Abstract
Asthma is a chronic inflammatory disease affecting over 300 million people worldwide. The common association with allergic rhinitis and the presence of proinflammatory cells and mediators in the circulation of patients qualify asthma as a systemic disease. This characteristic and the fact that the gold-standard therapy for persistent asthma, inhaled corticosteroids, cannot suppress all components of airway inflammation and fail to adequately penetrate into the small airways, warrant the quest for effective systemic anti-asthma therapies. This review describes the most important controlled studies of montelukast, a once-daily leukotriene receptor antagonist, in asthma and allergic rhinitis in both adults and children. Montelukast is a systemically active drug with a targeted, dual mechanism of action, acting both as a bronchodilator and anti-inflammatory. In patients of all ages, montelukast has shown a favorable safety profile and was well-tolerated. Both as monotherapy or in combination with inhaled corticosteroids, montelukast produced clinically relevant improvements in asthma-related parameters, including symptoms, lung function parameters, quality of life and the number of asthma exacerbations. Furthermore, bronchoprotective effects have been reported both against specific and nonspecific bronchoactive stimuli. Similarly, in patients with allergic rhinitis, montelukast produced substantial improvements in symptoms and quality of life. Long-term studies aimed to determine its effects on airway remodeling are still lacking.
Redrawn with permission from Citation[29].
![Figure 1. The multiple cellular effects of leukotrienes in the airways.Redrawn with permission from Citation[29].](/cms/asset/7800efb1-713f-45dd-81ea-704d188cce54/ierm_a_11212027_f0001_b.jpg)
(A) No difference could be seen between adding montelukast to budesonide 400 µg twice daily, compared with doubling the dose when all patients where included. (B) For those with concomittant rhinitis, montelukast proved to be superior.
LS: Least square; SEM: Standard error of the mean.
Redrawn with permission from Citation[87].
![Figure 3. Subanalysis of the Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) trial.(A) No difference could be seen between adding montelukast to budesonide 400 µg twice daily, compared with doubling the dose when all patients where included. (B) For those with concomittant rhinitis, montelukast proved to be superior.LS: Least square; SEM: Standard error of the mean.Redrawn with permission from Citation[87].](/cms/asset/ac7582e2-949a-4fa6-a014-76c4dd2a733f/ierm_a_11212027_f0003_b.jpg)
Open circles represent samples in which the CysLT concentration was less than the detection limit. Horizontal bars represent median values.
CysLT: Cysteinyl leukotriene; EIB: Exercise-induced bronchoconstriction.
Redrawn with permission from Citation[111].
![Figure 4. CysLT levels in exhaled breath condensate in asthmatic children, 7–18 years of age, with exercise-induced bronchoconstriction, without exercise-induced bronchoconstriction and healthy control children.Open circles represent samples in which the CysLT concentration was less than the detection limit. Horizontal bars represent median values.CysLT: Cysteinyl leukotriene; EIB: Exercise-induced bronchoconstriction.Redrawn with permission from Citation[111].](/cms/asset/0276e4d4-7728-471f-a36a-ff75283e2f6f/ierm_a_11212027_f0004_b.jpg)
After a 2.5-month baseline period, children were randomized to receive daily montelukast or placebo for 2.5 months (treatment interval). Urine samples were collected twice a day, on 8 consecutive days, during the baseline and the treatment interval and LTE4 and cotinine levels were measured. Use of albuterol was monitored daily. During the baseline interval, urine LTE4 levels in all subjects were strongly associated with increased albuterol use (21.9% increase in albuterol usage per LTE4 interquartile range increase [IQR; p = 0.003]) 2 days after urine collection. Shown are mean estimates and 95% CI for change in albuterol use per IQR in LTE4, modified by (A) sex and (B) mean cotinine. Estimates are given for BL and TRT.
BL: Baseline; IQR: Interquartile range; TRT: Treatment.
Redrawn with permission from Citation[124].
![Figure 5. Effect modifiers of the montelukast effect on LTE4-associated albuterol usage by children with asthma (n = 28; age 6–15 years).After a 2.5-month baseline period, children were randomized to receive daily montelukast or placebo for 2.5 months (treatment interval). Urine samples were collected twice a day, on 8 consecutive days, during the baseline and the treatment interval and LTE4 and cotinine levels were measured. Use of albuterol was monitored daily. During the baseline interval, urine LTE4 levels in all subjects were strongly associated with increased albuterol use (21.9% increase in albuterol usage per LTE4 interquartile range increase [IQR; p = 0.003]) 2 days after urine collection. Shown are mean estimates and 95% CI for change in albuterol use per IQR in LTE4, modified by (A) sex and (B) mean cotinine. Estimates are given for BL and TRT.BL: Baseline; IQR: Interquartile range; TRT: Treatment.Redrawn with permission from Citation[124].](/cms/asset/9651c569-3d91-4fa5-b6a3-e59448e84f03/ierm_a_11212027_f0005_b.jpg)
Figure 6. (A) Differences between montelukast and placebo treatment in percentages of days with worse asthma symptoms in boys according to age group. (B) Differences between montelukast and placebo treatment in percentages of days with worse asthma symptoms in girls according to age group.
NS: Not significant.
Redrawn with permission from Citation[134].
![Figure 6. (A) Differences between montelukast and placebo treatment in percentages of days with worse asthma symptoms in boys according to age group. (B) Differences between montelukast and placebo treatment in percentages of days with worse asthma symptoms in girls according to age group.NS: Not significant.Redrawn with permission from Citation[134].](/cms/asset/3ee103df-5888-46ee-93aa-7b194c1ac666/ierm_a_11212027_f0006_b.jpg)
Asthma is a highly prevalent disease and a major cause of morbidity and mortality. The Global Burden of Asthma Report Citation[1] states that one person in 20 suffers from asthma – a total of 300 million people of all ages worldwide. This figure is expected to rise in the next few years, particularly in urbanized areas. Our understanding of the pathophysiology of asthma has evolved from its initial perception as a disorder of the neuromusculature within the central airways, to subsequent recognition of the fundamental role of chronic airway inflammation. This inflammation was originally believed to be confined to the central airways; however, at present it is widely accepted that apart from the central, large- and intermediate-sized airways, the peripheral airways (<2 mm diameter) are of at least equal importance in the pathophysiology of asthma Citation[2]. Furthermore, there is an association with atopy, and although they are distinct disease entities, asthma is closely linked to allergic rhinitis (AR) Citation[3]. In addition, there is ample evidence for the systemic nature of asthma Citation[4], expressed by blood eosinophilia and other systemic associations, such as atopic dermatitis Citation[3,5], cognitive disorders and asthenia Citation[4,6].
Association with AR
For many years, the association between asthma and AR was primarily attributed to a shared allergic background. However, more recent evidence suggests that AR increases the risk of developing asthma by threefold in both atopic and nonatopic subjects Citation[7,8]. Indeed, approximately 50% of allergic rhinitics have seasonal airway hyperresponsiveness or full-blown asthma, while more than 80% of patients with allergic asthma suffer from concomitant AR Citation[3,9]. In these asthmatics, concomitant AR may be the cause of poor disease control Citation[10], and it is known that the risk for loss of asthma control increases with rhinitis severity Citation[11]. Apart from these epidemiological links, there is ample evidence that AR and asthma have similar pathogenetic and immunological mechanisms. AR is characterized by chronic inflammation of the nasal mucosa that shares many features of the lower airway inflammation observed in asthmatics, including accumulation of mast cells and eosinophils, T-lymphocyte activation and enhanced cytokine expression Citation[12]. In addition, noninvasive airway sampling has shown evidence of lower airway inflammation, in terms of enhanced sputum eosinophils and exhaled nitric oxide, in patients with seasonal AR both in and out of pollen season Citation[13,14]. Hence, modern guidelines advocate a combined strategy for diagnosis and treatment of allergic asthma with concomitant AR, often termed ‘allergic airways disease’ or combined AR and asthma syndrome (CARAS) Citation[3], i.e., AsthmaRhinitis.
Small airways inflammation & other systemic aspects
However, airway inflammation in asthma is not restricted to the upper airways, but involves the entire bronchial tree, including the peripheral small airways. Inflammation of the peripheral airways is present in asthma of all severities, even in very mild disease. Interestingly, the inflammation in the peripheral airways seems to be somewhat different from what is seen in the central airways, with increased numbers of mast cells in close vicinity to airway smooth muscle cells Citation[15] and high-affinity cysteinyl leukotriene-1 receptors (CysLT1R) Citation[2]. Furthermore, peripheral airway inflammation appears to be located within the peribronchiolar, parenchymal part of the lungs that are not readily accessible for inhaled therapies Citation[16]. In addition, clinically important features of remodeling seem to occur mainly in the small airways Citation[17], underscoring the necessity of targeting peripheral inflammation in order to achieve optimal disease control.
Furthermore, the presence of proinflammatory cells and mediators in the circulation qualifies asthma as a systemic disease and, therefore, warrants the use of systemic treatment modalities. Indeed, the early oral therapies (β2 agonists, theophylline and corticosteroids) already had the advantage of treating both the upper and small airways in combination with systemic aspects of asthma, but were limited by serious side effects Citation[4,18]. While in the 1970s asthma was primarily regarded as an inflammatory and bronchospastic disorder of the central airways, it was logical to aim for local anti-inflammatory and bronchodilator treatment by the inhaled route Citation[4,18]. At present, anti-inflammatory treatment with inhaled corticosteroids (ICS) is still the first-choice therapy, both aimed to control and to ‘cure’ persistent asthma Citation[201,202]. However, recent studies have demonstrated that the gold-standard therapy (ICS with or without long-acting β2-agonists) does not achieve optimal control in all asthmatics Citation[19], partly because ICS cannot fully reach all parts of the airways (i.e., the upper and the small airways) Citation[20] and partly due to corticosteroid-refractory mechanisms Citation[4]. In this respect, it has been shown that neither high doses of inhaled nor oral corticosteroids can suppress the production of leukotrienes, important proinflammatory mediators of inflammatory conditions, such as asthma Citation[21,22]. Hence, there is a need for therapeutic strategies that identify and target systemic components of asthma not sufficiently controlled by ICS alone.
Cysteinyl leukotrienes
In 1938, the cysteinyl leukotrienes (CysLTs: LTC4, LTD4 and LTE4) were first described as ‘slow-reacting substance of anaphylaxis (SRS-A)’ by Kellaway and Trethewie Citation[23], while their molecular structures were not identified until some 40 years later Citation[24]. CysLTs are eicosanoids derived from arachidonic acid through the 5-lipoxygenase pathway within inflammatory and structural cells, including mast cells, eosinophils, basophils and bronchial epithelial cells. These potent bronchoconstrictor and proinflammatory mediators exert their biological effects through interaction with their receptors, CysLT1R and CysLT2R Citation[25,26]. CysLT1Rs have been cloned and characterized by two research groups Citation[27,28]. These receptors have been found on the surface of several structural cells (e.g., epithelial cells and smooth muscle cells) and inflammatory cells (including macrophages, monocytes and eosinophils). CysLT1Rs are known to mediate processes involved in the pathophysiology of asthma and AR, including submucosal edema, airway smooth muscle cell proliferation and contraction, mucus production, as well as recruitment and activation of inflammatory cells, mainly eosinophils Citation[29]. In mice, excessive expression of CysLT1R has been shown to be associated with profound airway eosinophilia, Th2 cytokine production and airway hyperresponsiveness Citation[30]. This is in line with observations by Laitinen and colleagues, who found increased numbers of eosinophils in airway mucosal biopsies of asthmatic patients after LTE4 inhalation Citation[31] and Diamant et al., who demonstrated sputum eosinophilia following inhalations of LTD4 in patients with similar asthma characteristics Citation[32]. Recent evidence suggests that CysLTs may also play an important role in the process of airway remodeling through a CysLT1R-mediated increase of matrix proteins, including tenascin and laminin Citation[33]. Furthermore, CysLT2R has also been found on a number of inflammatory cells, including monocytes and eosinophils as well as on structural cells, such as epithelial cells, endothelial cells, smooth muscle cells and fibroblasts. Although its role in the pathophysiology of asthma is much less specified, the CysLT2R may be implicated in the process of airway remodeling Citation[34].
