Abstract
Spitz nevus was first described in 1948 by Dr Sophie Spitz, who termed it juvenile melanoma based on its striking cytologic resemblance to melanoma, being composed of large epithelioid and spindle cells, and its prevalence among children. Several changes in terminology have since occurred with increased knowledge and experience, particularly regarding its occurrence in adult populations and rather indolent clinical behavior. Nevertheless, the distinction of Spitz nevus from melanoma still remains a significant problem in dermatopathology. Most classic examples of Spitz nevi can be easily distinguished histopathologically from melanoma, although lesions with unusual or atypical features still pose diagnostic difficulties, particularly in small or partial samples. In general, poor interobserver diagnostic concordance is not uncommon. Herein, we review the histopathological features of Spitz nevi, including common and recently reported variants, and spitzoid melanoma, and discuss recent advances in ancillary studies that may prove useful in their diagnosis.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscapecme.com/journal/expertderm; (4) view/print certificate.
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe clinical features and classification of Spitz nevi.
• Describe histopathologic features distinguishing Spitz nevi from atypical Spitz tumor and spitzoid melanoma.
• Describe immunohistochemistry and other ancillary studies that may help differentiate these three lesion types.
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD, Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay has disclosed no relevant financial relationships.
AUTHOR
Stephen H Olsen, MD,Dermatopathology Section, Department of Pathology, University of Michigan, MI, USA
Disclosure:Stephen H Olsen has disclosed no relevant financial relationships.
Rajiv M Patel, MD,Dermatopathology Section, Department of Pathology, University of Michigan, MI, USA
Disclosure:Rajiv M Patel has disclosed no relevant financial relationships.
Linglei Ma, MD, PhD,Dermatopathology Section, Department of Pathology, University of Michigan, MI, USA
Disclosure:Linglei Ma has disclosed no relevant financial relationships.
Douglas R Fullen, MD,Dermatopathology Section, Department of Pathology, University of Michigan, MI, USA
Disclosure:Douglas R Fullen has disclosed no relevant financial relationships.
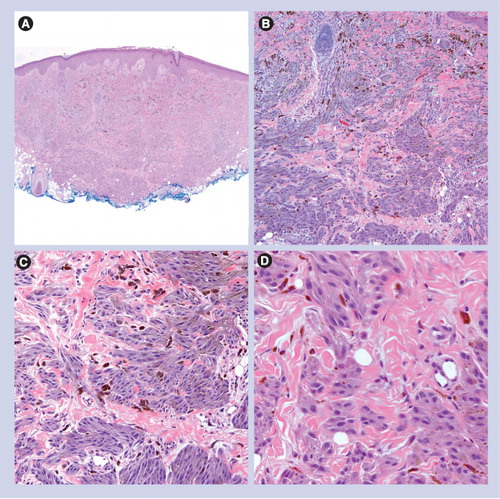
(A) Low power circumscription and symmetry with epidermal hyperplasia (40×). (B) Nests of epithelioid melanocytes are arranged vertically to the skin surface with ‘raining down’ appearance (100×). (C) Higher magnification shows epithelioid melanocytes, Kamino body and occasional pagetoid scattering (200×).
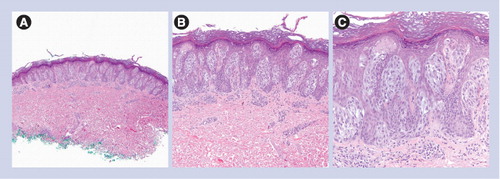
(A) Circumscribed and symmetric lesion (40×). (B) Mostly junctional nevus composed of heavily pigmented spindled melanocytes (100×). (C) Heavily pigmented and spindled melanocytes without cytologic atypia (200×).
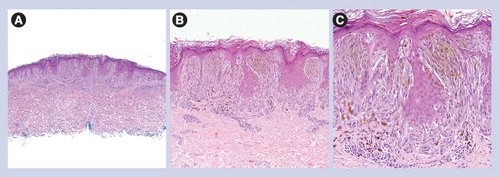
(A) Dermal-based lesion (40×). (B & C) Spindled and epithelioid melanocytes are arranged as fascicles or single units between sclerotic collagen bundles (100× and 200×, respectively). (D) Sclerotic collagen and epithelioid/spindled melanocytes (400×).
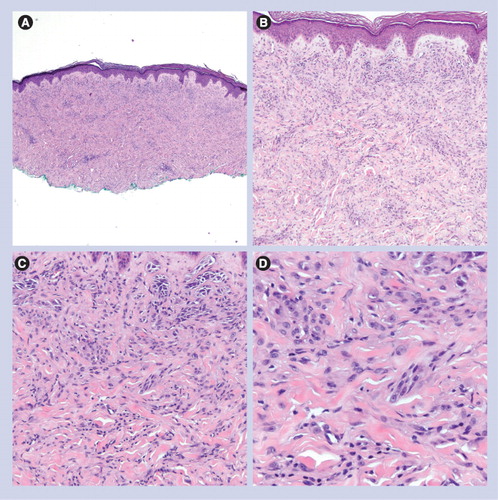
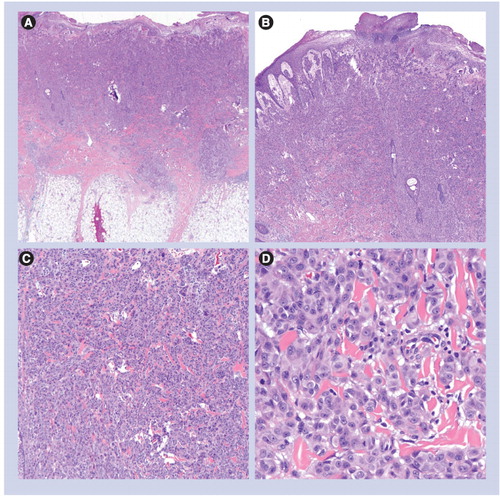
(A) Dermal-based lesion (40×). (B & C) Spindled and epithelioid melanocytes are set in a dense fibrous stroma, associated with prominent blood vessels (100× and 200×, respectively). (D) Epithelioid/spindled melanocytes and prominent vessels (400×).
