA1c <7.0% were comparable between both treatment groups. The incidence of nausea and vomiting was lower with lixisenatide compared with exenatide (24.5 vs 35.1% and 10 vs 13%, respectively). In addition, three-times fewer patients experienced symptomatic hypoglycemia (eight patients [2.5%] vs 25 patients [7.9%]) and six-times fewer patients experienced symptomatic hypoglycemic events with lixisenatide vs exenatide Citation[39].
In a 28-day, randomized, open-label, parallel-group, multicenter pharmacodynamics study, lixisenatide produced a significantly greater reduction in PPG (AUC) during a morning meal test vs pre-breakfast, when compared with liraglutide. Patients receiving lixisenatide experienced significant decreases in postprandial insulin, C-peptide (vs an increase with liraglutide), glucagon and had better gastrointestinal (GI) tolerability vs liraglutide Citation[40].
A recent meta-analysis assessed the efficacy and safety of lixisenatide in 1198 patients with T2DM treated with lixisenatide compared with placebo. A significant number of patients receiving lixisenatide as an add-on to basal insulin ± oral antihyperglycemic medications achieved HbA1c <7% compared with placebo (odds ratio [OR]: 3.67 [1.64, 8.19]; p = 0.002), were 2.5-times as likely to achieve HbA1c <7% with no documented hypoglycemia compared with placebo (OR: 2.65 [1.30, 5.38]; p = 0.007), and were 2.5-times as likely to achieve HbA1c <7% with no documented symptomatic hypoglycemia and no weight gain compared with placebo (OR: 2.65 [1.48, 4.70]; p = 0.0009) Citation[41]. In a pooled analysis of GetGoal-L, GetGoal-L-Asia and GetGoal-Duo-1 studies, postprandial hyperglycemia was reduced more with lixisenatide compared with placebo at 24 weeks (p < 0.0001). Where persisting postprandial hyperglycemia is an issue, lixisenatide may complement the effect of basal insulin control in T2DM, decreasing HbA1c mainly by reducing postprandial hyperglycemia Citation[42].
Safety & tolerability
Side effects related to GLP-1RAs are GI in nature, specifically nausea. The main safety concern with GLP-1RA usage is pancreas safety Citation[43].
Safety of short-acting GLP-1RAs
GI side effects have been reported in studies of lixisenatide as an add-on therapy to basal insulin.
In the GetGoal-Duo-1 study, nausea and vomiting (27.4 and 9.4% vs 4.9 and 1.3% for placebo, respectively) were the most common GI adverse events (AEs). In the GetGoal-L study, nausea, vomiting and diarrhea occurred in 26.2, 8.2 and 7.3% of patients with lixisenatide compared with 8.4, 0.6 and 5.4% with placebo, respectively. Most reports of nausea in the GetGoal-L study occurred in the first 2 months of treatment in both groups. In the GetGoal-L-Asia study, nausea and vomiting were reported in 39.6 and 18.2% vs 4.5 and 1.9% for lixisenatide and placebo, respectively. Reports of deaths and serious events are rare with GLP-1RAs as an add-on to basal insulin, and the incidence of serious AEs in lixisenatide, exenatide and placebo were similar Citation[44]. The most common AEs in one study of exenatide vs placebo were nausea (12.0 vs 2.0%; p = 0.037), vomiting (8.0 vs 0.0%; p = 0.031) and headache (4.0 vs 4.0%) Citation[45]. There have been no reports of pancreatitis with lixisenatide Citation[103].
Safety of long-acting GLP-1RAs
Therapies with long-acting GLP-1RAs appear to have slightly lower GI side effects compared with short-acting GLP-1RAs Citation[11]. In one randomized, multicenter, double-blind, parallel-group study, the incidence of GI AEs in subjects receiving albiglutide 30 mg weekly was less (29.0%) than that observed for the highest biweekly (54.3%) and monthly doses (55.9%) of albiglutide or exenatide Citation[46]. In a randomized, placebo-controlled, double-blind study of overweight patients with T2DM, nausea occurred in 13.6% of those in the dulaglutide group compared with 7.6% in the placebo group Citation[47]. One study examining the US FDA database of reported AEs from 2004 to 2009 found evidence of increased risk of pancreatitis with exenatide and cautioned about long-term use Citation[48]. The FDA warns that both GLP-1RAs and DDP-4 inhibitors should be stopped if patients develop signs of pancreatitis, and should be used with caution in patients with a history of the disease Citation[104]. In addition, liraglutide and exenatide once-weekly carry a black box warning for thyroid cancer risk.
