ABSTRACT
After the introduction of anti-tumor necrosis factor (anti-TNF) agents, the clinical outcome of patients with Inflammatory Bowel Disease (IBD) has improved significantly. However, use of anti-TNF therapy is complicated by loss of response. In order to maintain remission, adequate serum levels are required. Hence, therapeutic drug monitoring (TDM) is important in order to optimize serum drug levels, especially in patients with loss of response to these agents. Optimization of anti-TNF therapy by applying TDM enables clinicians to regain response to TNF blockers in a significant proportion of patients. It is important to use anti-TNF agents in their most optimal way, since these therapeutic agents are expensive and the medical options after failing anti-TNF therapy are limited. Here, we will discuss how to optimize treatment with anti-TNF agents in IBD patients in order to improve treatment efficacy, prevent anti-drug antibody formation, reduce side effects, discontinue unnecessary treatment and manage costs.
Introduction
In recent years, medical treatment of patients suffering from inflammatory bowel disease (IBD) has changed significantly. Novel therapeutic agents have become available and our knowledge how to optimize treatment strategies for IBD patients has improved markedly. The need for individualized treatment regimens is becoming more and more important.
Identification of the pro-inflammatory cytokine tumor necrosis factor (TNF) as an important inflammatory mediator in several chronic inflammatory conditions paved the way for the development of TNF antagonists. These anti-TNF agents not only bind and neutralize TNF, but they also down regulate inflammation by inducing apoptosis.[Citation1] Currently, there are four anti-TNF agents available for the treatment of IBD, namely infliximab (IFX), adalimumab (ADL), golimumab (GLM, only for ulcerative colitis (UC)) and certolizumab pegol (CZP). CZP has only been approved for Crohn’s Disease (CD) in the United States and Switzerland, and will not be discussed here.[Citation2] IFX and ADL are efficacious in inducing and maintaining clinical remission in patients with CD and UC.[Citation3–Citation7] Introduction of these two agents has led to a decrease in bowel-related surgery and hospitalization rates. IFX is an effective drug to treat fistulizing CD, but for ADL there are less data available.[Citation8,Citation9] GLM is an effective agent to induce and maintain remission in patients with moderate-to-severe UC.[Citation10,Citation11] Treatment with anti-TNF agents contributed to a better disease control with a reduction in (late) complications of the disease and improved quality of life in IBD patients.[Citation12] Unfortunately, a considerable proportion of patients fail to respond to anti-TNF induction therapy and these patients are labeled as primary non-responders. Up to 50% of patients who initially respond to anti-TNF therapy show loss of response (LOR) over time (i.e. secondary LOR), which often leads to discontinuation of treatment.[Citation13] An important factor that contributes to secondary LOR is the development of anti-drug antibodies (ADA). The so-called ‘immunogenicity’ can result in faster clearance of the drug subsequently leading to lower serum drug levels and LOR.[Citation14–Citation16] Formation of ADA is also associated with infusion reactions.[Citation17,Citation18]
Therapeutic options for IBD patients who fail anti-TNF therapy are limited, although novel agents such as anti-interleukin-23 antibodies and Janus kinase inhibitors may offer alternatives in the foreseeable future. Nonetheless, it is important to use anti-TNF agents in their most optimal way in order to improve treatment efficacy, reduce side-effects and manage costs.[Citation19] Here, we will discuss how to optimize treatment with anti-TNF therapy in patients with IBD.