From the 1980s onwards, ample evidence has been provided that CysLTs and CysLT1R-mediated effects are both involved in key mechanisms of the pathophysiology in asthma Citation[35,36] and in the allergic upper airway response in AR Citation[3,37]. As a result, potent CysLT1R antagonists have been developed and marketed for the treatment of allergic airways disease.
Presently, there are three specific leukotriene receptor antagonists (LTRAs), all blocking the CysLT1R, licensed for clinical use: montelukast (Singulair™ [USA]; Singulair® [Europe], Merck), zafirlukast (AccolateTM, AstraZeneca) and pranlukast (Ultair®, Ono Pharmaceutical Co., Ltd).
Global guidelines for the treatment of persistent asthma with or without AR
The Global Initiative for Asthma guidelines (GINA) Citation[202] recommend a stepwise approach to asthma treatment, with an ICS as the cornerstone of the treatment of mild and moderate persistent asthma in adults, with the addition of a long-acting β2-agonist (LABA) if insufficiently controlled. The guidelines address asthma as one organ disease and less attention is paid to the systemic aspects of the disease. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines were developed to address the management of AR, a common comorbidity of asthma Citation[3]. Asthma in children is often associated with clinically important, systemic manifestations and as such represents a therapeutic challenge. Severe asthma in children has been shown to be strongly related to concomitant rhinosinusitis Citation[38]. Moreover, allergic sensitization often involves several organs including the skin, GI tract, nose and lungs Citation[39]. While in older children, exercise-induced symptoms and bronchoconstriction are common and treatment compliance may be difficult to achieve with inhaled therapy Citation[40], in infants, viral wheezing illnesses, particularly those caused by rhinoviruses are the most significant predictors of the subsequent development of asthma at the age of 6 years, in a birth cohort at high risk for atopy Citation[41]. Indeed, several studies demonstrated that interaction between allergic sensitization in infancy and viral-induced bronchiolitis (mostly caused by rhinoviruses and respiratory syncytial virus) may result in the future development of asthma Citation[42], with leukotriene-induced airway inflammation as part of the clinical phenotype Citation[43].
With the apparent need to re-assess the role of systemic treatment modalities in the management of asthma and AR, AsthmaRhinitis as well as asthma and small airway inflammation, this paper provides an updated overview of recent clinical studies of the LTRA montelukast, a highly potent, once-daily CysLT1R antagonist.
Pharmacology
Montelukast sodium: chemistry & structure
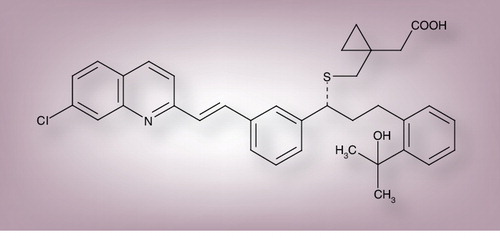
Montelukast sodium is an oral, potent and selective antagonist at CysLT1Rs, which mediates the bronchoconstrictor and proinflammatory actions of the CysLTs in asthma and AR. Montelukast sodium (MK-0476, L-706631) is marketed as Singulair by Merck & Co. in the USA and by Merck Sharp & Dohme elsewhere. The empirical formula is C35H35Cl.N.Na.O3S with a relative molecular mass of 608.18. The structural formula is (R)-1-[[1-(3-(2-(7-chloro-2-quinolinyl)ethynyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl]thiomethyl] cyclopropaneacetic acid, sodium salt, shown in .
Available & recommended dosing
Dose-finding studies indicate an optimum therapeutic dose of 10 mg/day in adults and adolescents Citation[44,45]. Determination of the optimum daily dose of 5 mg in children aged 6–14 years Citation[46] and 4 mg in infants as young as 6 months of age Citation[47,203] is based on serum blood levels of the drug. For adults over 14 years of age, montelukast is provided as beige, rounded-square (7.9 × 7.9 mm), film-coated tablets, engraved ‘SINGULAIR’ on one side and MSD 117 on the other. Each film-coated tablet contains 10.4 mg of montelukast sodium, equivalent to 10 mg of montelukast-free acid. For children (6–14 years), montelukast sodium (Singulair Paediatric 5 mg) is provided as pink, round (9.5-mm diameter), biconvex cherry-flavored chewable tablets, engraved SINGULAIR on one side and MSD 276 on the other. Each chewable tablet contains 5.2 mg of montelukast sodium, equivalent to 5 mg of montelukast-free acid. For young children (2–5 years), montelukast (Singulair Paediatric 4 mg) is provided as pink, oval, biconvex, cherry-flavored chewable tablets engraved SINGULAIR on one side and MSD 711 on the other. For infants (6–24 months) and young children (2–5 years) 4 mg montelukast is also provided in a granule form, packed in an envelope and it is administered with food. The 4 mg tablet formulation, unlike the others, should be administered either 1 h before or 2 h after food, as it may be better absorbed in an empty stomach Citation[48]. The dosage for all formulations is one tablet/dose daily at bedtime. The pediatric formulations for infants 6 months and older are presently registered in the USA and in all European countries.
Pharmacokinetics
In adults, montelukast (10 mg) has a mean oral bioavailability of 64%, and it is rapidly absorbed with a peak plasma concentration (Cmax) being reached within 3–4 h (Tmax) Citation[203]. In children up to 8 years of age, administered 4 or 5 mg montelukast (chewable tablet), Tmax is approximately 2.6 h Citation[49]. Cmax was shown to be 0.50 mg/l in children aged 6–8 years (5 mg), and 0.47 mg/l in those aged 2–5 years (4 mg) Citation[49]. In infants 6–24 months old given a 4 mg dose of montelukast by oral granules, Tmax was approximately 2.2 h and Cmax was 514 ng/ml Citation[47]. Overall, the pharmacokinetic profile of montelukast in patients aged as young as 6 months is comparable with that observed in adults Citation[44].
The pharmacokinetics of montelukast are similar whether dosed in the morning or evening and are not significantly affected by food ingestion Citation[203]. Following absorption, montelukast is more than 99% bound to plasma proteins, and is extensively metabolized by cytochrome P450 isoenzymes 3A4 and 2C9. Plasma clearance of montelukast is approximately 45 ml/min in healthy adults and is excreted along with its metabolites almost exclusively via the bile Citation[203].
Drug interactions
Cytochrome P450 3A4, 2A6 and 2C9 are involved in the oxidation of montelukast in human liver microsomes. Therapeutic concentrations of montelukast do not inhibit cytochrome P450 3A4, 2C9, 1A2, 2A6, 2C19 or 2D6, suggesting that self-induction of montelukast metabolism is unlikely to occur. Since montelukast sodium is metabolized by cytochrome P450 3A4 Citation[50], caution should be taken when montelukast is coadministered with inducers of 3A4 such as phenytoin, phenobarbitone and rifampicin.
Clinical pharmacology
Kinetic binding studies have demonstrated the following rank order of potency for binding to the CysLT1R (LTD4 site) for LTRAs: montelukast is equal to zafirlukast and both are more potent than pranlukast Citation[51]. The specific and reversible antagonism of the CysLT1R by montelukast exerts two important effects, acting on airway smooth muscle cells and inflammatory processes. Unlike asthma drugs that act directly as bronchodilators, evidence from animal studies shows that montelukast is a very potent blocker of LTD4-mediated bronchoconstriction Citation[52]. Initial proof-of-concept studies of montelukast examined its ability to prevent LTD4-induced bronchoconstriction. In a placebo-controlled study in patients with mild persistent asthma, LTD4-induced bronchoconstriction was assessed by the provocation concentration required to reduce airway conductance (sGaw) by 50% (LTD4 PC50) Citation[44]. After 4 h following single oral doses of 5, 20, 100 and 250 mg of montelukast, median LTD4 PC50 values were increased 85-, 113-, 161- and 181-fold, respectively, when compared with placebo. After 20 h following single oral doses of 40 or 200 mg montelukast, LTD4 PC50 values remained more than 50-fold higher than after placebo, indicating that montelukast produces sustained antagonism of LTD4-induced bronchoconstriction Citation[44]. In susceptible individuals, montelukast has been shown to inhibit bronchoconstriction provoked by aspirin Citation[53], inhaled allergen Citation[54] and exercise (both in children and adults) Citation[55]. Furthermore, maintenance therapy with 10 mg once-daily montelukast for 8 weeks provided greater and more sustained bronchoprotection against exercise-induced bronchoconstriction (EIB) than the LABA salmeterol (50 µg twice daily), both independent of or in addition to concurrent ICS treatment Citation[56–58]. Both montelukast and salmeterol offered comparable protection against EIB at days 1–3, but a significant loss of bronchoprotection was noted at weeks 4 and 8 in the salmeterol group due to tachyphylaxis.
In addition, montelukast has anti-inflammatory, mainly anti-eosinophil, properties Citation[36,59]. Although some studies suggested that this effect may be limited to steroid-naive patients Citation[60,61]; in asthmatics inadequately controlled by ICS, addition of montelukast led to a significant reduction in peripheral blood and induced sputum eosinophil counts Citation[62], suggesting that leukotriene-driven inflammation is not fully suppressed by corticosteroid treatment. One potential explanation for this effect is the observation that in addition to inhibiting the growth of eosinophils locally, montelukast has similar effects centrally on bone marrow progenitor cells Citation[63]. In children with asthma, montelukast has been shown to reduce levels of exhaled nitric oxide (eNO), another marker of airway inflammation, both as monotherapy and on top of inhaled corticosteroids Citation[64,65].
Apart from modulating the bronchoactive and proinflammatory effects of CysLTs, LTRA may also protect against several aspects of CysLT-induced airway hyperresponsiveness and airway remodeling Citation[66,67], as CysLT1Rs have been found on the airway smooth muscle in both central and peripheral airways Citation[25]. In line with these findings, in a study by Riccioni and colleagues, 12 weeks of treatment with montelukast (10 mg once daily) both as monotherapy and in combination with inhaled corticosteroids (budesonide 400 µg twice daily) decreased airway hyperresponsiveness in patients with mild-to-moderate persistent asthma Citation[68]. Similarly, 4 weeks of treatment with montelukast significantly reduced regional air-trapping, and hence improved small airway function in patients with similar asthma characteristics Citation[69]. In addition, two recent studies demonstrated improvements in markers of small airways function and inflammation in both children and adults with asthma after only short-term treatment (4 and 3 weeks, respectively) with montelukast, either as monotherapy or added to a fixed dose of ICS Citation[70,71]. These observations represent the outcome of what might be termed ‘double complementary effects’, since first, LTRAs have been shown to possess anti-inflammatory properties complementary to ICS Citation[72,73] and second, being systematically active, LTRA may reach places, for example upper and small airways, that are not easily accessed by inhaled formulations Citation[3,74].