Regulatory affairs
Lixisenatide was granted marketing authorization in February 2013 by the European Medicines Agency and is under consideration by other markets, including the US FDA Citation[105,106].
Conclusion
This review provides an overview of the q.d. prandial GLP-1RA lixisenatide as an add-on to basal insulin for use in glycemic control optimization. Although the efficacy of available treatments for the management of T2DM continues to improve, the need for glycemic control remains a challenge. Addition of basal insulin to the treatment therapy may help to reduce FPG levels but has a limited effect on PPG levels. However, PPG plays a significant role in glycemic control and in the complications of diabetes. With the action of basal insulin on FPG, the addition of a short-acting GLP-1RA provides a complementary effect on overall glycemic control. The combination of GLP-1RAs and basal insulin may help to optimize glycemic control without contributing to increased side effects of hypoglycemia and weight gain and may have a positive effect on CV by improving lipid profiles, decreasing blood pressure and other mechanisms. Short-acting prandial GLP-1RAs (exenatide, lixisenatide) and long-acting GLP-1RAs (albiglutide, dulaglutide, exenatide-LAR and liraglutide) lower blood glucose levels. Long-acting GLP-1RAs, in particular, reduce FPG, whereas short-acting GLP-1RAs reduce PPG levels. Modest reductions in postprandial hyperglycemia have also been reported with long-acting GLP-1RAs, but unlike short-acting GLP-1RAs, they have minimal impact on gastric emptying over long-term use, due to tachyphylaxis. Lixisenatide, once-daily, prandial GLP-1RA as an add-on to basal insulin therapy, lowers PPG levels, improves HbA1c control and has a beneficial effect on body weight, while limiting the risk of hypoglycemia with an acceptable safety/benefit ratio in patients with T2DM. Therefore, lixisenatide added on to basal insulin improves overall glycemic control in patients with T2DM.
Expert commentary
The incretin family – specifically, GLP-1RAs – have been shown to effectively improve glycemia with minimal impact on the AEs typically seen with rapid-acting insulin and sulphonylureas, such as hypoglycemia and weight gain. Long-acting GLP-1RAs have the additional benefit of lowering the likelihood of GLP-1-specific AEs such as nausea and vomiting, but they have reduced efficacy on PPG. PPG response is directly related to the extent of gastric emptying delay, and tachyphylaxis generally results in the use of longer-acting GLP-1RAs. The decision to use short- or long-acting GLP-1RAs partly depends on the T2DM disease profile, as the contribution of FPG and PPG hyperglycemia to overall glycemic control changes with the degree of hyperglycemia. In patients with moderately uncontrolled T2DM, PPG excursions contribute more to overall hyperglycemia, especially when HbA1c is approximately 7–8% Citation[1]. For patients where FPG is the primary treatment goal, long-acting non-prandial GLP-1RAs may be more suitable, whereas short-acting prandial GLP-1RAs have a stronger reducing effect on PPG levels owing to their effect on delaying gastric emptying. Thus, short-acting prandial GLP-1RAs may be more suitable for moderate degrees of hyperglycemia, whereas long-acting non-prandial GLP-1RAs may be more effective in patients with severe hyperglycemia Citation[49]. The q.d. prandial GLP-1RA lixisenatide may have the dual benefit of being a long-acting agent, allowing q.d. injection and a lower side-effect profile, while also providing rapid onset of action. The rapid onset may help to avoid tachyphylaxis, thereby maintaining the beneficial impact on delaying gastric emptying, improving PPG and having a beneficial effect on body weight.