Development of anti-TNF treatment
Our knowledge regarding the effective use of anti-TNF agents has improved considerably during recent years. Instead of switching to other agents in the case of LOR, prevention of secondary LOR by applying the right intervention is a more common approach nowadays. Importance of adequate serum levels of anti-TNF antibodies has been well established and several factors that may influence serum drug concentrations have been identified.[Citation20–Citation27] Rutgeerts et al. showed that patients who received scheduled IFX treatment instead of episodic treatment had fewer hospitalizations, higher rates of mucosal healing and a reduction in the formation of Ada compared to patients who received episodic treatment.[Citation28] Maser et al. demonstrated a positive association between detectable IFX trough levels (TLs), higher rates of clinical remission and endoscopic improvement in CD patients on maintenance treatment with IFX.[Citation28,Citation29] Since serum drug levels seem to be positively associated with clinical outcome, it is essential to define appropriate cut-off levels. From ACCENT-1 it was concluded that the therapeutic threshold of IFX serum levels should be above 3.5 µg/ml.[Citation21] It remains unclear if there is a maximum to the therapeutic range. However, recent work from our group showed that high IFX and ADL TLs (i.e. > 5.5 and 6.6 µg/ml, respectively) were associated with a lower disease-specific quality of life in IBD patients,[Citation30] particularly, regarding systemic symptoms and emotional status. A trend towards lower SF-36 and higher fatigue scores was observed in the higher anti-TNF TL group, but this difference was not significant. Based on available literature, it appears that a TL of 3 µg/ml should be sufficient for IBD patients who are in clinical remission. Vande Casteele et al. showed in the TAXIT study that in patients with a TL > 7 ug/ml, the IFX dosage could safely be de-escalated.[Citation31] In clinical practice, this means that IFX dosages may be reduced to achieve a TL of 3 µg/ml, which might result in a decrease in side-effects, reduction of costs and a higher proportion of patients who maintain clinical remission. The PRECISION study, a currently ongoing prospective trial at our institute, is performed to test these hypotheses. For ADL, a serum concentration at trough above 6–7 µg/ml is considered to be sufficient and a TL above 4.9 µg/ml is associated with mucosal healing.[Citation14,Citation32]
One of the latest additions to the anti-TNF class of drugs is GLM, a subcutaneous human anti-TNF agent that was recently approved by the Food and Drug Administration and European Medicines Agency for the treatment of moderately to severely active UC. The therapeutic window for GLM remains to be determined in IBD patients.[Citation10,Citation11] Data on the pharmacokinetic profile of GLM are based on trials in patients with ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis.[Citation33–Citation35] We are currently performing a pharmacokinetic study in UC patients who receive GLM treatment in order to increase our knowledge about the pharmacokinetic profile of this agent. A meta-analysis from Thorlund et al. suggested that IFX and GLM are comparable in efficacy and that both agents may statistically be superior to ADL, but more research is needed to confirm these findings.[Citation36]
Risk stratification
Appropriate selection of patients before initiating anti-TNF therapy is essential. Disease phenotype (severity, extent of the disease, etc.) and the medical history, such as previous (non)response to other therapeutic agents, should be taken into account. Poor prognostic factors that are associated with disabling disease can be used to select patients who benefit from early intervention with anti-TNF agents, so-called top-down therapy. Especially, adult patients with a young age at diagnosis (i.e. < 40 years), extensive small bowel involvement and/or perianal CD benefit from early introduction with anti-TNF therapy.[Citation37] However, the majority of patients have a relatively milder disease course and show a favorable response to conventional step-up care that consists of sequential treatment with 5-aminosalicylic acid, corticosteroids, immunomodulators and biologicals (mainly anti-TNF agents). However, incorporation of an algorithm with response assessment guiding treatment escalation (‘accelerated step-care’) was recently shown to be beneficial.[Citation38] Besides correct risk stratification before starting anti-TNF therapy, the increased risk of malignancy and opportunistic infections when starting combination therapy is important to take into account.[Citation39]
Screening before starting anti-TNF treatment
Screening procedures that should be done before anti-TNF treatment is started are partly based on evidence (guidelines) and on expert opinion. Screening for infectious diseases should be performed at baseline, including screening for tuberculosis (using Mantoux, chest X-ray and Interferon Gamma Release Assay (IGRA)) as well as viral serology for hepatitis B, hepatitis C, cytomegalovirus (CMV), Epstein-barr virus (EBV), varicella zoster virus (VZV) and human immunodeficiency virus (the latter two only on indication). Pneumococcal vaccination and annual influenza is advised before anti-TNF therapy is started. Yellow fever (in the case of traveling to endemic areas), hepatitis B (in high-risk groups: homosexual men, those traveling to endemic areas and intravenous drug abuse) and VZV (in the case of seronegativity or increased exposure risk, e.g. young children) should be considered. Stool tests for Salmonella/Shigella/Yersinia/Campylobacter (SSCY)/Escherichia coli and Clostridium difficile toxins should be routinely done. Screening for other infectious diseases should be performed according to geographical location and prevalence.