Clinical efficacy
Evaluation of clinical efficacy: disease-related markers
For the assessment of efficacy of anti-asthma treatment, a combination of clinical and functional parameters can be used, sometimes in combination with inflammatory disease markers Citation[2,75]. Functional end points that are widely used in clinical trials include forced expiratory volume in 1 s (FEV1) and peak expiratory flow (PEF). However, these end points mostly focus on the larger airways and hence may not adequately address the pathophysiology within the small airways. Clinical correlates of asthma, notably exacerbations, nocturnal worsening and exercise-induced symptoms may more accurately reflect small airway function Citation[76]. Consequently, current guidelines focus on improvement of disease control – that is, preventing asthma exacerbations Citation[3,202]. End points that are commonly used in the assessment of AR include nasal symptoms such as congestion, rhinorrhea, itching and sneezing, and eye symptoms Citation[3]. In addition, validated (surrogate) markers of airway inflammation, such as eNO and eosinophil counts in sputum or nasal samplings, are increasingly included in proof-of-efficacy trials and in clinical monitoring Citation[77,78].
Studies in adults & adolescents (>14 years of age)
Clinical efficacy of montelukast (10 mg once daily) in asthma was first demonstrated in three 12-week placebo-controlled studies involving 2471 patients with mild-to-moderate disease, in whom symptom scores and FEV1 were assessed as primary end points Citation[54,79,80]. In these studies, monotherapy with montelukast showed similar clinical efficacy to low-dose inhaled corticosteroids Citation[81–83]. Although in the studies by Israel et al. and Malmstrom et al. beclomethasone (200 µg twice daily) had a greater effect on lung function (FEV1 and PEF), montelukast showed similar improvements in all other outcome measures, including the percentage of asthma control days Citation[82] and blood eosinophils Citation[81], underscoring that lung function parameters may not fully reflect clinical efficacy. Interestingly, in all comparative studies in patients with mild-to-moderate persistent asthma, the numbers of responders and nonresponders appeared to be very similar in both treatment arms. In a comparative study, both montelukast and fluticasone propionate (FP) equally improved lung function, although unsurprisingly, FP more effectively reduced the steroid-sensitive inflammatory markers, eNO and sputum eosinophils Citation[84]. Interestingly, when looking at the response patterns of both treatment modalities, it appeared that, although the majority of patients responded equally well to both treatments, some responded better to FP while others had a better response to montelukast Citation[85]. Asthma control days (ACD) were significantly increased both by FP (2.8 days/week) and montelukast (2.1 days/week). When comparing responses within individuals, 29.3% of participants achieved at least one more ACD per week while on treatment with FP than during treatment with montelukast, whereas 12.2% achieved at least one more ACD per week during treatment with montelukast than during treatment with FP.
In a 16-week study in 639 patients with mild-to-moderate persistent asthma, addition of montelukast to inhaled budesonide (400–1600 µg/day) significantly reduced the median percentage of asthma exacerbation days by on average 35%, as compared with placebo, independent of the ICS dose Citation[86]. Additionally, montelukast also produced significant improvements in the secondary end points of the study (asthma-free days, nocturnal awakenings, β-agonist use and morning PEF). In a 12-week study of almost 900 patients receiving inhaled budesonide (800 µg/day), the effect of add-on montelukast therapy was compared with doubling the dose of ICS Citation[87]. Overall, add-on montelukast was at least as effective as doubling the dose of budesonide, with improvement in PEF occurring more rapidly in the montelukast group, while in the double-dose ICS group significantly more respiratory adverse events (AEs), mainly upper airways infections, were seen Citation[87]. In a subanalysis of this study involving 410 patients who were identified as having asthma with concomitant AR, the change in morning PEF from baseline was significantly higher (p < 0.03) in the group receiving add-on montelukast compared with doubling the dose of ICS , underscoring the need to treat both airway compartments to achieve optimal asthma control in patients with AsthmaRhinitis Citation[88].
In patients not adequately responding to ICS, current guidelines recommend complementary therapy either with LABA or LTRA Citation[202]. In a large, multicenter study of 1490 patients with mild-to-moderate persistent asthma, montelukast (10 mg once daily) was compared with salmeterol (50 µg twice daily) as add-on treatment to fluticasone (100 µg twice daily) Citation[62]. The primary outcome measure consisted of the number of severe asthma exacerbations, with symptom control, quality of life and lung function as secondary measures during 1 year of the respective treatments. After 52 weeks, montelukast was shown to be noninferior to salmeterol with regard to the proportion of patients experiencing an exacerbation (20.1 vs 19.1%, respectively; risk ratio: 1.05; 95% CI: 0.86–1.29) Citation[62]. Furthermore, both add-on treatments provided clinically relevant, significant improvements in nocturnal awakening and quality of life. While add-on montelukast significantly reduced eosinophil counts in induced sputum and in peripheral blood by virtue of its anti-inflammatory properties, add-on salmeterol significantly increased lung function parameters.
Exercise-induced bronchoconstriction represents a specific challenge in asthma management. The bronchoconstrictive trigger is believed to be osmotic changes in the respiratory epithelium caused by hyperventilation and it has been shown to induce increased resistance within the peripheral airways Citation[89]. Montelukast has been shown to effectively inhibit EIB in both adults and children with asthma Citation[14]. Comparison between montelukast and salmeterol has convincingly shown that montelukast is superior to salmeterol for chronic treatment of asthmatics with EIB, as the effect by salmeterol was reduced due to the development of tolerance Citation[56,57]. More recently, the effects of add-on treatment with montelukast and salmeterol were compared in fluticasone-treated asthmatics with EIB Citation[58]. In these patients, the recovery from EIB and response to rescue medication was slower with add-on salmeterol as compared with add-on montelukast, while patients developed tolerance to salmeterol Citation[58]. In another study, montelukast was compared with salmeterol evaluating exercise performance and gas exchange Citation[90]. Montelukast was found to be superior to salmeterol with better bronchoprotective activity and improved oxygen pulse (i.e., oxygen uptake related to heart rate), indicating a better gas exchange with less ventilation perfusion mismatch Citation[90]. In addition, concomitant treatment with ICS did not affect these outcomes Citation[90].
Asthma with concomitant AR (AsthmaRhinitis)
Apart from asthma, CysLTs have been found to play an important role in the pathophysiology of AR. Consequently, a series of controlled clinical trials in seasonal AR compared the efficacy of montelukast (10 mg once daily) with antihistamines and/or intranasal steroids or tested the efficacy of combination therapy with montelukast plus an antihistamine. Although montelukast has shown efficacy in AR alone, preferably when combined with an antihistamine Citation[91–93], the clinical indication is to treat asthma with concomitant rhinitis, the so-called AsthmaRhinitis.
In a large study in allergic rhinitics with concomitant asthma (n = 815), 2 weeks of treatment with montelukast (10 mg) significantly improved disease-related parameters as compared with placebo Citation[94]. Interestingly, patients with more active and symptomatic disease in particular showed a superior response to treatment with montelukast. This is in line with previous data obtained from a subgroup analysis in the large Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy trial comparing add-on montelukast (10 mg) to budesonide (800 µg/daily) versus doubling the budesonide dose (1600 µg/daily) Citation[88]. Indeed, in the subgroup of asthmatic patients with concomitant rhinitis, combination therapy with montelukast provided significantly greater improvements both in the nasal parameters and in lung function measures as compared with doubling the dose of budesonide Citation[88]. These observations underscore the validity of the unified approach recommended by the ARIA guidelines Citation[3] stated already by Grossman et al. 10 years earlier Citation[95].
At present, there is still an apparent need to further study the pathophysiology of AsthmaRhinitis, as more severe upper airways inflammation has been shown to increase the risk of worsening of disease control with more severe exacerbations within the lower airways of asthmatics with concomitant AR Citation[11].
Airway inflammation & remodeling
Inflammation with structural changes in the lower airways (airway remodeling) represents a future challenge in asthma management. Fewer than 1% of adult asthmatics recover from their disease either spontaneously or after treatment Citation[96]. ICS may affect some, but not all aspects of the remodeling process, even when introduced early in the course of the disease Citation[97,98]. These aspects include long-term disease control and lung function decline (for a review, see Citation[4]). One explanation for this relative lack of response could be that ICS do not sufficiently control inflammation within the peripheral ‘small airways’ Citation[99]. Another, perhaps more important reason is that ICS treatment per se does not affect aspects of the peripheral inflammation. Antileukotriene therapy has a potential role in this process. LTD4 and LTE4 augment the expression of tenascin and laminin, important components of the reticuloepithelial basement membrane, and this process is completely inhibited by montelukast Citation[33]. LTD4 potentiate fibronectin-induced migration of human lung fibroblasts Citation[100] and stimulate human smooth muscle cell proliferation Citation[101]. Mast cells, the majority of which express LTC4-synthase, a key enzyme in CysLT production, are believed to play a key role in this remodeling inflammation Citation[102]. Applying repeated allergen challenge in mice as a model for asthmatic airway remodeling, Henderson et al. demonstrated that this remodeling process could be substantially suppressed by montelukast Citation[103]. Moreover, montelukast was able to reverse some of these changes even when introduced later in the course, after the remodeling process had been initiated Citation[104]. In a biopsy study in patients with mild persistent asthma, 8 weeks of treatment with montelukast has been shown to inhibit the airway remodeling effects (increase in myofibroblasts) following low-dose allergen challenge Citation[46]. Furthermore, there have been occasional case reports indicating that montelukast may have a beneficial effect reversing structural changes within the small airways Citation[105].
However, despite good evidence from both human studies and animal experimental models of asthma, there are still insufficient data to support the clinical role of leukotriene modulator therapy in preventing airway remodeling and its consequences in clinical asthma. Convincing randomized long-term studies are still missing.
Studies in children (aged 6–14 years)
In children in particular, asthma management is often hampered by poor compliance to inhaled controller formulations. In view of its favorable safety profile and once-daily dosing regimen, montelukast seemed a promising candidate and hence in the past decade, many studies applying this drug in asthmatic children of different ages have been performed. In a study involving 336 asthmatic children (6–14 years, FEV1 50–85% of predicted), Knorr and colleagues Citation[106] demonstrated a significant increase in FEV1 and a decrease in days with asthma exacerbations following 8 weeks of treatment with montelukast (5 mg). A subsequent analysis in 87 patients with milder disease (FEV1 >75% predicted) confirmed that montelukast effectively improved FEV1, nocturnal awakenings and quality of life Citation[107]. In a crossover study including 279 children (mean age ± SD: 10.4 ± 2.2) with mild persistent asthma receiving ICS, addition of montelukast (5 mg) for 4 weeks significantly increased FEV1, while significantly decreasing exacerbation days, β2-agonist usage and blood eosinophil counts Citation[108]. In a 12-week, randomized, active-comparator study, treatment with montelukast (5 mg) was compared with that of inhaled budesonide (400 µg twice daily) in 63 children aged 8–14 years with mild persistent asthma and FEV1 greater than 80% of the predicted value Citation[109]. In addition to reductions in urinary LTE4 levels, montelukast produced clinically relevant improvements in airway function, daily symptom scores, nocturnal awakenings, β2-agonist usage and asthma exacerbations, similar to budesonide Citation[109].
Although ample evidence has been provided for a role of CysLTs in the pathophysiology of EIB, until recently, no increases in CysLTs could be measured in patients with EIB following exercise Citation[110]. In 2005, Carraro and colleagues found enhanced levels of CysLTs in exhaled breath condensate of asthmatic children with EIB following exercise as compared with asthmatic children without EIB or healthy controls , thus establishing the role of CysLTs in the pathophysiology of EIB Citation[111]. In the past decade, several studies have evaluated the efficacy of montelukast on EIB in children with asthma. One study in asthmatic children (age range: 7–13 years) showed that one single dose of montelukast (5 mg) significantly reduced EIB with the maximal protective effect obtained at 12 h after dosing (p = 0.02) Citation[112]. In children with atopic asthma, 1 week of treatment with montelukast (5 mg) produced significant reductions in both the immediate- and the late-phase airway response to exercise Citation[113]. In a placebo-controlled, crossover study in 64 children, 8 weeks of treatment with montelukast significantly improved asthma symptom scores, the maximum percentage fall in FEV1 after exercise, and the time to recovery Citation[114]. These improvements were maintained even 8 weeks after discontinuation of treatment, suggesting a sustained effect of montelukast on airway hyperresponsiveness. Moreover, in another study in asthmatic children (6–12 years), 4 weeks of treatment with montelukast (5 mg) significantly protected against EIB without the development of tolerance to the bronchoprotective effect Citation[115]. More recently, a 4-week randomized, placebo-controlled study in children (aged 6–18 years) with atopic asthma, compared the protective effect against EIB of monotherapy with montelukast (5 or 10 mg), with budesonide (200 µg; n = 20), budesonide (200 µg) plus montelukast (5 or 10 mg; n = 20), and budesonide (200 µg) plus salmeterol (9 µg; n = 20), respectively Citation[116]. Although all active treatment regimens appeared effective against EIB, montelukast offered the greatest protection, both as a single and a combination therapy Citation[116].