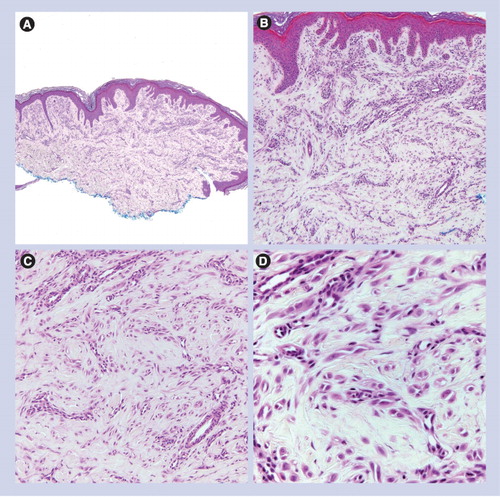
(A) Small and circumscribed lesion (20×). (B & C) Epidermal hyperplasia and bridging of junctional nests (40× and 100×, respectively). (D) Horizontal bridging of junctional nests with concentric fibroplasia and mild dermal inflammation. The melanocytes are epithelioid. Kamino bodies are present (200×).
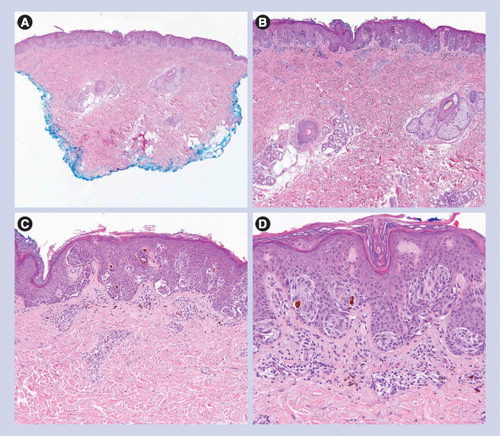
Classic Spitz nevus & variants
Classic Spitz nevus
Spitzoid lesions are composed of varying proportions of spindled and epithelioid cells that display a characteristic cytomorphology that is the common thread between the classic Spitz nevus and all its variants Citation[1]. The cells are large, contain abundant eosinophilic to amphophilic cytoplasm, and have nuclei with smooth nuclear membranes, delicate chromatin and prominent central nucleoli. Within a given lesion, spitzoid cells are generally uniform in size and appearance.
Classic Spitz nevi can be compound (most common), junctional or intradermal Citation[2]. Although originally thought to arise in children, patients older than 20 years may account for up to 66% of cases, and these patients are predominantly female Citation[3]. As with other benign nevi, Spitz nevi are symmetrical from side to side, but typically have a wedge-shaped silhouette when compound or intradermal. They are generally sharply circumscribed – that is, lesions with junctional components terminate as nests on each side. There are associated epidermal changes, ranging from mild acanthosis to pseudoepitheliomatous hyperplasia, which are more prominent in lesions with junctional components. Kamino bodies, or globular collections of eosinophilic (periodic acid-Schiff-positive) basement membrane material, are often present. Nevus cells are distributed mostly in nests along the dermal–epidermal junction with only minor components of single units, except in occasional examples of early or evolving junctional Spitz nevi. Nests often exhibit artifactual peripheral clefting from the surrounding epidermis. When spindled, cells within junctional nests can be oriented perpendicular to the surface, thereby appearing to ‘rain down’. Nests can extend into the upper levels of the epidermis, a phenomenon referred to as transepidermal elimination. Single cells, if present, tend to remain low within the epidermis, lack pagetoid scatter, and are confined to the center of the lesion. Dermal nevus cells are nested superficially and most exhibit maturation, characterized by diminution in cell size and dispersion from nests between collagen bundles, with deeper descent into the dermis. Moreover, the dermal component does not distort the normal dermal architecture. Mitotic activity can be found in some lesions; however, it is usually sparse, when present, and limited to the spitzoid cells of the junctional and superficial dermal components. Pigment is generally absent, but cells can demonstrate light ‘dusty’ pigmentation occasionally. Inflammation is seen in approximately 70% of lesions and when heavy, the findings are consistent with a ‘halo Spitz nevus’. Multinucleated melanocytes are present in 25% of Spitz nevi and are seen more often in adults Citation[3] and in nevi with epithelioid morphology Citation[2].
Pigmented spindle cell nevus
Many dermatopathologists believe the pigmented spindle cell nevus (PSCN) of Reed, or Reed’s nevus, represents one end of the spectrum of Spitz nevi, composed exclusively of spindled cells with the aforementioned spitzoid cytomorphology. These lesions tend to be junctional Citation[2]. Like classic Spitz nevi, individual PSCN lesions are sharply circumscribed, associated with epidermal hyperplasia, and form flat-topped, heavily pigmented papules. The cells form moderate-to-large-sized nests along the dermal–epidermal junction. Within these nests, spindle cells can be arranged in a vertically oriented or ‘raining down’ fashion, a whirling pattern, or less commonly oriented parallel to the epidermal surface. Copious amounts of granular melanin pigment are present within the cytoplasm of nevus cells, as well as in adjacent keratinocytes. Pigment is also present within the parakeratotic scale of the stratum corneum and within dermal melanophages. These lesions must be distinguished from melanoma in situ. While this is usually easily accomplished, it may be a challenge in partially sampled lesions, or those with superimposed features of irritation. When a PSCN is compound, the dermal component is often cellular, fills the papillary dermis and has a broad and pushing border along the base of the lesion.