Combination therapy
In addition to the implementation of scheduled treatment with anti-TNF agents, the importance of combination therapy with an immunomodulator in order to enhance anti-TNF treatment efficacy and in order to prevent immunogenicity has been recognized. The impact of combination therapy on IFX monotherapy is well understood.[Citation40–Citation42] For ADL, there is less evidence that combination therapy is superior to monotherapy.[Citation43] Several randomized controlled trials showed that concomitant use of immunomodulators (azathioprine, 6-mercaptopurine or methotrexate) increases anti-TNF serum levels and reduces immunogenicity thereby improving clinical outcome.[Citation40–Citation42] However, in the COMMIT trial IFX in combination with methotrexate was not more effective than IFX monotherapy, but it must be recognized that all patients received a high dose of prednisolone at the time induction treatment with IFX was started.[Citation44] Furthermore, combination therapy might also reduce the incidence and severity of infusion reactions, which are (at least partially) related to immunogenicity.[Citation16,Citation45,Citation46] Evidence suggests that combination treatment is important, especially within the first 12 months after starting anti-TNF therapy in order to reduce the risk of developing immunogenicity.[Citation47] Furthermore, there is evidence to support the idea that combination therapy with IFX and azathioprine is more cost-effective compared to IFX monotherapy.[Citation48] Hence, starting anti-TNF therapy in combination with an immunomodulator should be the preferred strategy, but this decision has to be made on an individual basis since combination treatment is associated with an increased risk of malignancies and opportunistic infections.[Citation39,Citation49–Citation55] An immunomodulator can also be introduced in patients with LOR while receiving anti-TNF monotherapy, since recapturing response after addition of immunomodulators has been described.[Citation56,Citation57] In our center, a significant proportion of patients with LOR on anti-TNF monotherapy regained response after addition of an immunomodulator. In these patients, we observed an accompanied increase in serum drug concentrations and in most patients ADA levels disappeared. Further research should be performed in order to identify patient- and disease-specific characteristics that could predict which patients would benefit from combination therapy. Besides ADA at a continuous measurable level, transient ADA can also appear during treatment, but their clinical contribution seems not significant.[Citation47,Citation58]
Appropriate dosing of anti-TNF agents in patients with active IBD
When optimizing serum concentrations of anti-TNF agents in order to obtain therapeutic drug levels, it is important to take the severity of the disease into account, which can be reflected by increased C-reactive protein (CRP) and low serum albumin levels, amongst others. A low body weight and the presence of ADA also have a negative impact on the pharmacokinetic profile of these drugs by increasing their clearance.[Citation59,Citation60] Recent studies by Brandse et al. and Yarur et al. investigated the role of luminal (feces) and mucosal (tissue) compartments in the pharmacokinetics of IFX.[Citation27,Citation61] Brandse et al. showed that a significant proportion of IFX is lost through leakage from the gut into the feces, especially during induction treatment in patients with severe colitis and this was associated with impaired clinical outcomes. Interestingly, no correlation was found between fecal and serum concentrations of IFX, although strict quantification of fecal loss of IFX was not performed.[Citation61] Furthermore, therapeutic antibodies can also be degraded by proteases in the mucosal compartment, in particular metalloproteinases.[Citation62] In order to fully understand the pharmacokinetic profile of anti-TNF agents, a pharmacokinetic model is being developed consisting of three physiological compartments (i.e. tissue, serum and feces).
Induction therapy with anti-TNF agents in IBD patients using standard dosages is sufficient in the majority of patients. For IFX, the induction phase consists of 5 mg/kg infusions that are administered at week 0, 2 and 6. For ADL, usually 160 mg subcutaneously (s.c) is used as starting doses although in some countries 80 mg is the preferred starting dose. For GLM, the weight of the patient should be taken into account. Induction treatment consists of 200 mg s.c. followed by 100 mg s.c. after two weeks. In Europe, after the induction phase, patients ≥ 80 kg receive 100 mg every 4 weeks and patients < 80 kg receive maintenance treatment with 50 mg GLM every four weeks. However, there is evidence that IBD patients with severe inflammation should receive higher doses of anti-TNF agents in the acute phase of the disease.[Citation63] The goals of administering higher doses of anti-TNF induction treatment are to neutralize circulating and tissue TNF, to correct for fecal loss and protease inactivation and to avoid occurrence of low serum drug levels and rapid ADA formation. Based on our own experience, hospitalized patients who have low albumin and high CRP levels benefit most from this approach. In patients who show a partial response with inadequate drug levels during the induction phase, treatment should be intensified by giving additional doses or by shortening the treatment interval, instead of immediately switching to another agent. Serum CRP and fecal calprotectin are valuable monitoring tools in this context.[Citation64–Citation66] In the case of switching between different anti-TNF agents, it is known that patients who developed ADA to IFX more often develop ADA to ADL versus anti-TNF naïve patients.[Citation67,Citation68] Identification of these patients on beforehand is not possible yet, but there is evidence that immunogenicity to IFX is associated with HLA-DRB1.[Citation69] A systematic review performed by Gisbert et al. showed that therapeutic efficacy of a second anti-TNF agent in CD patients mainly depends on the reason for switching.[Citation70] When primary or secondary failure was the reason to switch within class, relatively low remission rates were seen (30% and 45%, respectively) compared to patients who were intolerant to the first anti-TNF agent.[Citation70] In our opinion, a second (or third) anti-TNF agent can be efficacious in patients with prior response to an anti-TNF agent and subsequent intolerance to these agents.