Several studies have examined the effect of montelukast as add-on therapy in children with uncontrolled asthma despite treatment with inhaled steroids. In a 4-week study in 36 patients with asthma maintained on ICS, the addition of montelukast led to a significant increase in the number of rescue-free days (p < 0.002) compared with placebo Citation[117]. Following a 20-week extension phase, the ICS dose in the montelukast group could safely be reduced by 17%, while in the placebo group an increase in ICS of 64% was observed. By contrast, in a 1-year active comparator study, in 6–14-year-old children with mild persistent asthma, montelukast (n = 495) was shown to be noninferior to inhaled fluticasone (100 µg twice daily; n = 499) in the primary end point of percentage asthma rescue-free days Citation[118]. Alternatively, in a smaller study in children (5–15 years, mild persistent asthma) 12 weeks of treatment with montelukast (n = 30), when compared with inhaled budesonide (200 µg twice daily; n = 32), was similarly effective on symptom scores, lung function and rescue drugs Citation[119].
Following treatment with montelukast (5 or 10 mg) for 8 weeks, children with mild-to-moderate persistent asthma (n = 21; aged 9–18 years) had significantly lower residual volume, residual volume-total lung capacity ratio, airway resistance (Raw), better specific conductance (sGaw) and lower serum eosinophilic cationic protein (ECP) levels, as compared with placebo, suggesting an association between anti-inflammatory effects and decreased air trapping Citation[120]. In another open study using forced impulse oscillometry (IOS) as a tool to estimate peripheral and central airway resistance, 46 children aged 6–14 years were treated with montelukast or placebo for 4 weeks. Montelukast produced a significant improvement in all IOS parameters, especially in the distal capacitive reactance Xrs5, believed to best reflect the peripheral airways Citation[121].
In another crossover trial, Verini and colleagues evaluated the effects of 2 weeks of add-on therapy with montelukast versus salmeterol in 12 children with mild-to-moderate persistent asthma on low-dose inhaled fluticasone. Not unexpectedly, add-on salmeterol significantly increased FEV1 and decreased the residual volume, while add-on montelukast significantly decreased residual volume and eNO Citation[122].
The effect of montelukast (5 or 10 mg; n = 29) on central and peripheral airway function was compared with that of budesonide (200 µg; n = 29), budesonide (200 µg) plus montelukast (5 or 10 mg; n = 29), and budesonide (200 µg) plus salmeterol (9 µg; n = 29) in a randomized, placebo-controlled (n = 27), 4-week study in children 6–18 years old, with moderate atopic asthma. At the end of treatment, interrupted resistance R(int), specific airway resistance SR(aw) and FEV1 improved significantly in all active treatment groups while forced expiratory flow (FEF) 25–75% improved significantly only in the budesonide plus montelukast group and the montelukast group Citation[70].
Another 2-months’ crossover study in steroid-naive, atopic asthmatic children (aged 7–11 years; n = 48) compared the effect of budesonide (200 µg twice daily), budesonide 200 µg (twice daily) plus montelukast 5 mg daily, budesonide 200 µg plus formoterol 9 µg (twice daily) and budesonide (400 µg twice daily) on lung function and fractional exhaled nitric oxide (FENO). As compared with baseline, all treatments significantly increased FEV1 and decreased FENO. However, the combination of montelukast and budesonide was superior in reducing FENO levels as compared with the other treatment regimen Citation[123].
In another trial, school children with moderate-to-severe persistent asthma (n = 27) after a run-in period of 2.5 months with daily measurements of urinary LTE4, cotinine, FENO and monitoring of albuterol use were randomized to add either montelukast or placebo to their regular controller medications, for another 2.5 months. At baseline, LTE4 levels were positively associated with albuterol use. After 2.5 months, treatment with montelukast significantly reduced albuterol usage, especially in girls and in children with higher cotinine levels . In addition, children with high LTE4 levels relative to FENO demonstrated significant declines in albuterol usage Citation[124]. In an 18-week study, Szefler and colleagues evaluated markers associated with clinical response to ICS and LTRA in 126 children (aged 6–17 years) with mild-to-moderate persistent asthma in a double-blind, two-way crossover study, including 8 weeks’ treatment with either fluticasone (100 µg twice daily) or montelukast (5–10 mg once daily). Response to both treatments was assessed by FEV1 (defined as an increase of at least 7.5% from baseline) and relevant biomarkers (eNO, PC20 methacholine, serum IgE, ECP and total eosinophil counts) Citation[125].
In terms of increases in baseline FEV1, 17% of 126 children responded to both medications, 23% responded to fluticasone alone and 5% responded to montelukast alone, while 55% did not respond to either medication. Compared with children who did not respond to either medication, favorable response to fluticasone monotherapy appeared to be associated with overall higher baseline levels of eNO, serum IgE, serum ECP, total eosinophil counts, and lower baseline FEV1 and PC20 to inhaled methacholine. By contrast, favorable response to monotherapy with montelukast was associated overall with younger age and shorter disease duration. Based on the results of their study, the authors concluded that asthmatic children with mild-to-moderate persistent asthma with low pulmonary function and/or high levels of markers of allergic inflammation should primarily receive ICS therapy, while children with another asthma phenotype could receive either ICS or LTRA Citation[125].
Studies in children (aged ∼5 years)
The effect of montelukast (4 mg) has been studied in preschool- children (∼5 years) with symptoms consistent with asthma or with recurrent viral respiratory infections, a known trigger of childhood asthma. In a large multicenter, placebo-controlled study in 689 children (aged 2–5 years) with persistent asthma, treatment with montelukast for 12 weeks produced significant improvements in day- and night-time asthma symptoms, asthma-free days, use of relievers and ICS, and physician global evaluation score Citation[126]. In a placebo-controlled 1-year study in 549 children, aged 2–5 years, with viral-induced asthma, montelukast (n = 278) produced a significant reduction (mean: 31.9%) in asthma exacerbations, delaying the median time to first exacerbation by approximately 2 months along with the number of ICS courses Citation[127].
The efficacy and safety of montelukast (4 and 8 mg) in treating recurrent respiratory symptoms in children was examined in a large, multicenter study of 3–24-month-old children, previously hospitalized for one or two episodes of respiratory syncytial virus (RSV) bronchiolitis with a positive test for RSV. Patients (n = 979) were randomized to placebo or to montelukast at 4 or 8 mg/day for 4 weeks (period I) and 20 weeks (period II). The primary end point was the percentage of symptom-free days (% SFD). No significant differences were seen between montelukast and placebo in % SFD over period I Citation[128]. In contrast to the previous study of similar design but with a limited number of patients, montelukast did not show any beneficial effects in children with RSV bronchiolitis, possibly due to a different disease severity or phenotype in this multicenter cohort Citation[128] compared with that of the previous study Citation[129].
Interestingly, in an open-label study in 506 asthmatic children aged 2–5 years, long-term therapy with montelukast (4 mg once daily) mean duration 330 days, yielded similar rates of asthma-related healthcare resource utilization Citation[126], compared with those receiving ‘standard care’ with cromolyn or ICS Citation[129].
Another randomized study in 63 children (aged 2–6 years) with asthma-like symptoms compared the clinical efficacy of 3 months’ treatment with montelukast (n = 18) versus FP (n = 25) or placebo (n = 20).The primary outcome of the study comprised of daily symptom scores, while the secondary outcomes were rescue medication-free days, blood eosinophils and lung function. Although symptoms were improved in all treatment groups, only FP produced a statistically significant improvement compared with placebo, whereas only montelukast significantly reduced blood eosinophils Citation[130].
In a 4-week, randomized, placebo-controlled study, the effect of montelukast (4 mg once daily) was evaluated in 24 children (aged 10–26 months) with early childhood asthma (wheeze, allergy and a positive family history of asthma). As compared with placebo, patients treated with montelukast showed significant improvements in symptom scores, lung function (FEV1) and FENO Citation[131].
Similarly, in a placebo-controlled crossover study, montelukast (4 mg once daily) for 4 weeks has been shown to significantly reduce airway hyperresponsiveness in 26 preschool children (aged 3.3–6.0 years) with mild, persistent asthma, as compared with placebo Citation[132].
Several studies have examined whether a short course of treatment with montelukast at the beginning of a viral upper respiratory tract infection (URTI) or an asthma episode would be able to modify the severity of the episode. In one recent study, children aged 2–14 years with intermittent asthma participated in a randomized, placebo-controlled trial over a 12-month period Citation[133]. Montelukast (n = 107) or placebo (n = 113) was initiated by parents at the onset of each URTI or asthma symptoms and continued for 7 days or until symptoms had resolved for 48 h. There were 681 treated episodes (345 montelukast and 336 placebo) experienced by 202 patients. The montelukast group had 163 unscheduled healthcare visits for asthma, compared with 228 unscheduled visits in the placebo group (odds ratio: 0.65; 95% CI: 0.47–0.89). There was a nonsignificant reduction in hospitalizations, specialist attendances, duration of episode, β-agonist and prednisolone use. However, montelukast significantly reduced symptoms (14%), night-time awakenings (8.6%), days off from school or childcare (37%) and parents’ time off work (33%) Citation[133].
Another large study in 194 asthmatic children (aged 2–14 years) examined the impact of add-on montelukast versus placebo to their usual asthma therapy on viral asthma exacerbations between September 1 and October 15 2005. Children treated with montelukast compared with those with placebo experienced a 53% reduction in days with worse asthma symptoms and a 78% reduction in unscheduled visits for asthma . Montelukast was beneficial for all patients, irrespective of regular use of ICS and irrespective of colds during the trial Citation[134].
Another study examined whether administration of montelukast, early in the course of a viral infection, might change the course of the disease. A total of 53 infants (mean age: 3.8 ± 3.5 months) with a first episode of acute bronchiolitis were randomly assigned to daily treatment of either 4-mg montelukast sachets or placebo, during all hospitalization days. The primary outcome was the length of stay, and secondary outcomes included the clinical severity score and changes in type 1 and 2 cytokine levels in nasal lavage. There were neither differences in length of stay nor in clinical severity score or in cytokine levels or types (Th2/Th1) between the two treatment groups Citation[135]. It is possible that montelukast has no effect on healthy infants who experience their first episode of bronchiolitis when it is given after the disease has established. In this study, infants with previous episodes of bronchiolitis or ever treated with asthma medication prior to the episode of bronchiolitis were excluded from the study.
In a recent randomized trial, 238 children aged 12–59 months with moderate-to-severe intermittent wheezing were assigned to receive 7 days of either budesonide inhalation suspension (1 mg twice daily), montelukast (4 mg daily), or placebo in addition to albuterol with each identified respiratory tract illness (RTI). The primary outcome was the proportion of episode-free days (EFDs) during the 12-month trial. The three treatment groups did not differ in proportions of EFDs (p = 0.66), in oral corticosteroid use, healthcare use, quality of life or linear growth. However, during RTIs, budesonide and montelukast therapy led to modest reductions in trouble breathing (38 [p = 0.003] and 37% [p = 0.003], respectively) and interference with activity scores (32% [p = 0.01] and 40% [p = 0.001], respectively) that were most evident in patients with positive asthma predictive indices Citation[136].