Desmoplastic Spitz nevus
Desmoplastic Spitz nevi reside on the opposite end of the spectrum from PSCN (Reed’s nevus), being composed mostly of epithelioid cells . They most often occur on the extremities of adults, predominantly females, in their third decade Citation[4]. Lesions are most commonly intradermal, but may rarely be compound, albeit with minor junctional components. From scanning magnification, these lesions are usually symmetrical, circumscribed and wedge-shaped, with the apex of the lesion pointing into the mid- or deep-dermis. Epidermal hyperplasia is not pronounced, and Kamino bodies are not a typical feature. The cells in the dermis may cluster and form small nests superficially, but often are arrayed as single cells and cords. They are set, at least partially, within a sclerotic dermal stroma consisting of thickened and hyalinized collagen bundles that may resemble keloidal collagen. As with classic Spitz nevi, the cells exhibit some histologic evidence of maturation with descent into the dermis. Mitotic activity, when present, is rare and limited to the upper dermis. The differential diagnosis rests mainly with desmoplastic melanoma. Desmoplastic melanoma has an infiltrative growth pattern (in contrast to the wedge-shaped and symmetrical growth of a desmoplastic Spitz nevus), patchy dermal inflammation and often an atypical junctional melanocytic proliferation overlying the invasive melanoma from low magnification. The melanoma cells have cytologic atypia, are often predominantly spindled and lack spitzoid cytomorphology.
Angiomatoid Spitz nevus
The angiomatoid Spitz nevus is a rare lesion thought to be a variant of desmoplastic Spitz nevus Citation[5]. Similarly, the angiomatoid variant occurs in adults, most commonly females in their third decade, on the extremities. Lesions are symmetrical and circumscribed, and are predominantly intradermal processes, although a minor junctional component can be found occasionally. Most lesions are composed of epithelioid cells disposed singly or in small clusters, set within a dense fibrous stroma . These epithelioid melanocytes can be rare and difficult to observe in some lesions. Mitotic activity is rare and, when present, limited to the superficial dermis. A variable but often prominent number of slightly thick-walled blood vessels are embedded within the desmoplastic stroma and accompanied by a variably dense lymphocyte-predominant inflammatory host response that may or may not contain plasma cells. The pattern of the inflammatory infiltrate is usually perivascular, but can be diffuse. The significance of this variant is in its differential diagnosis with partially regressed or desmoplastic melanoma, but it must also be distinguished from a vascular lesion and epithelioid fibrous histiocytoma. Regressed melanoma can usually be distinguished from angiomatoid Spitz nevus by the smaller size and circumscription, lack of a significant junctional component in most cases, more uniform cytomorphology of the spitzoid cells, and maturation of the spitzoid cells as they descend in the dermis for the latter. Vascular and fibrohistiocytic lesions can be differentiated from angiomatoid Spitz nevus by evaluation of appropriate immunohistochemical stains.
Spitzoid dysplastic (Spark’s) nevus
The Spark’s nevus (melanocytic nevus with features of Spitz and Clark’s/dysplastic nevus) combines cytomorphologic features of Spitz nevi with the altered architectural patterning commonly associated with Clark’s dysplastic nevi . In practice, they can be referred to by the more descriptive term spitzoid dysplastic nevi. In a series reported by Ko et al., these lesions occurred in adults, mostly female, and were most commonly located on the trunk and lower extremities Citation[6]. Spitzoid dysplastic nevi are either junctional or compound, and are composed of variable proportions of spindled and epithelioid spitzoid-appearing melanocytes. The spitzoid melanocytes are disposed in regularly sized nests irregularly on the sides and tips of often hyperplastic rete ridges along the dermal–epidermal junction, with extensive bridging of nests between rete ridges in a horizontal or plaque-like pattern. The junctional component extends laterally or ‘shoulders’ beyond the dermal component in compound lesions. Foci of small clusters and single-unit melanocytes can be found in the epidermis primarily within the center of the lesion. Variable numbers of Kamino bodies can also be seen in the epidermis. Fibroplasia is present in the papillary dermis, although less commonly in lesions containing more epithelioid cells. In compound spitzoid dysplastic nevi, the dermal component is usually small and limited to the papillary dermis. Maturation of the dermal component with deeper descent into the dermis is common. Mitotic activity is rare.
In contrast to melanoma, spitzoid dysplastic nevi generally lack significant melanocyte involvement of suprapapillary plates and, therefore, junctional confluence or pagetoid scatter. Moreover, spitzoid dysplastic nevi are symmetrical, laterally circumscribed and typically smaller lesions than superficial spreading melanoma. However, similar lesions with high-grade cytologic atypia, pleomorphism and pagetoid scattering have been described in contiguity with superficial spreading melanoma Citation[7]. This finding suggests a potential for malignant degeneration of spitzoid dysplastic nevi and underscores the importance of distinguishing them from melanoma and achieving complete excision of these lesions. If only a portion of the lesion is present for interpretation in the initial biopsy, and diagnostic uncertainty exists as to whether the lesion is a spitzoid dysplastic nevus or melanoma, then it is advisable to issue a provisional diagnosis with a differential diagnosis that includes melanoma and recommend complete excision for complete histologic examination.
Atypical Spitz tumor
The atypical Spitz tumor (AST) is a term designated for borderline lesions and, in practice, conveys a degree of uncertainty or unpredictability regarding the biologic behavior of an individual lesion. Clinical and particularly histologic features deviate significantly from those attributed to the classic Spitz nevus and can overlap with those of melanoma Citation[8]. However, the vast majority of patients, particularly those who are prepubertal, have behaved in a clinically indolent fashion Citation[9].
