Abstract
Dermoscopy is a noninvasive technique that allows for the visualization of subsurface colors and structures within pigmented melanocytic skin lesions that are otherwise imperceptible to the naked eye. Dermoscopic findings have been formulated into diagnostic criteria that assist experienced clinicians in differentiating benign and malignant neoplasms. In essence, dermoscopy can help identify cutaneous malignancies, while at the same time minimizing the frequency of unwarranted biopsies of benign cutaneous lesions. In this article, we examine the clinical morphology of melanocytic nevi and melanoma in the pediatric population, and describe the relevant dermoscopic findings and histopathologic correlates that aid in the diagnosis and management of these lesions.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and poli¬cies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/expertderm (4) view/print certificate.
Release date: February 7, 2011; Expiration date: February 7, 2012
Learning objectives
Upon completion of this activity, participants should be able to:
• Describe the potential advantages and disadvantages of dermoscopy of melanocytic lesions in children
• Describe how dermoscopy can help differentiate benign from malignant melanocytic lesions in children
• Describe how dermoscopy can facilitate work-up and management of melanocytic lesions in children
• Describe classification of melanocytic lesions in children
Financial & competing interests disclosure
EDITOR
Elisa Manzotti,Editorial Director, Future Science Group, London, UK
Disclosure:Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Laurie Barclay, MD,Freelance writer and reviewer, Medscape, LLC
Disclosure:Laurie Barclay, MD, has disclosed no relevant financial relationships.
AUTHOR
Christine Brooks, DO,St Joseph Mercy Health System, Pontiac, MI, USA
Disclosure:Christine Brooks, DO, has disclosed no relevant financial relationships.
Alon Scope, MD,Sheba Medical Center, Tel Hashomer, Israel
Disclosure:Alon Scope has disclosed no relevant financial relationships.
Ralph P Braun, MD,Department of Dermatology, University Hospital Zurich, Zurich, Switzerland
Disclosure:Ralph P Braun, MD, has disclosed no relevant financial relationships.
Ashfaq A Marghoob, MD,Memorial Sloan-Kettering Cancer Center, NY, USA
Disclosure:Ashfaq A Marghoob, MD, has disclosed no relevant financial relationships.
The congenital melanocytic nevus displays an organized and symmetric pattern with a peripheral network pattern with central globules. The lesion also has a few milia cysts and hypertrichosis, which are structures commonly observed in congenital melanocytic nevus.
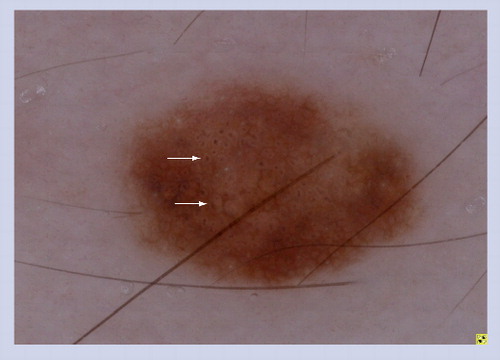
In the presence of a focal network, irregular globules and an irregular blotch, melanoma should be definitively ruled out.
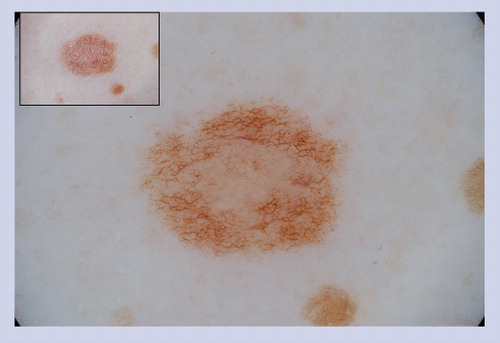
A reticular pattern is seen and the network is interrupted by follicular openings (hypertrichosis). Perifollicular hypopigmentation is a common feature seen in congenital melanocytic nevus.
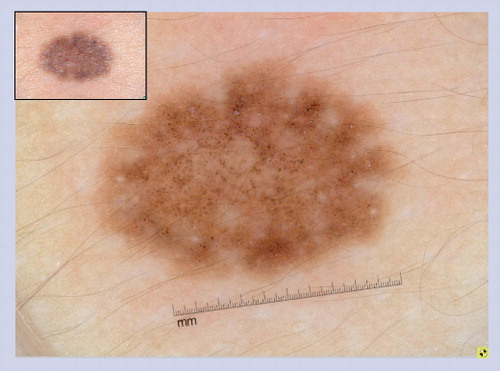
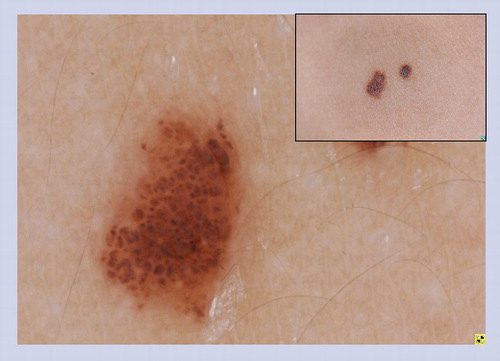
Globules of differing sizes with angulation and crowding of the globules create a characteristic cobblestone pattern.