Recommendations for young children
Based on the discrepancies in response to ICS or LTRAs in children with wheezing within this age group, in whom a diagnosis of asthma is difficult to make, a European Respiratory Society (ERS) task force was created in order to conclude on the phenotypes and the different treatment choices for recurrent wheezing in preschoolers. Two ‘wheezing phenotypes’ were identified for this age group: The ‘episodic’ (viral) and the ‘multi-triggered’ wheezing. According to the ERS task force, montelukast was recommended for the treatment of episodic (viral) wheeze and can be started when symptoms of a viral cold develop. Given the large overlap in phenotypes and the fact that preschoolers can possibly move from one phenotype to another, inhaled corticosteroids and montelukast may be considered on a trial basis in almost any preschool child with recurrent wheeze, but should be discontinued if there is no clear clinical benefit Citation[137].
Postmarketing experience
In a retrospective, cross-sectional survey of routinely collected clinical information from 56 centers in the UK, data were reviewed from 1351 eligible patients with asthma who had been treated with montelukast Citation[138]. According to independent assessment, 66.4% of patients experienced an improvement in their asthma while on montelukast treatment. In these patients, montelukast produced clinically relevant decreases in asthma-related sleep disturbance (64.3%) and activity symptoms (63.9%). Interestingly, a relatively larger proportion of children presented with ‘much’ or ‘dramatically’ improved disease (41.3 vs 33.5% of adults). The largest proportion of ‘responders’ was seen among those with mild-to-moderate disease. In addition, 40.4% of patients were recorded as having coexisting rhinitis; among 211 patients for whom a response was recorded, 54.5% showed an improvement in rhinitis Citation[138].
Another observational post hoc study included 344 patients with asthma and rhinitis from Italy, Spain and Poland. Patients with mild persistent asthma and concomitant rhinitis treated with ICS/LABA (n = 163) or ICS alone (n = 181) received montelukast (10 mg once daily) as add-on treatment for 1 year. The outcome variables included the need for additional medication for asthma and/or rhinitis and the occurrence of severe asthma exacerbations during the year after initiation of montelukast add-on therapy. Overall, as compared with the previous year, during the add-montelukast treatment significant reductions were observed in most of the studied variables, including use of oral corticosteroids (from 13.1 to 2.3%), severe asthma exacerbations (from 27 to 5.5%) and emergency room visits (from 16.6 to 2.6%). There was no significant difference between the two treatment groups, indicating that montelukast was effective as add-on therapy independent of LABA Citation[139]. These observations were supported by the findings from a large Belgian open study including 5769 patients insufficiently controlled on ICS/LABA. Approximately 50% of the patients (n = 2442) were treated for concomitant rhinitis. Addition of montelukast significantly improved asthma-related quality of life and 82% of those with concomitant rhinitis reported improvement in their rhinitis symptoms Citation[140].
Safety & tolerability
Adults & adolescents (aged ∼15 years)
Overall, oral montelukast has demonstrated an excellent tolerability profile in placebo-controlled studies. In a meta-analysis of safety data among adults with asthma enrolled in ten randomized, controlled studies (Phase IIb/III), discontinuations due to AEs occurred in 73 out of 1955 patients (3.7%) treated with montelukast and 61 out of 1180 patients (5.2%) receiving placebo Citation[141]. In total, 1300 patients (66.5%) treated with montelukast and 841 patients (71.3%) receiving placebo reported an AE. There were no clinically relevant differences between the rates of individual AEs in each group . Similarly, in four long-term extension studies, 559 out of 698 adults (80.1%) receiving montelukast for up to 4.1 years reported an AE, compared with 186 out of 250 patients (74.4%) receiving beclomethasone Citation[141]. Even at doses of up to 200 mg, being substantially higher than the (maximum) marketed dose of 10 mg, montelukast neither increased the discontinuations nor the frequency of AEs in two studies of adult patients with asthma Citation[141]. A safety analysis of three large randomized, controlled studies of patients with seasonal AR further supports the favorable tolerability profile of montelukast in adults Citation[142]. There were no substantial differences in the reporting of individual AEs between groups receiving montelukast, loratadine or placebo.
Pregnancy
Montelukast has been classified as pregnancy category ‘B’. No teratogenicity was observed in animal studies using doses up to 100-times higher than the maximum recommended dose in adults Citation[204]. Although data on pregnancies occurring in patients taking montelukast during clinical studies did not show any toxicity Citation[141], so far, no adequate well-controlled studies in pregnant women have been conducted. Therefore, Merck Pharmaceutical maintains the Pregnancy Registry Program for Singulair (montelukast) Citation[205]. As of July 2006, data from 203 prospective records have identified eight major congenital anomalies Citation[204]. The birth prevalence of major structural anomalies in the Merck Registry was 3.5%, which is similar to the 3–4% rate in the general US population. One recent controlled epidemiological study compared perinatal outcomes among 96 women who took LTRAs (montelukast or zafirlukast) during pregnancy, with those of women who exclusively took short-acting β2-agonists (n = 122) and of women without asthma (n = 346). The study demonstrated that the use of LTRAs in pregnancy was not associated with a specific pattern of major structural anomalies in offspring, or a large risk of other adverse perinatal outcomes. However, results should be interpreted with caution because of limited sample sizes Citation[143].
Children (aged 6–14 years)
The safety profile of montelukast in children aged 6–14 years is similar to placebo and the adult safety profile. In the largest clinical study published to date, discontinuations due to AEs were seen in eight out of 201 patients (4.0%) treated with montelukast and three out of 135 patients (2.2%) receiving placebo, while 151 patients (75.1%) treated with montelukast and 102 patients (75.6%) receiving placebo reported an AE Citation[106]. Two studies compared montelukast with ICS and showed that montelukast did not affect growth over 12 months of therapy in either study. One study compared montelukast with inhaled fluticasone (100 µg twice daily) in children with mild asthma and showed that the growth rate over 12 months was significantly greater in the montelukast group (treatment difference = 0.41 cm/year; p = 0.018) Citation[118]. The second study compared montelukast with inhaled beclomethasone (200 µg twice daily) or placebo. The results showed that linear growth velocity in the beclomethasone group was significantly less than in the placebo group (difference = -0.78 cm/year; p < 0.001) or the montelukast group (0.81 cm/year; p < 0.001). The growth rate was similar for both montelukast and placebo Citation[144]. One recent study assessed the influence of montelukast on short-term lower leg growth rate (LLGR) in 71 prepubertal children with asthma. Children were randomized to one of two crossover arms, with two treatment sequences per arm: montelukast 5 mg once daily/placebo or inhaled dry powder budesonide 200 µg twice daily/placebo. Mean LLGR was similar between patients receiving montelukast and placebo treatments, showing no effect of montelukast on short-term leg growth rate in prepubertal children Citation[145].
Young children (aged ∼5 years)
The safety profile of montelukast in children aged approximately 5 years is generally similar to that in older children. In the largest published clinical study in children aged 2 to 5 years, 16 out of 461 patients (3.5%) treated with montelukast and seven out of 228 patients (3.1%) receiving placebo discontinued due to an AE Citation[126]. There were no clinically meaningful differences between the two groups in overall frequency of AEs; asthma, fever and URTI were the most commonly reported AEs. In a 6-month, placebo-controlled evaluation of 124 patients aged 12–23 months, montelukast was generally well tolerated Citation[203], while in a study of infants as young as 6 months old, 13 out of 32 patients (40.6%) treated with montelukast reported an AE; the most commonly reported AE was diarrhea, in six out of 32 patients (18.8%) Citation[46].
In the largest published clinical study in children 3–24 months of age, AEs leading to discontinuation from the study occurred in 2.8, 1.9 and 2.8%, for the placebo (n = 318), montelukast 4 mg (n = 315) and 8 mg (n = 319) groups, respectively Citation[128]. The safety profiles of both doses of montelukast (4 and 8 mg) assessed over period I (4 weeks) and II (20 weeks) were comparable to placebo. The percentage of patients who presented with one or more clinical AEs for the placebo, montelukast 4 and 8 mg group were 81.4, 84.1 and 82.1%, respectively; drug-related AEs were 4.1, 4.4 and 2.8%, respectively; and serious AEs were 16.0, 13.0 and 16.6%, respectively. The type of AEs and their occurence were similar to those of placebo. The five most frequent AEs in either dose group of montelukast included diarrhea, pyrexia, nasopharyngitis, rhinitis and upper respiratory infection, with similar percentages to those in the placebo group. Serious clinical adverse experiences in either dose group of montelukast were bronchiolitis, gastroenteritis, pneumonia, overdose and accidental overdose in percentages similar to those of placebo Citation[128].
Churg–Strauss syndrome
Some asthma medications, including LTRAs, have been associated with systemic eosinophilia, which has focused interest on the potential development of Churg–Strauss syndrome (CSS). In a combined analysis of 11 randomized, controlled (long-term) studies including four extension trials in adults and children, no patients experienced vasculitis or CSS Citation[141]. To date, approximately 25 million patients have received montelukast worldwide. Although postmarketing experience with the drug has identified rare case reports of eosinophilic conditions suggestive of CSS, the incidence of 3.7 cases per 100,000,000 is consistent with the incidence of CSS in the general asthmatic population Citation[141]. In a recent epidemiological study in which asthma patients who had not used an LTRA were identified (n = 36,230), the background incidence rate of definite CSS was in the range 0–67 per 1,000,000 person-years, depending on the definition used Citation[146].
Churg–Strauss syndrome generally occurs in adult patients with significant asthma histories who have received numerous asthma medications, often including systemic corticosteroids. Clinical signs of CSS in these patients usually begin or worsen during or following corticosteroid reduction, which in some cases was accomplished by adding LTRA. In these cases, therefore, LTRA unmasked rather than induced CSS, since a causal relationship between LTRA and eosinophilic conditions has not been established Citation[141]. In a recent case crossover study in 78 patients taking montelukast, there was a 2.8-fold increased chance to develop CSS within 3 months of therapy. This figure should be compared with a 3.6-fold increased chance after adding LABA or fourfold increased chance after adding oral corticosteroids. Only the latter reached statistical significance. The authors conclude that the risk for developing CSS was mainly associated with a gradual increase in asthma therapy as the disease progressed to a more advanced stage Citation[147]. At present, there are no substantial data supporting a link between montelukast therapy and CSS.
Neuropsychiatric events
During the last few years, postmarketing surveillance has revealed rare but possibly drug-related side effects, including anxiousness, depression and suicidality (suicidal thinking and behavior). This has been communicated to the US FDA, resulting in a subsequent statement of a possible association between the use of leukotriene modifiers and neuropsychiatric conditions Citation[148]. The company performed a careful analysis of all data available from clinical trials with montelukast as a test drug and could not identify any signs of increased neuropsychiatric side effects associated with the use of montelukast. In a company-independent analysis of three clinical trials including 1469 patients of whom 569 were treated with montelukast, follow-up with quality of life including scales of emotional wellbeing did not reveal any negative effect on emotional status Citation[149]. However, strictly selected clinical trial patients may not fully represent the patients treated in ‘real life’. In addition, patients with asthma are known to have a higher prevalence of both anxiety and depressions, more prevalent with more severe disease Citation[150] and idiosyncratic reaction to montelukast cannot be fully excluded.