Atypical Spitz tumors are usually compound lesions composed predominantly of epithelioid cells. While there may be histologic features of Spitz nevus, lesions tend to be larger, asymmetric and not as circumscribed as classic Spitz nevus. They demonstrate cytologic atypia, including nuclear pleomorphism, and aberrant sheet-like growth resulting from a dense, compact cellularity that distorts the dermal architecture . Maturation with dermal descent is lacking and mitotic activity is often present, including in the deep dermis. Cerroni et al. recently found the presence of mitoses, and mitoses near the base of the lesion to be significant features in distinguishing lesions with unfavorable clinical behavior from those with favorable behavior, corroborating previous reports Citation[8,10–12]. Other findings that can be seen include ulceration, extension into the deep dermis or upper subcutis, and a bulbous or expansile and pushing border at the base of the lesion. While many of these histologic features can be seen in melanoma, they are often present in conjunction with other conventionally reassuring criteria, making these lesions exceedingly difficult to classify with certainty. This issue is underscored by the well-documented low diagnostic concordance among expert dermatopathologists in classifying these lesions as benign or malignant Citation[10,13]. Pertinent histopathologic findings of Spitz nevus, AST and spitzoid melanoma are summarized in . Due to the ambiguous nature of this diagnosis, patients are often treated as for melanoma, with sentinel lymph node biopsies (SLNB) undertaken for lesions deeper than 1 mm. Multiple reports show a relatively high proportion of positive sentinel lymph nodes (SLNs), up to 40–50%. In the past, the presence of any atypical cells in SLNs in this setting was taken as evidence of aggressive behavior and the primary skin lesions were retrospectively classified as melanoma. However, as multiple series demonstrate, the vast majority of these lesions do not show a similar aggressive behavior to conventional melanoma Citation[9,14–16]. The pattern of lymph node involvement, tumor burden and presence of mitotic activity are important features to identify when evaluating melanocytic deposits in this setting. In general, lymph node deposits within the parenchyma and large size of a deposit that effaces the nodal architecture are adverse features. Furthermore, characterization by molecular methods, such as comparative genomic hybridization (CGH) or FISH, may give additional information to help guide therapy (see later).
Spitzoid melanoma
Spitzoid melanoma can arise de novo or within a pre-existing nevus Citation[17]; thus, features of a Spitz nevus or its variants can be present in the background of a melanoma and confound the diagnosis. The junctional component may be nested, although nests are larger and more irregular and predominated in areas by single-unit melanocytes with pagetoid scattering. Spitzoid melanomas are more often associated with epidermal atrophy, resulting from consumption of the epidermis, rather than the hyperplasia commonly seen in Spitz nevi . There may be clefting around nests, as is observed in Spitz nevi, but dyscohesion of melanocytes within nests is more characteristic of spitzoid melanoma. Kamino bodies can also be seen, but they are scarce and not well formed when compared with Spitz nevi. Ulceration may be present and is more common in melanoma. The invasive dermal component demonstrates similar features to those listed for AST. It can form large nodules of compact, sheet-like growth, resulting from high cellularity, which may compress or disrupt surrounding structures of the dermis. The cells exhibit cytologic atypia, which is often high grade, and includes prominent nuclear pleomorphism. There is no maturation of the spitzoid melanocytes with descent into the dermis. Mitotic activity is conspicuous, including in deep aspects of the lesion, features recently found to be significantly associated with unfavorable clinical behavior Citation[10]. In fact, some authors believe that more than six mitoses per mm2 or three mitoses per high power field are concerning for or favor melanoma Citation[8,17]. An inflammatory host response often presents as a banded infiltrate of lymphocytes and plasma cells, which can be associated with regression and melanosis. More adverse diagnostic features include necrosis, angiolymphatic spread and perineural invasion. Although spitzoid melanoma in children may have a better prognosis than in adults, some young patients still develop metastases and die from the disease Citation[18].
Ancillary studies
Although careful evaluation of hematoxylin and eosin-stained sections remains the gold standard for the diagnosis of spitzoid lesions, ancillary studies have become increasingly important, particularly when the pathologist is faced with a limited specimen, such as a partial punch or superficial shave biopsy.
Immunohistochemistry
Many markers have been proposed as useful for distinguishing spitzoid lesions from conventional nevi and melanoma. Common and recent promising markers are reviewed later.
Spitz nevi and melanoma show diffuse immunoreactivity for S-100 protein and Melan-A. Studies of HMB-45 and Ki-67 (MIB-1) in spitzoid lesions have yielded varying results, with some groups finding these stains useful and others finding them not useful. Bergman and colleagues reported that Spitz nevi demonstrate a ‘stratification’ pattern of HMB-45 staining manifested as decreasing immunoreactivity as the lesional cells progress from the junction and superficial dermis to the base of the lesion Citation[19]. By contrast, spitzoid melanomas tend to have greater HMB-45 expression in the deep dermal component than Spitz nevi. Others have asserted that classic Spitz nevi have a more heterogeneous staining pattern Citation[20]. Although there is some debate, increased immunoreactivity for HMB-45 in the deep portions of a lesion, in conjunction with ambiguous or worrisome clinical and histopathologic findings, may be helpful in distinguishing spitzoid melanoma from compound Spitz nevi Citation[19]. The utility of HMB-45 stratification for distinguishing between Spitz nevi and AST has not been well-characterized. Pure desmoplastic melanoma is generally, although not always, negative for markers other than S100, and, therefore, positivity for Melan-A and HMB-45 could help to distinguish it from desmoplastic Spitz nevus.
Measurement of the mitotic rate in the dermal component of spitzoid lesions is one of the most important factors in determining biologic potential Citation[8]. Immunohistochemical labeling for Ki-67 (MIB-1) provides useful information beyond mitotic count. A recent study demonstrated a Ki-67 proliferation index of 10% in ASTs relative to 0.53% for ordinary nevi, 5.04% in conventional Spitz nevi and 36.83% in melanoma Citation[21]. Caution must be exercised when interpreting this marker because Ki-67 immunoreactivity may be found in keratinocytes, mesenchymal cells and inflammatory cells. As a result, direct evaluation of mitoses on hematoxylin and eosin-stained sections may be more useful than a Ki-67 index in inflamed spitzoid lesions Citation[17]. We have found HMB-45 and Ki-67 immunohistochemistry helpful in a subset of cases.