Management of loss of response
Before the era of TDM, the management of secondary LOR to anti-TNF agents consisted of empiric treatment intensification by either increasing the dose or by shortening the interval (based on patient’s/physician’s preference). Alternatively, patients could be switched to another drug (out or within class). This approach was largely based on trial and error rather than on evidence and many patients probably did not receive optimal treatment. Nowadays, TDM is more and more used in daily practice in order to improve treatment efficacy and to reduce side-effects. This is done by individually adjusting the dose within a relatively narrow therapeutic range, because treatment with anti-TNF agents can easily be over or under dosed.
In IBD patients who receive treatment with anti-TNF agents, TDM is usually applied in the case of suspicion of active disease and secondary LOR (). The first step is to determine clinical and biochemical disease activity. CRP testing is relatively cheap and widely available, but an elevated serum CRP concentration is not specific for intestinal inflammation. Fecal calprotectin has a greater specificity for intestinal inflammation compared to CRP and correlates significantly with endoscopic disease activity, especially in UC.[Citation71,Citation72] Combining CRP, calprotectin and additional information seen at endoscopy, in most cases allows the physician to determine disease activity.[Citation73–Citation76] Endoscopy and imaging modalities (such as magnetic resonance imaging) are particularly useful in order to differentiate between active inflammation and other causes of abdominal pain such as fibrostenotic strictures, gastrointestinal infections or functional pain (Irritable Bowel Syndrome). If active disease is confirmed, the next step is to perform TDM according to the LOR algorithm that we use in our clinic ().
Figure 1. TDM algorithm (LOR=Loss of response, ADA=anti-drug antibodies). In patients with positive ADA, an immunomodulator can be introduced or therapy intensification (increasing dose or decreasing interval) can be applied. If therapy intensification is not sufficient, switching to another agent (out or within class) is recommended.
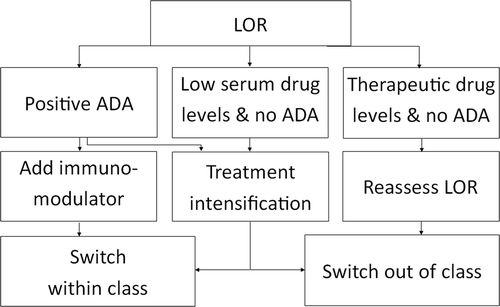
If patients fail to respond to induction treatment with anti-TNF agents or lose response over time (primary or secondary LOR, respectively), the first question that should be answered is whether the patient may benefit from dose escalation. Depending on the combination of results, patients can be divided into three different groups: (I) patients with low or undetectable serum concentrations with ADA; (II) patients with low or undetectable serum drug levels without ADA; and (III) patients with therapeutic drug levels without ADA. In the first group, increased serum concentrations and a decline in ADA can be achieved by adding an immunomodulator.[Citation56,Citation57] If patients already receive combination therapy, it seems valid to intensify anti-TNF treatment by doubling the dose or decreasing the treatment interval, but this seems only a successful strategy in patients with low ADA titers.[Citation77–Citation79] Katz et al. showed that in patients with CD, doubling the IFX dose was not superior to halving the treatment interval in terms of regaining response. However, dose-doubling may be the preferable choice in terms of costs.[Citation80] When deciding which type of dose intensification should be applied, it is important to take the symptoms of the patient into account. If patients have symptoms during the entire treatment interval, increasing the dose is recommended. If symptoms appear during the treatment interval, for example, at week 6 after the last infusion, shortening the interval seems more logical. When none of these options are available, treatment should be switched to another agent (within or out of class).
In the second situation (i.e. low or undetectable serum drug levels without ADA), dose intensification may suffice to regain response.[Citation24,Citation77–Citation79] Again, two different strategies of dose intensification can be applied: increasing the dose or shortening the treatment interval. If dose intensification is not sufficient, switching to another agent is recommended.