Conclusion
There is growing evidence for a systemic nature of asthma, which has increased the need for systemic treatments that are both effective and safe. Successful treatment with montelukast of asthmatics with concomitant AR has confirmed a link between these inflammatory conditions of the lower and upper airways. CysLTs are produced in increased amounts only during disease, making them disease-specific mediators that are preferably blocked through the systemic route. Asthmatic children can be particularly challenging to treat. In this respect, many studies have shown relevant improvements in CysLT-driven asthma-related conditions, including EIB and viral-induced asthma.
Finally, montelukast possesses an excellent safety profile in patients of all ages and its oral formulation may offer better adherence to therapy compared with inhaled medications.
Expert commentary
The LTRA montelukast differs from other classes of pharmacological treatments of asthma by its targeted mechanism of action that acts throughout the entire bronchial tree: that is, from the upper airways down into the small airways. Currently, it is recognized that histamine (upper airway) and the CysLTs (upper and lower airways) are integral factors in the disease process of AR and asthma Citation[3]. CysLTs are known to be more related to mast cell-induced inflammation, an important and integral part of the inflammation also occurring in the small airways with clinical correlates such as nocturnal asthma, EIB and asthma exacerbations Citation[4].
Treating asthma is far from the ‘one size fits all’ concept and future drug development will be more phenotype-driven focusing on individual responses and treatment outcomes Citation[151]. It is also clear that inhaled corticosteroid therapy does not cover all aspects of the asthmatic inflammation. Thus, future approaches to asthma treatment are likely to include combinations of targets in the pathophysiological processes across the different phenotypes. For example, a synergistic effect between montelukast and an anti-thromboxane agent has recently been demonstrated in a human lung slice model Citation[152]. Furthermore, there is increasing evidence of the roles of multiple cytokines in orchestrating and perpetuating the inflammation in asthma, leading to the evaluation of novel anticytokine therapies. In addition, targeting the cell surface adhesion molecules involved in the trafficking of white blood cells to sites of inflammation may represent an alternative target of novel anti-inflammatory drugs. Further exploration of these novel pharmacological targets and careful timing of treatments is likely to be an important intervention to effectively prevent lung function decline associated with asthma.
Five-year view
To our belief, the rising trend in the treatment of asthma is based on ideas presented in the recently published ‘Brussel’s declaration’ Citation[151]. Treatment guidelines based on mean data from strictly selected patient populations will be more oriented towards a ‘real-life population’ with different, specified phenotypes. Children are not ‘small adults’ and new treatment modalities need to address specific pediatric aspects and needs. The systemic nature of the disease and the involvement of the entire respiratory tract from the nose to the small peripheral airways represent specific challenges and favor the use of a systemic treatment, targeting disease-specific mechanisms.
Phenotyping is a recent multifaceted approach aimed at linking distinct asthma entities to ‘customized’ treatment. Many adults diagnosed with severe disease appear to have poor asthma control rather than treatment-resistant disease Citation[152]. The main reasons for poor asthma control are decreased compliance, untreated comorbidities (mainly chronic rhinosinusitis) and environmental factors (airway pollution, cigarette smoke and allergens). Rhinosinusitis usually results from neglected, untreated rhinitis. Montelukast can treat rhinitis, prevent its progression to sinusitis and can result in better treatment compliance compared with ICS. Montelukast-responsive asthma phenotypes are characterized by an exercise-induced disease component, aspirin-induced asthma and asthma with concomitant AR.
Traditionally, phenotypes have been mainly defined by clinical and pathophysiological characteristics. In the future, we will need to combine clinical, pathophysiological, epidemiological and immunological aspects. This approach has proven its applicability in unraveling the complexity and heterogeneity of more severe asthma Citation[153]. In this manner, the following subtypes of severe asthma have been identified: ‘symptom predominant/early onset’, ‘noneosinophilic type’, ‘early-onset atopic’ and ‘inflammation predominant’ (eosinophilic) Citation[153]. Based on sputum inflammatory cells, patients with an inflammation predominant phenotype reached better disease control on high doses of ICS, while the noneosinophilic and the symptom-predominant phenotypes improved despite a decrease in ICS treatment. In children, asthma phenotypes defined by low inflammatory markers, virally triggered and/or a short duration of disease, appeared well-responsive to montelukast Citation[125,137]. By contrast, ICS are the drug of choice for phenotypes with high inflammatory markers and multi-triggered disease Citation[125,137]. Thus, careful phenotyping, as part of customized therapy, will eventually replace the traditional one size fits all therapeutic approach. In addition, it may also help to reveal new underlying mechanisms and unmet therapeutic needs.
Information resources
• GINA guidelines. www.ginasthma.com
• Pediatric GINA guidelines, updated 2009, in press. www.ginasthma.org
• ARIA guidelines. www.whiar.org
• Merck prescribing information. www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf
Table 1. Adverse events occurring in 3% or more of patients regardless of relationship to study medication, in a pooled analysis of ten Phase IIb/III trials.
Key issues
• Asthma is a systemic, inflammatory disorder, in close association with allergic rhinitis (‘AsthmaRhinitis’).
• AsthmaRhinitis should be treated and handled as one clinical entity in order to achieve optimal disease control.
• Cysteinyl leukotrienes (CysLTs) play an important role in the pathophysiology of asthma and rhinitis.
• Montelukast represents an oral anti-asthma drug that targets leukotriene activity at the CysLT1 receptor with dual effects: both on airway smooth muscle cells (resulting in modest bronchodilator effect) and on inflammatory process (resulting in an anti-inflammatory effect).
• Clinical efficacy of montelukast is achieved by virtue of its bronchoprotective and anti-inflammatory effects.
• Montelukast is ‘double complementary’ to inhaled corticosteroids: having both add-on anti-inflammatory properties and acting systemically.
• Numerous randomized, controlled studies support the efficacy and tolerability of montelukast in adults and children with asthma (with or without concomitant allergic rhinitis), exercise-induced and viral-induced asthma.
• Small airways are important in all stages of asthma severity and controlling their inflammation is an important key to asthma control.
• Clinical correlates to small airway inflammation are exercise-induced asthma, nocturnal asthma and risk for asthma exacerbations; these clinical ‘markers’ are known to respond to leukotriene receptor antagonist treatment.
• Follow-up data from long-term treatment studies are eagerly awaited to determine whether montelukast has any clinically relevant effects on airway remodeling.
• In preschool children who wheeze during recurrent viral episodes and who are asymptomatic between episodes (episodic or viral wheezing phenotype), a leukotriene modifier, montelukast, is the treatment of choice and can be started when symptoms of viral cold develop.
• In asthmatic children aged 5 years or younger, low-dose inhaled corticosteroids and/or a leukotriene modifier, such as montelukast, are the first choice in controller therapy (GINA 2009).
• Inhaled corticosteroids and montelukast may be considered on a trial basis (for at least 2–3 months) in almost any preschool child with recurrent wheeze, but should be discontinued if there is no clear clinical benefit.
Acknowledgements
The contents of this manuscript have been supported by our European colleagues: D Cataldo, C Gratziou, M Mei-Zahav, H Kurz, JM Lopes dos Santos, A Möller, M Morais de Almeida and W Pohl.
Financial & competing interests disclosure
Leif Bjermer has received lecture fees from AstraZeneca, GlaxoSmithKline (GSK), Merck, Pfizer and UCB during the last 2 years. He is a member of a national advisory board for AstraZeneca and GSK, and for the international board of Merck and UCB pharma. He runs a clinical trial unit that has received grants to perform Phase II trials from AIrsonette, AstraZeneca, Boehringer, GSK, Novartis and Pfizer. Zuzana Diamant worked from 2004 to 2009 as the Research Director of Respiratory and Allergy at the Centre for Human Drug Research in Leiden, The Netherlands, and as such she participated on advisory boards (Actelion, Movetis, Merck Sharp and Dohme), and was principle investigator on several clinical trials of asthma, a.o. Sanofi-Aventis, Actelion, Merck Sharp and Dohme, Centocor, Veronapharma and GSK. Eva Mantzoranis has participated as the principle investigator in several clinical trials sponsored by GSK, AstraZeneca, Wyeth, Merck Sharp and Dhome, and Roche. She has not received fees for lectures and declares no conflict of interest with any pharmaceutical company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy59(5), 469–478 (2004).
- Bjermer L. History and future perspectives of treating asthma as a systemic and small airways disease. Respir. Med.95(9), 703–719 (2001).
- Bousquet J, Khaltaev N, Cruz AA et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy63(Suppl. 86), 8–160 (2008).
- Bjermer L. Time for a paradigm shift in asthma treatment: from relieving bronchospasm to controlling systemic inflammation. J. Allergy Clin. Immunol.120(6), 1269–1275 (2007).
- Kyllönen H, Malmberg P, Remitz A et al. Respiratory symptoms, bronchial hyper-responsiveness, and eosinophilic airway inflammation in patients with moderate-to-severe atopic dermatitis. Clin. Exp. Allergy36(2), 192–197 (2006).
- Ten Brinke A, Ouwerkerk ME, Zwinderman AH, Spinhoven P, Bel EH. Psychopathology in patients with severe asthma is associated with increased health care utilization. Am. J. Respir. Crit. Care Med.163(5), 1093–1096 (2001).
- Leynaert B, Bousquet J, Neukirch C, Liard R, Neukirch F. Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J. Allergy Clin. Immunol.104(2 Pt 1), 301–304 (1999).
- Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J. Allergy Clin. Immunol.109(3), 419–425 (2002).
- Greisner WA, Settipane RJ, Settipane GA. Co-existence of asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Asthma Proc.19(4), 185–188 (1998).
- Bousquet J, Gaugris S, Kocevar VS et al. Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: a subgroup analysis of the investigation of montelukast as a partner agent for complementary therapy corrected. Clin. Exp. Allergy35(6), 723–727 (2005).
- Ponte EV, Franco R, Nascimento HF et al. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy63(5), 564–569 (2008).
- Chanez P, Vignola AM, Vic P et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am. J. Respir. Crit. Care Med.159(2), 588–595 (1999).
- Gratziou C, Rovina N, Lignos M, Vogiatzis I, Roussos C. Exhaled nitric oxide in seasonal allergic rhinitis: influence of pollen season and therapy. Clin. Exp. Allergy31(3), 409–416 (2001).
- de Benedictis FM, Vaccher S, de Benedictis D. Montelukast sodium for exercise-induced asthma. Drugs Today (Barc.)44(11), 845–855 (2008).
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med.346(22), 1699–1705 (2002).
- Balzar S, Chu HW, Strand M, Wenzel S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am. J. Respir. Crit. Care Med.171(5), 431–439 (2005).
- Dolhnikoff M, da Silva LF, de Araujo BB et al. The outer wall of small airways is a major site of remodeling in fatal asthma. J. Allergy Clin. Immunol. (2009) (Epub ahead of print).
- Diamant Z, Boot JD, Virchow JC. Summing up 100 years of asthma. Respir. Med.101(3), 378–388 (2007).
- Bateman ED, Boushey HA, Bousquet J et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am. J. Respir. Crit. Care Med.170(8), 836–844 (2004).
- Bourdin A, Paganin F, Préfaut C, Kieseler D, Godard P, Chanez P. Nitrogen washout slope in poorly controlled asthma. Allergy61(1), 85–89 (2006).
- Dworski R, Fitzgerald GA, Oates JA, Sheller JR. Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am. J. Respir. Crit. Care Med.149(4 Pt 1), 953–959 (1994).
- Pavord ID, Ward R, Woltmann G, Wardlaw AJ, Sheller JR, Dworski R. Induced sputum eicosanoid concentrations in asthma. Am. J. Respir. Crit. Care Med.160(6), 1905–1909 (1999).
- Feldberg W, Kellaway CH. Liberation of histamine and formation of lyscithin-like substances by cobra venom. J. Physiol.94, 187–226 (1938).