S100A6, a member of the S100 protein family of calcium-binding proteins, has shown promise for differentiating between Spitz nevi, melanomas and melanocytic nevi. Ribe and colleagues recently reported strong diffuse cytoplasmic staining in both the junctional and dermal components of 100% of analyzed Spitz nevi. By contrast, only 33% of melanomas demonstrated S100A6 expression, mainly in a weak or patchy pattern in the dermal component, with absent or minimal immunostaining in the junctional component. Moreover, 56% of different melanocytic nevi expressed this marker, nearly all of them weakly and in the dermal component only. The authors concluded that S100A6 could be used to distinguish Spitz nevi from melanoma in classic cases and in cases difficult to distinguish by light microscopy Citation[22].
Differentiating desmoplastic Spitz nevi from desmoplastic melanoma may be difficult, particularly when faced with a limited biopsy and incomplete clinical information. Loss of the CDKN2a gene product p16 is thought to be involved in melanomagenesis via dysregulation of the cell cycle and is indicative of a poorer prognosis in patients with melanoma. Hilliard and colleagues recently reported loss or weak expression of p16 in 81.8 and 18.2%, respectively, of desmoplastic melanomas by immunohistochemistry. All desmoplastic Spitz nevi studied were moderately or strongly immunoreactive for p16. In the authors’ experience, the p16 pattern, in conjunction with S100 staining, the Ki-67 index and clinical and histologic features, was useful in distinguishing between desmoplastic melanoma and desmoplastic Spitz nevus Citation[23].
Molecular studies
Melanoma typically contains complex genotypic abnormalities, in contrast to Spitz nevi, which tend to have a normal karyotype when analyzed by CGH Citation[24]. Some of the most exciting recent developments in the pathology of spitzoid lesions have been in the realm of molecular biology. Although these findings have by no means been consistent between studies thus far, techniques such as CGH and FISH show promise as ancillary techniques for the diagnosis of ambiguous melanocytic lesions.
BRAF/NRAS
BRAF (v-raf murine sarcoma viral oncogene homolog B1) mutations may be found in approximately 50% of conventional melanomas and 80% of ordinary nevi Citation[25,26], and a small subset of spitzoid melanomas Citation[27]. The vast majority of conventional Spitz nevi do not contain BRAF mutations Citation[26]. However, when the spectrum of spitzoid lesions is expanded to include unusual or atypical variants, such as spitzoid dysplastic nevi or Reed’s nevi, the incidence of BRAF mutations is increased Citation[27–29]. A limited number of studies have examined BRAF mutations in AST, and only one study reported finding a BRAF mutation in AST Citation[25–27]. Mutations in NRAS (neuroblastoma RAS viral [v-ras] oncogene homolog) have also been reported in melanoma Citation[25,26,28], but not in conventional Spitz nevi Citation[25,26,28]. The inconsistencies between published studies may be related to a lack of consensus among dermatopathologists on histologic criteria used in the diagnosis of spitzoid melanocytic lesions Citation[13]. Additional studies examining BRAF/NRAS mutations in a large series of well-characterized spitzoid melanocytic lesions with long-term follow-up are needed to clarify and expand on these findings.
HRAS
The majority of Spitz nevi have a normal karyotype. However, Bastian and colleagues recently used a combination of CGH and FISH to demonstrate amplification of chromosome 11p, corresponding to the location of HRAS (v-Ha-ras Harvey rat sarcoma viral oncogene homolog), in a small subset of Spitz nevi Citation[30]. Chromosome 11p copy number increases have also been noted in AST Citation[25], but not in spitzoid melanoma Citation[25,26]. Currently, there is no evidence to suggest that Spitz nevi with RAS activation are at risk for progression to melanoma. Further studies of spitzoid lesions with RAS activation are warranted to assess their biologic behavior Citation[24].
CDKN2A
Copy number loss of the cyclin-dependent kinase inhibitor 2A gene (CDKN2A) is frequently seen in melanoma, but not in conventional Spitz nevi. Takata and colleagues reported that three out of 16 ambiguous spitzoid lesions, most diagnosed as ASTs, had copy number loss of CDKN2A. A total of 12 unambiguous Spitz nevi lacked copy number loss of CDKN2A. The authors concluded that most ASTs show no evidence of genetic or epigenetic changes similar to conventional Spitz nevi, but a subset of ASTs may have genetic abnormalities, including copy number loss of CDKN2ACitation[25].
TRPM1 mRNA expression
The transient receptor potential cation channel, subfamily M, member 1 (TRMP1/melanstatin-1/MLSN-1) expression has been shown to have prognostic value in primary cutaneous melanoma. Regional loss or complete absence of TRMP1 mRNA was identified by Erickson and colleagues by in situ hybridization in 82% of melanomas, but only 1% of Spitz nevi. Of 16 patients with metastatic disease, 15 (94%) had primary tumors that demonstrated reduced MLSN-1 RNA expression by all or a portion of the dermal tumor Citation[31]. The pattern of TRMP1 mRNA expression may be helpful in differentiating Spitz nevi from melanoma, but additional studies are indicated.