In the third group (i.e. patients with therapeutic drug levels without ADA) anti-TNF treatment appears to be ineffective. For these patients, evaluation of disease activity is key, endoscopy being the most informative diagnostic tool. If active endoscopic disease is confirmed, anti-TNF therapy should be discontinued and switch out of class should be initiated (i.e. drug with another mode of action). It is possible that in such patients other cellular mediators than TNF play a dominant role. Vedolizumab, a monoclonal antibody which binds to α4β7 integrins, is a valuable option for CD and UC patients who fail anti-TNF therapy.[Citation81,Citation82] In the nearby future, ustekinumab (anti-IL12/ IL23) will likely become available for patients with refractory IBD.
When assessing LOR, adherence to treatment should also be determined. A systematic review performed by Lopez et al. showed that more than three-quarters of IBD patients adhere to biologics, but when assessing secondary LOR, non-adherence to treatment should be recognized since poor adherence may undermine the therapeutic benefit of biologics.[Citation83–Citation86]
Individualized treatment strategies
Personalized medicine is becoming more and more important in modern healthcare. Individualized anti-TNF treatment strategies are a major area of research and several strategies are gradually being implemented in clinical practice. We already discussed the use of TDM for the management of LOR, but TDM is also becoming increasingly important for treatment optimization. VandeCasteele et al. showed that targeting patient’s serum drug levels to the therapeutic range of IFX resulted in higher clinical remission rates in CD patients as compared to conventional dosing.[Citation31] After dose optimization, it seemed not beneficial to continue concentration-based dosing in order to maintain clinical remission. Clinical remission is an important outcome for patients, but mucosal healing (i.e. no ulcers seen at endoscopy) is the desired treatment goal, since this is associated with improved long-term outcomes and it can also be objectively measured.[Citation87,Citation88] There is evidence to support the idea that TDM predicts the likelihood of achieving mucosal healing following IFX dose intensification in CD and UC.[Citation24]
Anti-TNF agents are expensive and account for 64% and 31% of the total health costs in CD and UC in The Netherlands.[Citation19] TDM seems to be a cost-effective approach, as it was shown that costs of individualized treatment using algorithm based interventions were significantly lower compared to costs of empiric IFX dose intensification (5 mg/kg every four weeks) in CD patients failing IFX.[Citation89,Citation90] Hence, TDM perfectly fits in the personalized treatment approach in order to optimize treatment outcomes and manage costs.
Other valuable tools for personalization of anti-TNF therapy are so-called point-of-care diagnostic tests, since rapid diagnostic results might be helpful in certain clinical situations in order to make proactive treatment adjustments. Endoscopy is the gold standard for assessment of disease activity, but this cannot be implemented on a regular basis. Transabdominal ultrasound is a safe and relatively cheap technique that has proven to be valuable for follow-up of IBD patients and to monitor treatment efficacy.[Citation91,Citation92] Therefore, this modality is being used in our out-patient clinic for point-of-care assessment of disease activity. Moreover, MRI technology can be very useful for assessing small bowel disease and perianal disease in CD patients.[Citation93,Citation94] For optimization of anti-TNF therapy, point-of-care tests that measure serum drug concentrations in (capillary) blood seem to be a valuable tool as they would allow for rapid treatment adjustments, thereby avoiding unnecessary treatment delay. A quick test for fecal calprotectin is already available for ‘on the spot’ assessment of disease activity.[Citation95] Hence, introduction of point-of-care assays will allow for rapid treatment adjustments.
Discontinuation and reintroduction of anti-TNF treatment
In a proportion of patients anti-TNF therapy is discontinued because of longstanding remission. Discontinuation of anti-TNF therapy may be considered for various reasons, such as safety concerns, pregnancy, preference of the patient, etc. We believe that several criteria must be met before discontinuation of anti-TNF therapy is considered. In the case of deep remission (defined by clinical, biochemical and endoscopic remission), discontinuation of anti-TNF therapy may be considered, but this should be done on an individual basis. The long-term outcome of patients previously included in de STORI trial showed that after stopping anti-TNF treatment because of sustained remission under combination therapy, 85% of the patients had to restart treatment again. In the case of extensive small bowel disease and/or symptomatic perianal fistulas, long-term treatment with anti-TNF agents is indicated, because alternative medical therapies are limited.[Citation96–Citation98] Retreatment of patients that experience a relapse after cessation of IFX therapy is usually well tolerated and the success rate is high, ranging from 71% to 94% in different studies.[Citation96–Citation98] Nevertheless, for IFX it is known that patients have an increased risk of developing (severe) infusion reactions and delayed hypersensitivity during reintroduction with IFX.[Citation18,Citation99,Citation100] Despite limited evidence, we do recommend to always restart IFX together with corticosteroids and a H1-receptor antagonist as pre-medication in order to reduce the chance of developing an acute infusion reaction.[Citation101,Citation102] There is also evidence that reintroduction of anti-TNF therapy together with an immunomodulator decreases the risk of infusion reactions.[Citation102]
Expert commentary
Preventing and managing LOR remains one of the most challenging aspects in the management of IBD patients who receive treatment with anti-TNF agents. There is evidence that personalized strategies improve treatment outcomes with anti-TNF agents.[Citation24,Citation31] Low serum drug concentrations and ADA are associated with LOR. Therefore, optimization of serum drug concentrations and minimizing the chance of ADA formation should be the first step in preventing LOR. We recommend to start anti-TNF therapy in combination with an immunomodulator, if possible, and to incorporate TDM in the early phase of anti-TNF therapy (i.e. during the induction phase) in order to prevent primary and secondary LOR by optimizing serum drug concentrations in adult patients. In pediatric patients, there is too little evidence especially about the safety of combination therapy. During induction therapy, doubling the anti-TNF dose in the case of severe colitis seems to be of value in order to prevent primary LOR.