Five-year view
In 2012, more than US$471 billion were spent on healthcare for diabetes, with the cost of healthcare for patients with T2DM expected to rise by an additional 25% between 2035 and 2045. The relative economic burden of the disease can be expected to increase by 40–50% Citation[50], and CV disease accounts for nearly 70% of morbidity and mortality in patients with T2DM Citation[51]. Therefore, achieving and maintaining optimal glycemic control is vital in reducing macrovascular complications associated with T2DM.
The q.d. prandial GLP-1RA lixisenatide as an add-on therapy to basal insulin has been shown to lower HbA1c in T2DM patients, and also has a beneficial effect on weight, thereby improving patients’ likelihood of avoiding complications of diabetes.
The GetGoal-Duo-1 study examined the efficacy and safety of lixisenatide in patients with HbA1c that is still elevated after initiation of insulin glargine. Another upcoming study (GetGoal-Duo-2) will compare lixisenatide with rapid-acting insulin glulisine as an add-on to basal insulin therapy. The study will focus on HbA1c reduction effects and body weight change at week 26 in patients with T2DM not adequately controlled on insulin glargine ± metformin, and may further highlight the potential role that lixisenatide may play as an add-on to basal insulin therapy Citation[107].
An upcoming study (RISE Adult Medication Study; NCT01779362) is investigating the role of therapy using basal insulin and a GLP-1RA in adults presenting with prediabetes Citation[108]. Future models of prescribing for patients with T2DM include the addition of basal insulin for patients already receiving GLP-1RAs who have suboptimal control of T2DM due to the development of β-cell failure Citation[16]. Another area of interest is the potential for innovation in the delivery system for insulin/GLP-1 receptor analog combinations in order to reduce the potential complexity of these regimens Citation[52]. Finally, the incretin class of therapies appears to have positive effects on surrogates of CV disease. To date, trials investigating the effect of lixisenatide on CV outcomes have been animal based. However, the upcoming ELIXA trial (NCT01147250) is designed to evaluate CV outcomes in patients with T2DM after acute coronary syndrome during treatment with lixisenatide Citation[109].
Key issues
• Optimal glycemic management has yet to be achieved for all Type 2 diabetes mellitus (T2DM) patients.
• Both fasting plasma glucose and postprandial plasma glucose (PPG) levels contribute to overall glycemic control.
• In cases where oral antidiabetic drugs and lifestyle modifications are no longer capable of maintaining glycemic control, addition of basal insulin can restore HbA1c to 7.0% for 50–60% of patients with T2DM.
• Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) have been designed to restore and maintain incretin GLP-1 hormone levels and attenuate PPG excursions Citation[9].
• The effect of GLP-1RAs in dual therapy with basal insulin may improve overall glycemic control without additional weight gain and with less hypoglycemia.
• Four GLP-1RAs are currently available for the treatment of T2DM: exenatide twice daily; a long-acting formulation, once weekly; liraglutide q.d.; and q.d. prandial lixisenatide Citation[30].
• Lixisenatide is a once daily prandial GLP-1RA for the treatment of T2DM.
• Lixisenatide as an add-on to basal insulin therapy lowers PPG levels, improves HbA1c control and has a beneficial effect on weight in patients with T2DM, and has been shown to reduce the risk of hypoglycemia without the serious side effect of pancreatitis.
Financial & competing interests disclosure
R Aronson has received research support and/or consulting honoraria from Sanofi-Aventis, Novo Nordisk, Eli Lilly and Takeda. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial support was provided by Judith Leavy, Medicus International (London, UK) and funded by Sanofi-Aventis.
Notes
References
- Blevins T. Control of postprandial glucose levels with insulin in type 2 diabetes. Postgrad. Med. 123(4), 135–147 (2011).
- Nathan DM, Buse JB, Davidson MB et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32(1), 193–203 (2009).
- Kendall DM, Riddle MC, Rosenstock J et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes care 28(5), 1083–1091 (2005).
- Raccah D, Bretzel R, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough - what next? Diabetes Metab. Res. Rev. 23, 257–264 (2007).
- Riddle M, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes care 26, 3080–3086 (2003).
- Arnolds S, Dellweg S, Clair J et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof- of-concept study. Diabetes care 33(7), 1509–1515 (2010).