FISH
Using existing data on DNA copy number alterations, Gerami and colleagues recently identified a panel of FISH probes applicable to paraffin-embedded tissue that were useful as an ancillary diagnostic tool in the diagnosis of melanoma. A combination of four probes targeting 6q23, 6p25, 6 centromere and 11q13, correctly classified melanoma with 86.7% sensitivity and 95.4% specificity when compared with various nevi, including Spitz nevi. Their study set also included 27 lesions with ambiguous morphology, 24 of which were difficult to classify spitzoid lesions. Within this subset, their FISH probes identified six out of six lesions that later metastasized, although the details relating to the spitzoid cases are unclear. The authors suggested that a limited panel of FISH probes provided useful diagnostic information in cases that could not be classified reliably by histopathology Citation[32]. In a more recent study, this group concluded that molecular abnormalities in melanoma were heterogeneous and that the sensitivity of this probe set varied with the subtype of melanoma. This study included five difficult spitzoid lesions, four with benign and one with malignant clinical behavior on follow-up. Their FISH analysis was positive in only one of their cases that had a benign outcome Citation[33]. Subsequently, Gaiser and colleagues, utilizing an identical FISH probe set, reported only a sensitivity of 60% and specificity of 50% in discriminating melanomas from nevi based on a series of patients with histologically ambiguous lesions and long-term clinical follow-up. The authors concluded that this probe set did not achieve sufficient sensitivity and specificity to be clinically useful in this setting Citation[34]. Isaac and colleagues recently reported polyploidy in four out of 41 benign Spitz nevi. Polyploidy was more common in agminate Spitz nevi and Spitz nevi with an atypical epithelioid component Citation[35].
FISH represents a promising ancillary diagnostic test for problematic melanocytic lesions. However, results of recent studies suggest that validation in larger series of patients with histologically well-characterized lesions and long-term follow-up is necessary before their use in routine diagnostic practice can be recommended. Owing to differences in type and frequency of molecular abnormalities between different melanocytic lesions, the composition of probe sets for analyzing spitzoid lesions may prove to be different from those used in the analysis of conventional nevi and melanomas. CGH analysis of a large series and spectrum of spitzoid melanocytic lesions linked to clinical outcome may provide further insight into the molecular events that underpin these lesions and potentially form a rationale for identifying different FISH probes from those that are currently touted for distinguishing benign nevi from melanoma.
Sentinel lymph node biopsy
Unfortunately, little objective information on the frequency and biological implications of ‘ectopic’ melanocytic cells in SLNB from patients with conventional Spitz nevi exists in the literature Citation[36]. However, intracapsular nodal nevus cells have been found in staging lymphadenectomy specimens for epithelial malignancies in patients with and without a history of melanoma. Criteria for differentiating these deposits from melanoma have been previously proposed Citation[37]. Based on proven prognostic benefit in melanoma, melanocytic deposits in an extracapsular site within a SLN may lead surgeons to consider a completion lymphadenectomy in patients with ambiguous spitzoid melanocytic lesions. Recent data suggest that this finding does not have the same prognostic significance as in melanoma. Ludgate and colleagues reported that 27 out of 57 patients (47%) with atypical Spitz tumors had SLN involvement. All of these patients were alive and free of disease after a median follow-up of 43.8 months. These findings questioned the malignant potential of AST and the role of SLNB in their management. Interestingly, however, one patient in this series who did not undergo SLNB developed regional and distant metastases and died from metastatic disease Citation[9]. Busam and colleagues identified 11 individuals of young age with atypical spitzoid melanocytic tumor (ASMT) and positive SLN, and compared their clinical outcome to five young patients with unequivocal melanoma and either a positive SLNB or death from disseminated disease. All patients with ASMT remained free of disease after a median follow-up of 47 months. This study confirmed prior observations suggesting that children and teenagers with ASMT and positive SLN had a favorable prognosis, and again questioned the prognostic value of SLNB in this clinical setting Citation[16]. In a recent review of the literature on AST, approximately 17% of reported patients with AST and positive SLN also had non-SLN involvement. One patient had local recurrence, and all were alive and well after 6–57 months follow-up Citation[38]. The outcome of young patients with AST and positive lymph nodes is vastly superior to the overall 5-year survival of 68% seen in patients under the age of 19 years with regional metastatic melanoma Citation[39]. Determining the ultimate role SLNB plays in the management of patients with ambiguous spitzoid lesions will likely require the outcome of randomized controlled trials of SLNB versus non-SLNB in a large cohort of patients with long-term follow-up. Patients with unequivocal spitzoid melanoma should be managed clinically the same as other subtypes of melanoma, including consideration of a SLNB when the lesion is of sufficient Breslow depth Citation[17].
Expert commentary
Spitz nevi are uncommon nevi that can present with a variety of histologic appearances and can occur at any age, although they are most common in children, adolescents and young adults. The most common types of Spitz nevi include the classic spindled and epithelioid Spitz nevus, pigmented spindle cell nevus (Reed’s nevus), spitzoid dysplastic or Spark’s nevus, and desmoplastic Spitz nevus. The angiomatoid variant of Spitz nevus can be particularly difficult to diagnose. Spitz nevus may be admixed with other types of nevi to form a combined nevus.
The cytomorphology of Spitz nevus cells, including the large size of spitzoid cells and the presence of macronucleoli, differs significantly from the nevomelanocytes of other common nevi and may present a challenge in distinguishing Spitz nevus from melanoma for a given lesion. Adding further to the diagnostic difficulty is that a small subset of melanomas has some histologic features that resemble Spitz nevi, but have sufficient criteria for malignancy and, therefore, are best classified as spitzoid melanomas. However, a small subset of spitzoid lesions, referred to by some authors as melanocytic tumor of uncertain malignant potential or atypical Spitz tumor, have histologic features that sufficiently deviate from the usual histology of Spitz nevi yet lack definitive criteria for malignancy, such that the biologic behavior of the lesions still cannot be reliably predicted on histologic grounds. SLNB has been performed for this type of lesion in many academic centers and has a surprisingly high rate of positivity, approaching 50% in some studies, although adverse outcomes are remarkably rare. There remains a lack of general consensus on the best diagnostic term to use for these lesions and the most appropriate treatment for these patients. We favor the term ‘atypical Spitz tumor’, for its specificity, over ‘melanocytic tumor of uncertain malignant potential’, which is a broader designation incorporating unrelated lesions, such as those in the blue nevus family.