Nowadays, TDM is mainly used to assess secondary LOR. But optimization of serum drug levels during induction therapy is not implemented on a regular basis yet. In our view, TDM should be applied more often since it may result in preventing secondary LOR, especially for patients that may not have received the optimal dose in the first place. In order to ensure that patients respond to treatment with anti-TNF agents, endoscopic evaluation after starting anti-TNF therapy is mandatory in our opinion. There are no data to answer the question what the best timing is for performing endoscopy. For CD patients who embark on anti-TNF therapy, we evaluate endoscopic disease activity after approximately 6 months. For UC patients, we evaluate endoscopic responses at week 10–18 after starting anti-TNF therapy. Moreover, fecal calprotectin is often used as a surrogate marker to assess disease activity in our outpatient clinic as it has been shown that there is a high correlation between fecal calprotectin and endoscopic disease activity in IBD patients.[Citation72,Citation103] The ultimate goal of all these strategies is to discontinue ineffective or unnecessary treatment, improve treatment efficacy and reduce side-effects of anti-TNF therapy.
Five-year view
After the recent introduction of vedolizumab, other biologicals such as ustekinumab (IL-12/ IL-23 blocking antibody) will be introduced for the treatment of IBD in the nearby future. Personalized treatment with biologicals (anti-TNF treatment being at the forefront) will become more and more important and this type of treatment will be facilitated by point-of-care tests, such as on the spot measurements of serum drug concentrations, CRP and fecal calprotectin that allow for immediate treatment decisions. It is likely that disease activity will also be monitored via E-health which may lead to less hospital visits.[Citation104] Another example of such technology is the development of so-called dashboard systems (i.e. software packages that integrate information and calculations about therapeutics from multiple components into a single interface for use in the clinical environment), which are currently being developed in order to simplify TDM of anti-TNF agents.[Citation105] This will likely improve implementation of TDM on a larger scale and should enable gastroenterologists to make proactive treatment adjustments in order to maintain a targeted TL. TDM will also be increasingly used in order to prevent primary non-response instead of only managing secondary LOR.
Biosimilar IFX has recently entered the IBD arena. Efficacy and safety data from clinical trials in rheumatoid arthritis and spondyloarthritis have been extrapolated to IBD. We believe that ongoing clinical trials that investigate treatment efficacy and switching strategies in IBD patients with anti-TNF biosimilars will increase our knowledge and confidence in using these agents. The use of anti-TNF biosimilars will significantly reduce treatment costs which will result in the ability to treat more patients with anti-TNF therapy. In conclusion, the future of IBD treatment with anti-TNF agents will become more personalized, the number of available therapeutic agents will increase in the next five years end the use of anti-TNF biosimilars will expand.
Conflicts of interest
The authors have no conflicts of interest to declare.
Financial & competing interests disclosure
M. Löwenberg has served as speaker and/or principal investigator for AbbVie, Covidien, Dr. Falk, Ferring Pharmaceuticals, Merck Sharp & Dohme, Receptos, Takeda, Tillots and Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Achmea healthcare and ZonMW. A. Strik has served as speaker for Mundipharma, MSD, Abbvie and Takeda. G.R. D’Haens has served as advisor for Abbvie, Ablynx, Actogenix, Amakem, Amgen, AM Pharma, AstraZeneca, Avaxia, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Elan, Ferring, Dr FALK pharma, Centocor/Jansen Biologics, Engene, Ferring, Galapagos, Gilead, Glaxo Smith Kline, Hospira, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Novonordisk, Otsuka, Pfizer, Protein Design Laboratories, Prometheus laboratories/Nestle, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, UCB, Versant and Vifor and received speaker fees from Abbvie, Ferring, Jansen Biologics, Merck Sharp Dome, Mundipharma, Norgine, Shire, Takeda, Tillotts, UCB and Vifor. S. Bots has served as speaker for AbbVie, Merck Sharp & Dohme, Takeda and Tillotts.