- Harris S, Kapor J, Lank C, Wilan A, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam. Physician 56(12), e418–e424 (2010).
- Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul. Pept. 164(2–3), 58–64 (2010).
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548), 1696–1705 (2006).
- Visboll T, Holst J. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia 47(3), 357–366 (2004).
- Buse JB, Rosenstock J, Sesti G et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374(9683), 39–47 (2009).
- Mundil D, Cameron-Vendrig A, Husain M. GLP-1 receptor agonists: a clinical perspective on cardiovascular effects. Diab. Vasc. Dis. Res. 9(2), 95–108 (2012).
- Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35(6), 1364–1379 (2012).
- Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 34(12), 2508–2514 (2011).
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8(12), 728–742 (2012).
- Cowan J, McKay G, Fisher M. GLP-1 receptor agonists and insulin: where are we now? Pract. Diabetes 29(9), 351–352a (2012).
- Drucker DJ, Buse JB, Taylor K et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372(9645), 1240–1250 (2008).
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews D. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211). Diabetes Care 27(6), 1335–1342 (2004).
- Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60(5), 1561–1565 (2011).
- Marathe C, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 36(5), 1396–1405 (2013).
- Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of literature. Diab. Obes. Metab. 15, 485–502 (2013).
- UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes (UKPDS 28). BMJ 317, 703–713 (1998).
- Diamant M, Van Gaal L, Stranks S et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 375(9733), 2234–2243 (2010).
- Pratley RE, Nauck M, Bailey T et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375(9724), 1447–1456 (2010).
- Wang B, Zhong J, Lin H et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab 15(8), 737–749 (2013).
- Ahrén B, Leguizamo D, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 36(9), 2543–2550 (2013).
- Ratner RE, Hanefeld M, Shamanna P. Efficacy and safety of lixisenatide once-daily versus placebo in patients with type 2 diabetes mellitus insufficiently controlled on sulfonylurea ± metformin (GetGoal-S). Diabetologia 54 ( Suppl. 1), S317 (2011).
- Riddle M, Aronson R, Home P et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal inulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 36(9), 2489–2496 (2013).
- Barnett A. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid. 6, 67–79 (2011).
- Christensen M, Knop FK, Vilsboll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin. Investig. Drugs 20(4), 549–557 (2011).
- Distiller L, Ruus P. Pharmacokinetics and pharmacodynamics of GLP-1 agonist AVE0010 in type 2 diabetes patients. Diabetes Care 57( Suppl. 57), A154 (2008).
- Green BD, Flatt PR. Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract. Res. Clin. Endocrinol. Metab. 21(4), 497–516 (2007).
- Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diab. Obes. Metab. 14(8), 675–688 (2012).
- Horowitz M, Rayner CK, Jones KL. Mechanisms and clinical efficacy of lixisenatide for the management of type 2 diabetes. Adv. Ther. 30(2), 81–101 (2013).
- Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Hanefeld M. Efficacy and safety of lixisenatide once-daily versus placebo in patients with type 2 diabetes mellitus insufficiently controlled on metformin (GetGoal-F1). Diabt. Med. (2013) (In Press).
- Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diab. Obes. Metab. (2013) ( Epub ahead of print).
- Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes. Metab. 14(10), 910–917 (2012).
- Rosenstock JFT, Aronson R, Sauque-Reyna L, Souhami E, Ping L, Riddle M. Efficacy and safety of once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes: GetGoal-Duo-1 Study. Diabetes Care. 36(9), 2497–503 (2013).
- Rosenstock J, Raccah D, Koranyi L. Efficacy and safety of lixisenatide once-daily versus exenatide twice-daily in patients with type 2 diabetes insufficiently controlled on metformin (GetGoal-X). Diabetologia 54 ( Suppl. 1), S317(2011).
- Kapitza C, Forst T, Coester H, Poitiers F, Ruus P, Hincelin-Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled with metformin. Diab. Obes. Metab. 15(7), 642–649 (2013).
- Charbonnel B, Wang E, Lin J, Davies M, Fonseca V. Meta-analysis of randomized controlled trials of lixisenatide as add-on to basal insulin in patients with type 2 diabetes mellitus. Presented at: American Diabetes Association 73rd Scientific Session. Chicago, IL, USA, 21–25 June(2013).