Ancillary studies, such as immunohistochemistry for MIB-1 proliferation index and HMB-45 staining pattern for presence or absence of stratification of the dermal component, have been used with variable results. Molecular analysis of pigmented lesions of the skin has become increasingly popular as an adjunct to histology and immunohistochemistry in defining the biologic behavior of an individual lesion. CGH has been applied to diagnostically challenging spitzoid lesions to look for gains or losses in genes commonly seen in melanoma as a means of guiding subsequent therapy. Recently, FISH has been developed with probes targeting 6q23, 6p25, 6 centromere and 11q13, and the assay has been reported to have a sensitivity of approximately 87% and an approximate 95% specificity. It remains unclear, however, whether this probe set is ideally suited for defining the biologic behavior in histologically ambiguous spitzoid lesions or their lymph node deposits.
Five-year view
The major emphasis in the diagnostic difficulty of Spitz nevi will continue to center around distinguishing Spitz nevi from spitzoid melanoma and defining whether the continued classification of a borderline category of atypical Spitz tumors is clinically justified. Outcome studies with longer follow-up will continue to elucidate the biologic behavior of these histologically ambiguous lesions and help guide the management of these patients. Molecular studies, such as CGH and targeted FISH, with results linked to patient outcomes, will be beneficial in stratifying the risk of a diagnostically challenging cutaneous lesion and determining whether SLNB is appropriate for an individual patient. Moreover, these studies should lead to defining the most appropriate areas of the genome to target in molecular assays to aid in prognosis, and the most appropriate treatment of these patients.
Table 1. Pertinent histopathologic findings of Spitz nevus, atypical Spitz tumor and spitzoid melanoma.
Key issues
• Spitz nevi and their variants are uncommon melanocytic lesions with characteristic morphology that differs significantly from that of common acquired nevi and can be mistaken for melanoma.
• Most Spitz nevi can be easily classified and distinguished from melanoma; however, diagnostic difficulty remains in a subset of lesions, particularly when faced with small and incomplete sampling.
• Atypical Spitz tumor is a lesion with overlapping features of Spitz nevus and spitzoid melanoma, which make it difficult to classify with certainty; many such lesions display indolent clinical behavior.
• Despite difficulties, histologic examination of hematoxylin and eosin-stained sections remains the gold standard for diagnosis, although ancillary studies have been investigated and may prove even more useful in the future.
• Molecular studies such as comparative genomic hybridization and FISH have shown promise, but additional work remains in specific application to histologically ambiguous spitzoid lesions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Spitz S. Melanomas of childhood. Am. J. Pathol.24(3), 591–609 (1H948).
- Requena C, Requena L, Kutzner H, Sanchez Yus E. Spitz nevus: a clinicopathological study of 349 cases. Am. J. Dermatopathol.31(2), 107–116 (2009).
- Cesinaro AM, Foroni M, Sighinolfi P, Migaldi M, Trentini GP. Spitz nevus is relatively frequent in adults: a clinico-pathologic study of 247 cases related to patient’s age. Am. J. Dermatopathol.27(6), 469–475 (2005).
- Diaz-Cascajo C, Borghi S, Weyers W. Angiomatoid Spitz nevus: a distinct variant of desmoplastic Spitz nevus with prominent vasculature. Am. J. Dermatopathol.22(2), 135–139 (2000).
- Tetzlaff MT, Xu X, Elder DE, Elenitsas R. Angiomatoid Spitz nevus: a clinicopathological study of six cases and a review of the literature. J. Cutan. Pathol.36(4), 471–476 (2009).
- Ko CJ, McNiff JM, Glusac EJ. Melanocytic nevi with features of Spitz nevi and Clark’s/dysplastic nevi (‘Spark’s’ nevi). J. Cutan. Pathol.36(10), 1063–1068 (2009).
- Magro CM, Yaniv S, Mihm MC. The superficial atypical Spitz tumor and malignant melanoma of superficial spreading type arising in association with the superficial atypical Spitz tumor: a distinct form of dysplastic spitzoid nevomelanocytic proliferation. J. Am. Acad. Dermatol.60(5), 814–823 (2009).
- Barnhill RL. The spitzoid lesion: rethinking Spitz tumors, atypical variants, ‘spitzoid melanoma’ and risk assessment. Mod. Pathol.19(Suppl. 2), S21–S33 (2006).
- Ludgate MW, Fullen DR, Lee J et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer115(3), 631–641 (2009).
- Cerroni L, Barnhill R, Elder D et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the xxix symposium of the International Society of Dermatopathology in Graz, October 2008. Am. J. Surg. Pathol.34(3), 314–326 (2010).
- Spatz A, Calonje E, Handfield-Jones S, Barnhill RL. Spitz tumors in children: a grading system for risk stratification. Arch. Dermatol.135(3), 282–285 (1999).
- Walsh N, Crotty K, Palmer A, McCarthy S. Spitz nevus versus spitzoid malignant melanoma: an evaluation of the current distinguishing histopathologic criteria. Hum. Pathol.29(10), 1105–1112 (1998).
- Barnhill RL, Argenyi ZB, From L et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum. Pathol.30(5), 513–520 (1999).
- Su LD, Fullen DR, Sondak VK, Johnson TM, Lowe L. Sentinel lymph node biopsy for patients with problematic spitzoid melanocytic lesions: a report on 18 patients. Cancer97(2), 499–507 (2003).
- Murali R, Sharma RN, Thompson JF et al. Sentinel lymph node biopsy in histologically ambiguous melanocytic tumors with spitzoid features (so-called atypical spitzoid tumors). Ann. Surg. Oncol.15(1), 302–309 (2008).
- Busam KJ, Murali R, Pulitzer M et al. Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomas. Am. J. Surg. Pathol.33(9), 1386–1395 (2009).
- Kamino H. Spitzoid melanoma. Clin. Dermatol.27(6), 545–555 (2009).
- Paradela S, Fonseca E, Pita S et al. Spitzoid melanoma in children: clinicopathological study and application of immunohistochemistry as an adjunct diagnostic tool. J. Cutan. Pathol.36(7), 740–752 (2009).