Key issues
Combination therapy (anti-TNF agent plus immunomodulator) is more efficacious in CD and UC patients than anti-TNF monotherapy
The risk of developing ADA is lower in patients receiving combination therapy versus IFX monotherapy
Regaining response to anti-TNF agents by adding an immunomodulator is possible in secondary non-responders with low serum drug levels (even in the presence of ADA)
In IBD patients with an expected unfavorable disease course, top-down strategy (i.e. starting with anti-TNF treatment) should be the preferred treatment strategy
The use of biosimilars in anti-TNF therapy will expand the coming years
Personalized medicine of anti-TNF therapy will become more and more important for IBD patients. This consists of TDM, dashboard systems, point-of-care assessments and E-health
References
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279.
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. New England J Med. 2007;357(3):228–238.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–333; quiz 591.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56(9):1232–1239.
- Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–787.
- Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128(4):862–869.
- Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135(5):1493–1499.
- Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96–109.e1.
- Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95; quiz e14-5.
- Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–109.
- Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–995.
- Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137(5):1628–1640.
- West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28(9):1122–1126.
- Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–608.
- O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20(1):1–6.
- Steenholdt C, Svenson M, Bendtzen K, et al. Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(1):51–58.
- van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. 2014;63(1):72–79.
- Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59(1):49–54.
- Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721–1727.
- Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1126–1135.
- Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis. 2013;7(9):736–743.
- Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19(12):2568–2576.
- Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19(6):1112–1122.
- Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2014;12(3):423–31.e1.
- Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016 Feb;65(2):249–255. doi:10.1136/gutjnl-2014-308099.
- Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–413.
- Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2006;4(10):1248–1254.
- Brandse JF, Vos LM, Jansen J, et al. Serum concentration of anti-TNF antibodies, adverse effects and quality of life in patients with inflammatory bowel disease in remission on maintenance treatment. J Crohns Colitis. 2015;9(11):973–981.
- Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–9.e3.
- Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2014;12(1):80–4.e2.
- Xu ZH, Lee H, Vu T, et al. Population pharmacokinetics of golimumab in patients with ankylosing spondylitis: impact of body weight and immunogenicity. Int J Clin Pharmacol Ther. 2010;48(9):596–607.
- Xu Z, Vu T, Lee H, et al. Population pharmacokinetics of golimumab, an anti-tumor necrosis factor-alpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol. 2009;49(9):1056–1070.
- Hu C, Xu Z, Zhang Y, et al. Population approach for exposure-response modeling of golimumab in patients with rheumatoid arthritis. J Clin Pharmacol. 2011;51(5):639–648.
- Thorlund K, Druyts E, Toor K, et al. Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expert Rev Gastroenterol Hepatol. 2015;9(5):693–700.
- D’Haens G, Baert F, Van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660–667.
- Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015 Sep 2. pii: S0140-6736(15)00068-9. doi:10.1016/S0140-6736(15)00068-9
- Osterman MT, Sandborn WJ, Colombel JF, et al. Increased risk of malignancy with adalimumab combination therapy, compared with monotherapy, for Crohn’s disease. Gastroenterology. 2014;146(4):941–949.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395.
- Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.e3.
- Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130(4):1054–1061.
- van Schaik T, Maljaars JP, Roopram RK, et al. Influence of combination therapy with immune modulators on anti-TNF trough levels and antibodies in patients with IBD. Inflamm Bowel Dis. 2014;20(12):2292–2298.
- Feagan BG. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014 Mar;146(3):681–688.e1. doi:10.1053/j.gastro.2013.11.024.
- Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2004;2(7):542–553.
- Steenholdt C, Al-khalaf M, Brynskov J, et al. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2209–2217.
- Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63(8):1258–1264.
- Saito S, Shimizu U, Nan Z, et al. Economic impact of combination therapy with infliximab plus azathioprine for drug-refractory Crohn’s disease: a cost-effectiveness analysis. J Crohns Colitis. 2013;7(2):167–174.
- Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617–1625.
- Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121–1125.
- Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2009;7(8):874–881.
- Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64(8):753–767.
- Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118(4):705–713.
- Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30(3):210–226.