- Kellaway CH, Trethewie ER. The liberation of a slow reacting smooth-muscle stimulating substance in anaphylaxis. Q. J. Exp. Physiol.30(2), 121–145 (1940).
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J. Immunol.173(3), 1503–1510 (2004).
- Peres CM, Aronoff DM, Serezani CH, Flamand N, Faccioli LH, Peters-Golden M. Specific leukotriene receptors couple to distinct G proteins to effect stimulation of alveolar macrophage host defense functions. J. Immunol.179(8), 5454–5461 (2007).
- Lynch KR, O’Neill GP, Liu Q et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature399(6738), 789–793 (1999).
- Sarau HM, Ames RS, Chambers J et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol. Pharmacol.56(3), 657–663 (1999).
- Hay DW. Pharmacology of leukotriene receptor antagonists. More than inhibitors of bronchoconstriction. Chest111(2 Suppl.), 35S–45S (1997).
- Yang G, Haczku A, Chen H et al. Transgenic smooth muscle expression of the human CysLT1 receptor induces enhanced responsiveness of murine airways to leukotriene D4. Am. J. Physiol. Lung Cell Mol. Physiol.286(5), L992–L1001 (2004).
- Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet341(8851), 989–990 (1993).
- Diamant Z, Hiltermann JT, van Rensen EL et al. The effect of inhaled leukotriene D4 and methacholine on sputum cell differentials in asthma. Am. J. Respir. Crit. Care Med.155(4), 1247–1253 (1997).
- Altraja S, Kadai M, Rekker E, Altraja A. Synthesis of tenascin and laminin beta2 chain in human bronchial epithelial cells is enhanced by cysteinyl leukotrienes via CysLT1 receptor. Respir. Res.9, 44 (2008).
- Abdulamir AS, Hafidh RR, Abubakar F. Different inflammatory mechanisms in lungs of severe and mild asthma: crosstalk of NF-κ-B, TGFβ1, Bax, Bcl-2, IL-4 and IgE. Scand. J. Clin. Lab. Invest. (2009) (Epub ahead of print).
- Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am. Rev. Respir. Dis.142(1), 112–119 (1990).
- Diamant Z, Sampson AP. Anti-inflammatory mechanisms of leukotriene modulators. Clin. Exp. Allergy29(11), 1449–1453 (1999).
- Georgitis JW, Matthews BL, Stone B. Chronic sinusitis: characterization of cellular influx and inflammatory mediators in sinus lavage fluid. Int. Arch. Allergy Immunol.106(4), 416–421 (1995).
- Pawankar R, Zernotti ME. Rhinosinusitis in children and asthma severity. Curr. Opin. Allergy Clin. Immunol.9(2), 151–153 (2009).
- Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics108(2), E33 (2001).
- Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J. Allergy Clin. Immunol.98(6 Pt 1), 1051–1057 (1996).
- Jackson DJ, Gangnon RE, Evans MD et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med.178(7), 667–672 (2008).
- Kusel MM, de Klerk NH, Kebadze T et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol.119(5), 1105–1110 (2007).
- Piedimonte G, Renzetti G, Auais A et al. Leukotriene synthesis during respiratory syncytial virus bronchiolitis: influence of age and atopy. Pediatr. Pulmonol.40, 285–291 (2005).
- De Lepeleire I, Reiss TF, Rochette F et al. Montelukast causes prolonged, potent leukotriene D4-receptor antagonism in the airways of patients with asthma. Clin. Pharmacol. Ther.61(1), 83–92 (1997).
- Reiss TF, Sorkness CA, Stricker W et al. Effects of montelukast (MK-0476); a potent cysteinyl leukotriene receptor antagonist, on bronchodilation in asthmatic subjects treated with and without inhaled corticosteroids. Thorax52(1), 45–48 (1997).
- Kelly MM, Chakir J, Vethanayagam D et al. Montelukast treatment attenuates the increase in myofibroblasts following low-dose allergen challenge. Chest130(3), 741–753 (2006).
- Migoya E, Kearns GL, Hartford A et al. Pharmacokinetics of montelukast in asthmatic patients 6 to 24 months old. J. Clin. Pharmacol.44(5), 487–494 (2004).
- Sampson AP, Diamant Z. Monograph on montelukast. J. Drug Evaluation1(2), 53–88 (2002).
- Muijsers RB, Noble S. Spotlight on montelukast in asthma in children 2 to 14 years of age. Am. J. Respir. Med.1(3), 225–228 (2002).
- Chiba M, Xu X, Nishime JA, Balani SK, Lin JH. Hepatic microsomal metabolism of montelukast, a potent leukotriene D4 receptor antagonist, in humans. Drug Metab. Dispos.25(9), 1022–1031 (1997).
- Ravasi S, Capra V, Panigalli T, Rovati GE, Nicosia S. Pharmacological differences among CysLT(1) receptor antagonists with respect to LTC(4) and LTD(4) in human lung parenchyma. Biochem. Pharmacol.63(8), 1537–1546 (2002).
- Jones TR, Labelle M, Belley M et al. Pharmacology of montelukast sodium (Singulair), a potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol.73(2), 191–201 (1995).
- Dahlén SE, Malmström K, Nizankowska E et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am. J. Respir. Crit. Care Med.165(1), 9–14 (2002).
- Diamant Z, Grootendorst DC, Veselic-Charvat M et al. The effect of montelukast (MK-0476), a cysteinyl leukotriene receptor antagonist, on allergen-induced airway responses and sputum cell counts in asthma. Clin. Exp. Allergy29(1), 42–51 (1999).
- Leff JA, Busse WW, Pearlman D et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N. Engl. J. Med.339(3), 147–152 (1998).
- Villaran C, O’Neill SJ, Helbling A et al. Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J. Allergy Clin. Immunol.104(3 Pt 1), 547–553 (1999).
- Edelman JM, Turpin JA, Bronsky EA et al. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann. Intern. Med.132(2), 97–104 (2000).
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respir. Med.98(11), 1051–1062 (2004).
- Pizzichini E, Leff JA, Reiss TF et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur. Respir. J.14(1), 12–18 (1999).
- Jayaram L, Pizzichini E, Lemière C et al. Steroid naive eosinophilic asthma: anti-inflammatory effects of fluticasone and montelukast. Thorax60(2), 100–105 (2005).
- Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet357(9273), 2007–2011 (2001).
- Bjermer L, Bisgaard H, Bousquet J et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ327(7420), 891 (2003).
- Braccioni F, Dorman SC, O’Byrne PM et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J. Allergy Clin. Immunol.110(1), 96–101 (2002).
- Ghiro L, Zanconato S, Rampon O, Piovan V, Pasquale MF, Baraldi E. Effect of montelukast added to inhaled corticosteroids on fractional exhaled nitric oxide in asthmatic children. Eur. Respir. J.20(3), 630–634 (2002).
- Wahn U, Dass SB. Review of recent results of montelukast use as a monotherapy in children with mild asthma. Clin. Ther.30, 1026–1035 (2008).
- Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J. Allergy Clin. Immunol.111(Suppl. 1), S18–S34 (2003).
- Walters EH, Reid DW, Johns DP, Ward C. Nonpharmacological and pharmacological interventions to prevent or reduce airway remodelling. Eur. Respir. J.30(3), 574–588 (2007).
- Riccioni G, Vecchia RD, D’Orazio N, Sensi S, Guagnano MT. Comparison of montelukast and budesonide on bronchial reactivity in subjects with mild–moderate persistent asthma. Pulm. Pharmacol. Ther.16(2), 111–114 (2003).
- Zeidler MR, Kleerup EC, Goldin JG et al. Montelukast improves regional air-trapping due to small airways obstruction in asthma. Eur. Respir. J.27(2), 307–315 (2006).
- Stelmach I, Grzelewski T, Bobrowska-Korzeniowska M, Stelmach P, Kuna P. A randomized, double-blind trial of the effect of anti-asthma treatment on lung function in children with asthma. Pulm. Pharmacol. Ther.20(6), 691–700 (2007).
- Fritscher LG, Rodrigues MT, Zamel N, Chapman KR. The effect of montelukast on exhaled nitric oxide of alveolar and bronchial origin in inhaled corticosteroid-treated asthma. Respir. Med.103(2), 296–300 (2009).
- Currie GP, McLaughlin K. The expanding role of leukotriene receptor antagonists in chronic asthma. Ann. Allergy Asthma Immunol.97(6), 731–741 (2006).
- Bjermer L, Diamant Z. Complementary therapy in asthma: inhaled corticosteroids and what? Curr. Opin. Pulm. Med.15(1), 46–51 (2009).
- Kraft M, Cairns CB, Ellison MC, Pak J, Irvin C, Wenzel S. Improvements in distal lung function correlate with asthma symptoms after treatment with oral montelukast. Chest130(6), 1726–1732 (2006).
- Rosi E, Ronchi MC, Grazzini M, Duranti R, Scano G. Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J. Allergy Clin. Immunol.103(2 Pt 1), 232–237 (1999).
- Kraft M, Pak J, Martin RJ, Kaminsky D, Irvin CG. Distal lung dysfunction at night in nocturnal asthma. Am. J. Respir. Crit. Care Med.163(7), 1551–1556 (2001).
- Boot JD, Panzner P, Diamant Z. A critical appraisal of methods used in early clinical development of novel drugs for the treatment of asthma. Pulm. Pharmacol. Ther.20(3), 201–219 (2007).
- Boot JD, Chandoesing P, de Kam ML et al. Applicability and reproducibility of biomarkers for the evaluation of anti-inflammatory therapy in allergic rhinitis. J. Invest. Allergol. Clin. Immunol.18(6), 433–442 (2008).
- Noonan MJ, Chervinsky P, Brandon M et al. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Montelukast Asthma Study Group. Eur. Respir. J.11(6), 1232–1239 (1998).
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch. Intern. Med.158(11), 1213–1220 (1998).
- Malmstrom K, Rodriguez-Gomez G, Guerra J et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann. Intern. Med.130(6), 487–495 (1999).
- Israel E, Chervinsky PS, Friedman B et al. Effects of montelukast and beclomethasone on airway function and asthma control. J. Allergy Clin. Immunol.110(6), 847–854 (2002).
- Baumgartner RA, Martinez G, Edelman JM et al. Distribution of therapeutic response in asthma control between oral montelukast and inhaled beclomethasone. Eur. Respir. J.21(1), 123–128 (2003).
- Kanniess F, Richter K, Böhme S, Jörres RA, Magnussen H. Montelukast versus fluticasone: effects on lung function, airway responsiveness and inflammation in moderate asthma. Eur. Respir. J.20(4), 853–858 (2002).
- Zeiger RS, Szefler SJ, Phillips BR et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J. Allergy Clin. Immunol.117(1), 45–52 (2006).
- Vaquerizo MJ, Casan P, Castillo J et al. Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax58(3), 204–210 (2003).
- Price DB, Hernandez D, Magyar P et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax58(3), 211–216 (2003).
- Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy61(6), 737–742 (2006).
- Kaminsky DA, Bates JH, Irvin CG. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am. J. Respir. Crit. Care Med.162(1), 179–186 (2000).
- Steinshamn S, Sandsund M, Sue-Chu M, Bjermer L. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest126(4), 1154–1160 (2004).
- Meltzer EO. Clinical evidence for antileukotriene therapy in the management of allergic rhinitis. Ann. Allergy Asthma Immunol.88(4 Suppl. 1), 23–29 (2002).
- Wilson AM, Orr LC, Coutie WJ, Sims EJ, Lipworth BJ. A comparison of once daily fexofenadine versus the combination of montelukast plus loratadine on domiciliary nasal peak flow and symptoms in seasonal allergic rhinitis. Clin. Exp. Allergy32(1), 126–132 (2002).