- Bergman R, Dromi R, Trau H, Cohen I, Lichtig C. The pattern of HMB-45 antibody staining in compound Spitz nevi. Am. J. Dermatopathol.17(6), 542–546 (1995).
- Palazzo J, Duray PH. Typical, dysplastic, congenital, and Spitz nevi: a comparative immunohistochemical study. Hum. Pathol.20(4), 341–346 (1989).
- Kapur P, Selim MA, Roy LC, Yegappan M, Weinberg AG, Hoang MP. Spitz nevi and atypical Spitz nevi/tumors: a histologic and immunohistochemical analysis. Mod. Pathol.18(2), 197–204 (2005).
- Ribe A, McNutt NS. S100a6 protein expression is different in Spitz nevi and melanomas. Mod. Pathol.16(5), 505–511 (2003).
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J. Cutan. Pathol.36(7), 753–759 (2009).
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of hras in Spitz nevi with distinctive histopathological features. Am. J. Pathol.157(3), 967–972 (2000).
- Takata M, Lin J, Takayanagi S et al. Genetic and epigenetic alterations in the differential diagnosis of malignant melanoma and spitzoid lesion. Br. J. Dermatol.156(6), 1287–1294 (2007).
- van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am. J. Surg. Pathol.29(9), 1145–1151 (2005).
- Fullen DR, Poynter JN, Lowe L et al.Braf and nras mutations in spitzoid melanocytic lesions. Mod. Pathol.19(10), 1324–1332 (2006).
- Indsto JO, Kumar S, Wang L, Crotty KA, Arbuckle SM, Mann GJ. Low prevalence of ras–raf-activating mutations in Spitz melanocytic nevi compared with other melanocytic lesions. J. Cutan. Pathol.34(6), 448–455 (2007).
- La Porta CA, Cardano R, Facchetti F et al.Braf v599e mutation occurs in Spitz and Reed naevi. J. Eur. Acad. Dermatol. Venereol.20(9), 1164–1165 (2006).
- Bastian BC, Wesselmann U, Pinkel D, Leboit PE. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J. Invest. Dermatol.113(6), 1065–1069 (1999).
- Erickson LA, Letts GA, Shah SM, Shackelton JB, Duncan LM. Trpm1 (melastatin-1/mlsn1) mrna expression in Spitz nevi and nodular melanomas. Mod. Pathol.22(7), 969–976 (2009).
- Gerami P, Jewell SS, Morrison LE et al. Fluorescence in situ hybridization (fish) as an ancillary diagnostic tool in the diagnosis of melanoma. Am. J. Surg. Pathol.33(8), 1146–1156 (2009).
- Gerami P, Mafee M, Lurtsbarapa T, Guitart J, Haghighat Z, Newman M. Sensitivity of fluorescence in situ hybridization for melanoma diagnosis using rreb1, myb, cep6, and 11q13 probes in melanoma subtypes. Arch. Dermatol.146(3), 273–278 (2010).
- Gaiser T, Kutzner H, Palmedo G et al. Classifying ambiguous melanocytic lesions with fish and correlation with clinical long-term follow up. Mod. Pathol.23(3), 413–419 (2010).
- Isaac AK, Lertsburapa T, Pathria Mundi J, Martini M, Guitart J, Gerami P. Polyploidy in Spitz nevi: a not uncommon karyotypic abnormality identifiable by fluorescence in situ hybridization. Am. J. Dermatopathol.32(2), 144–148 (2010).
- LeBoit PE. What do these cells prove? Am. J. Dermatopathol.25(4), 355–356 (2003).
- Busam KJ, Pulitzer M. Sentinel lymph node biopsy for patients with diagnostically controversial spitzoid melanocytic tumors? Adv. Anat. Pathol.15(5), 253–262 (2008).
- Urso C, Borgognoni L, Doria M, Tinacci G, Zini E. Non-sentinel lymph node involvement in a patient with an atypical Spitz tumor and a positive sentinel node. Report of a case and review of the literature. J. Cutan. Pathol.36(5), 586–590 (2009).
- Lange JR, Palis BE, Chang DC, Soong SJ, Balch CM. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J. Clin. Oncol.25(11), 1363–1368 (2007).
Difficulties in the diagnosis of spitzoid melanocytic lesions
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscapecme.com/journal/expertderm. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation Where 1 is strongly disagree and 5 is strongly agree
1. Your patient is a 14-year-old white boy with a suspicious melanocytic lesion thought possibly to be a Spitz nevus. Based on the above review by Dr Olsen and colleagues, which of the following statements about clinical features and classification of Spitz nevi is correct?
□ A Spitz nevi and their variants are highly prevalent
□ B Morphology of Spitz nevi is very similar to that of common acquired nevi
□ C Usual age of onset is 65 years or older
□ D Types include classic spindled and epithelioid Spitz nevus, pigmented spindle cell nevus (Reed’s nevus), spitzoid dysplastic or ‘Spark’s’ nevus, desmoplastic Spitz nevus, and angiomatoid variant
2. The patient in question 1 undergoes biopsy of the melanocytic lesion. Based on the above review, which of the following features is most likely to suggest Spitz nevus rather than spitzoid melanoma?
□ A Size >1 cm
□ B Pleomorphism
□ C Kamino bodies
□ D Expansile nodules
3. Based on the above review, which of the following statements about immunohistochemistry and other ancillary studies that may help differentiate Spitz nevi from atypical Spitz tumor and spitzoid melanoma is correct?
□ A Fluorescence in situ hybridization (FISH) with probes targeting 6q23, 6p25, 6 centromere, and 11q13 has poor sensitivity and specificity
□ B FISH with probes targeting 6q23, 6p25, 6 centromere, and 11q13 has been proven to define biologic behavior in histologically ambiguous spitzoid lesions
□ C Comparative genomic hybridization has no apparent role in management
□ D Histologic examination of hematoxylin and eosin-stained sections is still the gold standard for diagnosis