- Rahier JF. Management of IBD patients with current immunosuppressive therapy and concurrent infections. Dig Dis. 2015;33(Suppl 1):50–56.
- Ong DE, Kamm MA, Hartono JL, et al. Addition of thiopurines can recapture response in patients with Crohn’s disease who have lost response to anti-tumor necrosis factor monotherapy. J Gastroenterol Hepatol. 2013;28(10):1595–1599.
- Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2013;11(4):444–447.
- Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9(7):525–531.
- Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20(12):2247–2259.
- Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297–308.
- Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149(2):350–5.e2.
- Biancheri P, Brezski RJ, Di Sabatino A, et al. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology. 2015;149(6):1564–74.e3.
- Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2015;13(2):330–5.e1.
- Bressler B, Panaccione R, Fedorak RN, et al. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2015;29(7):369–372.
- Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol: WJG. 2015;21(40):11246–11259.
- Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18(10):1894–1899.
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69(5):817–821.
- Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with de Novo development of antibodies to Adalimumab and therapeutic failure in Infliximab-to-Adalimumab switchers with IBD. Inflamm Bowel Dis. 2014;20:1714–1721.
- Billiet T, Vande Casteele N, Van Stappen T, et al. Immunogenicity to infliximab is associated with HLA-DRB1. Gut. 2015;64(8):1344–1345.
- Gisbert JP, Marin AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41(7):613–623.
- Summerton CB, Longlands MG, Wiener K, et al. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14(8):841–845.
- D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–2224.
- De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19(10):2111–2117.
- Travis S, Satsangi J, Lemann M. Predicting the need for colectomy in severe ulcerative colitis: a critical appraisal of clinical parameters and currently available biomarkers. Gut. 2011;60(1):3–9.
- Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6(10):991–1030.
- Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4(1):7–27.
- Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2015;13(3):522–30.e2.
- Afif W, Loftus EV Jr., Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105(5):1133–1139.
- Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109(8):1250–1256.
- Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18(11):2026–2033.
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721.
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114(1):39–43.
- Lopez A, Billioud V, Peyrin-Biroulet C, et al. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19(7):1528–1533.
- Billioud V, Laharie D, Filippi J, et al. Adherence to adalimumab therapy in Crohn’s disease: a French multicenter experience. Inflamm Bowel Dis. 2011;17(1):152–159.
- Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther. 2009;26(10):936–946.
- Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15(9):1295–1301.
- Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–1201.
- Steenholdt C, Brynskov J, Thomsen OO, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn’s disease patients failing infliximab. Dig Dis Sci. 2015;60(9):2762–2770.
- Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2013;11(6):654–666.
- Moreno N, Ripolles T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis. 2014;8(9):1079–1087.
- Castiglione F, Testa A, Rea M, et al. Transmural healing evaluated by bowel sonography in patients with Crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis. 2013;19(9):1928–1934.
- Koelbel G, Schmiedl U, Majer MC, et al. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152(5):999–1003.
- Shoenut JP, Semelka RC, Magro CM, et al. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol. 1994;19(1):31–35.
- Coorevits L, Baert FJ, Vanpoucke HJ. Faecal calprotectin: comparative study of the Quantum Blue rapid test and an established ELISA method. Clin Chem Lab Med: CCLM/FESCC. 2013;51(4):825–831.
- Molander P, Farkkila M, Salminen K, et al. Outcome after discontinuation of TNFalpha-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis. 2014;20(6):1021–1028.
- Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142(1):63–70.e5; quiz e31.
- Steenholdt C, Molazahi A, Ainsworth MA, et al. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. 2012;47(5):518–527.
- Duron C, Goutte M, Pereira B, et al. Factors influencing acute infusion reactions in inflammatory bowel disease patients treated with infliximab in the era of scheduled maintenance therapy. Eur J Gastroenterol Hepatol. 2015;27(6):705–711.
- Kugathasan S, Levy MB, Saeian K, et al. Infliximab retreatment in adults and children with Crohn’s disease: risk factors for the development of delayed severe systemic reaction. Am J Gastroenterol. 2002;97(6):1408–1414.
- Han PD, Cohen RD. Managing immunogenic responses to infliximab: treatment implications for patients with Crohn’s disease. Drugs. 2004;64(16):1767–1777.
- Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015;9(9):806–815.
- Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–819; quiz 20.
- Elkjaer M, Shuhaibar M, Burisch J, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652–1661.
- Mould DR, Upton RN, Wojciechowski J. Dashboard systems: implementing pharmacometrics from bench to bedside. AAPS J. 2014;16(5):925–937.