- Nayak AS, Philip G, Lu S, Malice MP, Reiss TF. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann. Allergy Asthma Immunol.88(6), 592–600 (2002).
- Philip G, Nayak AS, Berger WE et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr. Med. Res. Opin.20(10), 1549–1558 (2004).
- Grossman J. One airway, one disease. Chest111(2 Suppl.), 11S–16S (1997).
- Rönmark E, Lindberg A, Watson L, Lundbäck B. Outcome and severity of adult onset asthma – report from the obstructive lung disease in northern Sweden studies (OLIN). Respir. Med.101(11), 2370–2377 (2007).
- Murray CS. Can inhaled corticosteroids influence the natural history of asthma? Curr. Opin. Allergy Clin. Immunol.8(1), 77–81 (2008).
- Selroos O. Effect of disease duration on dose-response of inhaled budesonide in asthma. Respir. Med.102(7), 1065–1072 (2008).
- Berry M, Hargadon B, Morgan A et al. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur. Respir. J.25(6), 986–991 (2005).
- Kato J, Kohyama T, Okazaki H et al. Leukotriene D4 potentiates fibronectin-induced migration of human lung fibroblasts. Clin. Immunol.117(2), 177–181 (2005).
- Panettieri RA, Tan EM, Ciocca V, Luttmann MA, Leonard TB, Hay DW. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction in vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am. J. Respir. Cell. Mol. Biol.19(3), 453–461 (1998).
- Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S-expressing cells in aspirin-tolerant asthma. Am. J. Respir. Cell. Mol. Biol.29(6), 683–693 (2003).
- Henderson WR, Tang LO, Chu SJ et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am. J. Respir. Crit. Care Med.165(1), 108–116 (2002).
- Henderson WR, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am. J. Respir. Crit. Care Med.173(7), 718–728 (2006).
- Pifferi M, Caramella D, Ragazzo V, De Marco E, Pietrobelli A, Boner AL. Montelukast and airway remodeling in children with chronic persistent asthma: an open study. Pediatr. Allergy Immunol.15(5), 472–473 (2004).
- Knorr B, Matz J, Bernstein JA et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA279(15), 1181–1186 (1998).
- Becker A, Swern A, Tozzi CA, Yu Q, Reiss T, Knorr B. Montelukast in asthmatic patients 6 years-14 years old with an FEV1 > 75%. Curr. Med. Res. Opin.20(10), 1651–1659 (2004).
- Simons FE, Villa JR, Lee BW et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J. Pediatr.138(5), 694–698 (2001).
- Karaman O, Sünneli L, Uzuner N et al. Evaluation of montelukast in 8 to 14 year old children with mild persistent asthma and compared with inhaled corticosteroids. Allergol. Immunopathol. (Madr.)32(1), 21–27 (2004).
- Taylor IK, Wellings R, Taylor GW, Fuller RW. Urinary leukotriene E4 excretion in exercise-induced asthma. J. Appl. Physiol.73(2), 743–748 (1992).
- Carraro S, Corradi M, Zanconato S et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J. Allergy Clin. Immunol.115(4), 764–770 (2005).
- Peroni DG, Piacentini GL, Pietrobelli A et al. The combination of single-dose montelukast and loratadine on exercise-induced bronchospasm in children. Eur. Respir. J.20(1), 104–107 (2002).
- Melo RE, Solé D, Naspitz CK. Exercise-induced bronchoconstriction in children: montelukast attenuates the immediate-phase and late-phase responses. J. Allergy Clin. Immunol.111(2), 301–307 (2003).
- Kim JH, Lee SY, Kim HB et al. Prolonged effect of montelukast in asthmatic children with exercise-induced bronchoconstriction. Pediatr. Pulmonol.39(2), 162–166 (2005).
- de Benedictis FM, del Giudice MM, Forenza N, Decimo F, de Benedictis D, Capristo A. Lack of tolerance to the protective effect of montelukast in exercise-induced bronchoconstriction in children. Eur. Respir. J.28(2), 291–295 (2006).
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J. Allergy Clin. Immunol.121(2), 383–389 (2008).
- Phipatanakul W, Greene C, Downes SJ et al. Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Ann. Allergy Asthma Immunol.91(1), 49–54 (2003).
- Garcia Garcia ML, Wahn U, Gilles L, Swern A, Tozzi CA, Polos P. Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma: the MOSAIC study. Pediatrics116(2), 360–369 (2005).
- Kumar V, Ramesh P, Lodha R, Pandey RM, Kabra SK. Montelukast vs. inhaled low-dose budesonide as monotherapy in the treatment of mild persistent asthma: a randomized double blind controlled trial. J. Trop. Pediatr.53(5), 325–330 (2007).
- Spahn JD, Covar RA, Jain N et al. Effect of montelukast on peripheral airflow obstruction in children with asthma. Ann. Allergy Asthma Immunol.96(4), 541–549 (2006).
- Nieto A, Pamies R, Oliver F, Medina A, Caballero L, Mazon A. Montelukast improves pulmonary function measured by impulse oscillometry in children with asthma (Mio study). Respir. Med.100(7), 1180–1185 (2006).
- Verini M, Peroni DG, Piacentini GL et al. Comparison of add-on therapy to inhaled fluticasone propionate in children with asthma: residual volume and exhaled nitric oxide as outcome measures. Allergy Asthma Proc.28(6), 691–694 (2007).
- Miraglia del Giudice M, Piacentini GL, Capasso M et al. Formoterol, montelukast, and budesonide in asthmatic children: effect on lung function and exhaled nitric oxide. Respir. Med.101(8), 1809–1813 (2007).
- Rabinovitch N, Strand M, Stuhlman K, Gelfand EW. Exposure to tobacco smoke increases leukotriene E4-related albuterol usage and response to montelukast. J. Allergy Clin. Immunol.121(6), 1365–1371 (2008).
- Szefler SJ, Phillips BR, Martinez FD et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J. Allergy Clin. Immunol.115(2), 233–242 (2005).
- Knorr B, Franchi LM, Bisgaard H et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics108(3), E48 (2001).
- Bisgaard H, Zielen S, Garcia-Garcia ML et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am. J. Respir. Crit. Care Med.171(4), 315–322 (2005).
- Bisgaard H, Flores-Nunez A, Goh A et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am. J. Respir. Crit. Care Med.178(8), 854–860 (2008).
- Davies GM, Dasbach EJ, Santanello NC, Knorr BA, Bratton DL. The effect of montelukast versus usual care on health care resource utilization in children aged 2 to 5 years with asthma. Clin. Ther.26(11), 1895–1904 (2004).
- Kooi EM, Schokker S, Marike Boezen H et al. Fluticasone or montelukast for preschool children with asthma-like symptoms: randomized controlled trial. Pulm. Pharmacol. Ther.21(5), 798–804 (2008).
- Straub DA, Moeller A, Minocchieri S et al. The effect of montelukast on lung function and exhaled nitric oxide in infants with early childhood asthma. Eur. Respir. J.25(2), 289–294 (2005).
- Hakim F, Vilozni D, Adler A, Livnat G, Tal A, Bentur L. The effect of montelukast on bronchial hyperreactivity in preschool children. Chest131(1), 180–186 (2007).
- Robertson CF, Price D, Henry R et al. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am. J. Respir. Crit. Care Med.175(4), 323–329 (2007).
- Johnston NW, Mandhane PJ, Dai J et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics120(3), e702–e712 (2007).
- Amirav I, Luder AS, Kruger N et al. A double-blind, placebo-controlled, randomized trial of montelukast for acute bronchiolitis. Pediatrics122(6), e1249–e1255 (2008).
- Bacharier LB, Phillips BR, Zeiger RS et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J. Allergy Clin. Immunol.122(6), 1127–1135.e8 (2008).
- Brand PL, Baraldi E, Bisgaard H et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur. Respir. J.32(4), 1096–1110 (2008).
- Barnes N, Thomas M, Price D, Tate H. The national montelukast survey. J. Allergy Clin. Immunol.115(1), 47–54 (2005).
- Dal Negro RW, Borderias L, Zhang Q et al. Rates of asthma attacks in patients with previously inadequately controlled mild asthma treated in clinical practice with combination drug therapy: an exploratory post-hoc analysis. BMC Pulm. Med.9, 10 (2009).
- Korn D, Van den Brande P, Potvin E, Dramaix M, Herbots E, Peché R. Efficacy of add-on montelukast in patients with non-controlled asthma: a Belgian open-label study. Curr. Med. Res. Opin.25(2), 489–497 (2009).
- Storms W, Michele TM, Knorr B et al. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clin. Exp. Allergy31(1), 77–87 (2001).
- Chervinsky P, Philip G, Malice MP et al. Montelukast for treating fall allergic rhinitis: effect of pollen exposure in 3 studies. Ann. Allergy Asthma Immunol.92(3), 367–373 (2004).
- Bakhireva LN, Jones KL, Schatz M et al. Safety of leukotriene receptor antagonists in pregnancy. J. Allergy Clin. Immunol.119(3), 618–625 (2007).
- Becker AB, Kuznetsova O, Vermeulen J et al. Linear growth in prepubertal asthmatic children treated with montelukast, beclomethasone, or placebo: a 56-week randomized double-blind study. Ann. Allergy Asthma Immunol.96(6), 800–807 (2006).
- Pedersen S, Agertoft L, Williams-Herman D et al. Placebo-controlled study of montelukast and budesonide on short-term growth in prepubertal asthmatic children. Pediatr. Pulmonol.42(9), 838–843 (2007).
- Loughlin JE, Cole JA, Rothman KJ, Johnson ES. Prevalence of serious eosinophilia and incidence of Churg–Strauss syndrome in a cohort of asthma patients. Ann. Allergy Asthma Immunol.88(3), 319–325 (2002).
- Hauser T, Mahr A, Metzler C et al. The leucotriene receptor antagonist montelukast and the risk of Churg-Strauss syndrome: a case-crossover study. Thorax63(8), 677–682 (2008).
- Holbrook JT, Harik-Khan R. Montelukast and emotional well-being as a marker for depression: results from 3 randomized, double-masked clinical trials. J. Allergy Clin. Immunol.122(4), 828–829 (2008).
- Strine TW, Mokdad AH, Balluz LS, Berry JT, Gonzalez O. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J. Asthma45(2), 123–133 (2008).
- Holgate S, Bisgaard H, Bjermer L et al. The Brussels Declaration: the need for change in asthma management. Eur. Respir. J.32(6), 1433–1442 (2008).
- Wohlsen A, Martin C, Vollmer E et al. The early allergic response in small airways of human precision-cut lung slices. Eur. Respir. J.21(6), 1024–1032 (2003).
- ten Brinke A, Sterk PJ, Masclee AA et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur. Respir. J.26(5), 812–818 (2005).
- Haldar P, Pavord ID, Shaw DE et al. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med.178(3), 218–224 (2008).
Websites
- NAEPP (National Asthma Education and Preventive Program). Expert Panel Report 3: Guidelines for the diagnosis and management of asthma www.nhlbi.nih.gov/guidelines/asthma/index.htm
- GINA. Global Initiative for Asthma – Guidelines www.ginasthma.org
- Merck & Co. Inc. Singulair® Prescribing Information www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf
- Merck Pregnancy Registry Program. Eighth annual report on exposure during pregnancy from the Merck Pregnancy Registry for SINGULAIR (montelukast sodium): covering the period from US approval (February 20, 1998) through July 31 2006. North Wales (PA) www.merckpregnancyregistries.com
- Merck Pregnancy Registry Program. Seventh annual report on exposure during pregnancy from the Merck Pregnancy Registry for SINGULAIR (montelukast sodium). North Wales (PA), 2005 www.merckpregnancyregistries.com
- FDA communication Link.