- Riddle M, Seino Y, Cariou B et al. Once-daily lixisenatide as add-on to basal insulin ± OADs in patients with Type 2 diabetes selectively reduces postprandial hyperglycemic daytime exposure. Presented at: American Diabetes Association 73rd Scientific Session.Chicago, IL, USA (2013).
- Butler P, Elashoff M, Elashoff R, Gale EAM. A critical analysis of the clinical use of incretin-based therapies. Are the GLP-1 therapies safe?. Diabetes Care 36(7), 2118–2125 (2013).
- Buse JB, Bergenstal RM, Glass LC. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann. Int. Med. 154, 103–112 (2011).
- Liutkus J, Rosas-Guzman J, Norwood P et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diab. Obes. Metab. 12, 1058–1065 (2010).
- Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care 32(10), 1880–1860 (2009).
- Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ. The effects of LY2198265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diab. Obes. Metab. 13(5), 418–425 (2011).
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 14(1), 150–156 (2011).
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 26(3), 881–885 (2003).
- Bagust A, Hopkinson PK, Maslove L, Currie CJ. The projected health burden of type 2 diabetes in the UK from 2000 to 2060. Diabet. Med. 19(s4), 1–5 (2002).
- Khardori R, Nguyen DD. Glucose control and cardiovascular outcomes: reorienting approach. Front. Endocrinol. (Lausanne.) 3, 110 (2012).
- Jendle J, Martin SA, Milicevic Z. Insulin and GLP-1 analog combinations in type 2 diabetes mellitus: a critical review. Expert Opin. Investig. Drugs 21(10), 1463–1474 (2012).
- Liu YH, Ruus P. Effect of the GLP-1 agonist AVE0010 on absorption of concomitant oral drugs. Annual Meeting American Diabetes Association, 69th Scientific Session. New Orleans, USA 2009 (Abstract 495).
- Ratner RE, Rosenstock J, Boka G. A dose-finding study of the new GLP-1 AVE0010 in type 2 diabetes insufficiently controlled with metformin. Annual Meeting of the American Diabetes Association: Abstract 433-P (2008).
- Drucker D. The biology of incretin hormones. Cell. Metab. 3, 153–165 (2006).
Websites
- International Diabetes Federation. The Global Burden. www.idf.org/diabetesatlas/5e/the-global-burden ( Accessed 01 February 2013)
- Evaluate. Zealand Pharma Reports Successful Phase I/IIa Trial with ZP10 in Type 2 Diabetes Patients, 2002. www.evaluategroup.com/Universal/View.aspx?type=Story&id=199032 ( Accessed 20 June 2013)
- Lyxumia, INN-lixisenatide - European Medicines Agency. www.ema.europa.eu. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf
- FDA. Victoza (liraglutide [rDAN origin] injection) – Access data FDA. (2010). www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf ( Accessed 20 June 2013)
- Sanofi Press Release. Sanofi New Drug Application for Lixisenatide Accepted for Review by FDA. (2013). http://en.sanofi.com/Images/31904_20130219_LIXISENATIDE_en.pdf ( Accessed 20 February 2013)
- European Medicines Agency. Lyxumia (lixisenatide). Summary of opinion (initial authorisation). (EMA/CHMP/706420/2012). (2012). www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002445/WC500134872.pdf ( Accessed 20 June 2013)
- Clinicaltrials.gov. Efficacy and safety of lixisenatide versus insulin glulisine on top of insulin glargine with to without metformin in type 2 diabetic patients (GetGoal-Duo-2). www.clinicaltrials.gov/ct2/show/NCT01768559?term=12626&rank=1 ( Accessed 20 June 2013)
- ClinicalTrials.gov. RISE Medication Study. http://clinicaltrials.gov/ct2/show/NCT01779362?term=RISE&rank=7 ( Accessed 20 June 2013)
- ClinicalTrials.Gov. Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (Lixisenatide) (ELIXA). http://clinicaltrials.gov/ct2/show/study/NCT01147250 ( Accessed 20 June 2013)