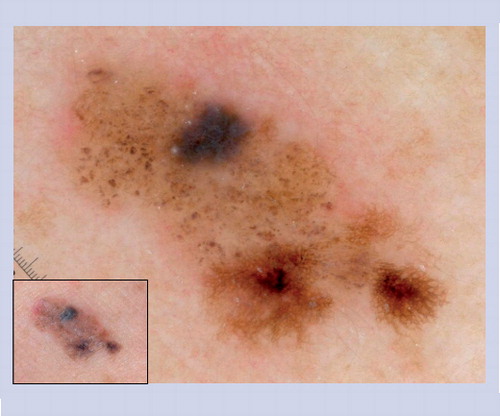
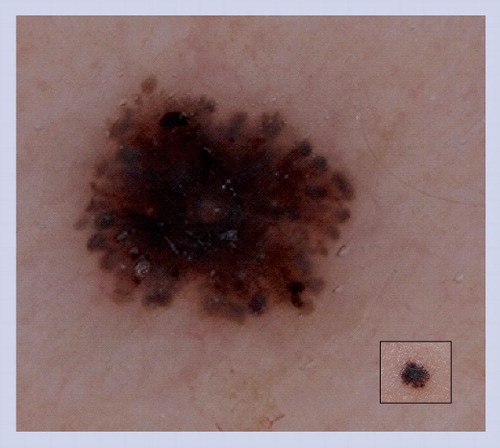
This lesion does not manifest the benign dermoscopic patterns illustrated in . Half of the lesion reveals an organized pattern with cobblestone globules, hypertrichosis and milia cysts, which are all common features of congenital melanocytic nevus. The other half of the lesion is disorganized with irregular network, dots and peppering. This lesion was excised and histopathology confirmed the diagnosis of melanoma arising in a small congenital melanocytic nevus.
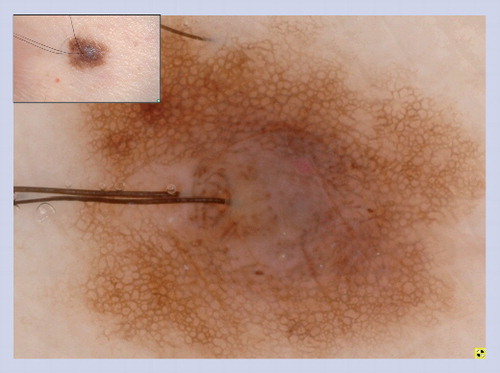
Acquired melanocytic nevus showing a peripheral network with central hyperpigmentation.
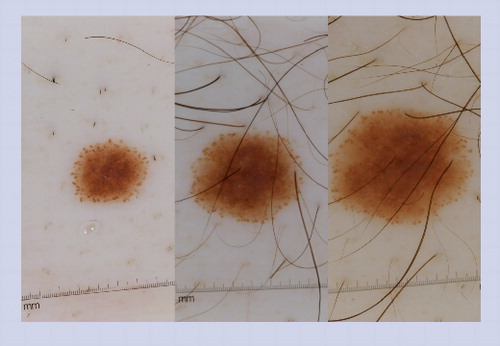
The presence of a peripheral rim of globules is an indicator that the nevus has not yet entered senescence. It can be predicted that nevi displaying this pattern will enlarge symmetrically over time. Once the nevus enters senescence, it will stop growing and the peripheral rim of globules will no longer be visible. The nevus in these images was followed over the course of 2 years. The first image was taken at baseline, the second image was acquired at 9-month follow-up and the last image was obtained at the 17-month follow-up visit.
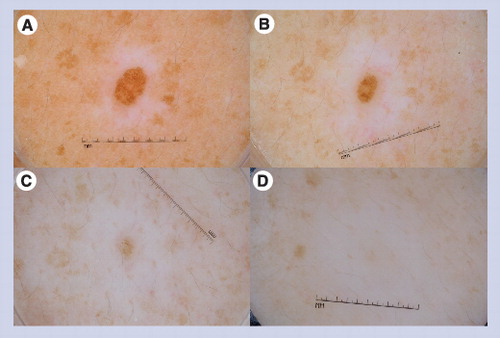
This globular nevus is surrounded by a halo of depigmentation. This nevus became smaller over time and eventually disappeared over the course of a 4-year period. The first image (A) was obtained at baseline, the second image (B) at the 1-year follow-up visit, the third image (C) at the 3-year follow-up visit, and the last image (D) at the 4-year follow-up visit.
The starburst pattern is the most common pattern manifest by typical pigmented Spitz nevi.
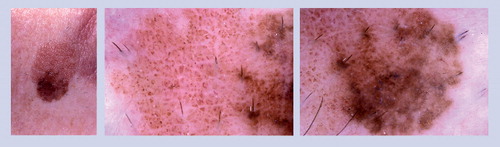
At baseline this nevus revealed a starburst pattern. The lesion was re-imaged at a 6-month follow-up appointment and another image was obtained at 2-year follow-up. The starburst pattern had transformed into a homogeneous pattern.

A focal area with a negative network is visible on nonpolarized dermoscopy (A) and polarized dermoscopy (B). The negative network is located on the right side of the lesion at approximately 3 o’clock.
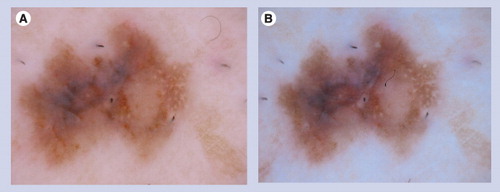
These fine, white streaks are only visualized with polarized dermoscopy (B) and are not visible with nonpolarized dermoscopy (A).
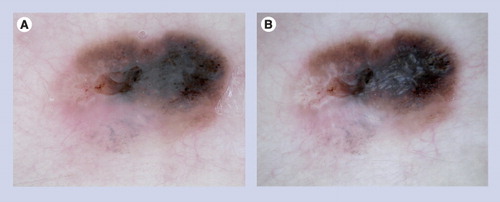
This asymmetric pigmented lesion reveals focal pseudopods, irregular blue–white veil and atypical network. This constellation of findings raises concern for melanoma. The lesion was excised and the histopathology was deemed to be consistent with a Spitz nevus.
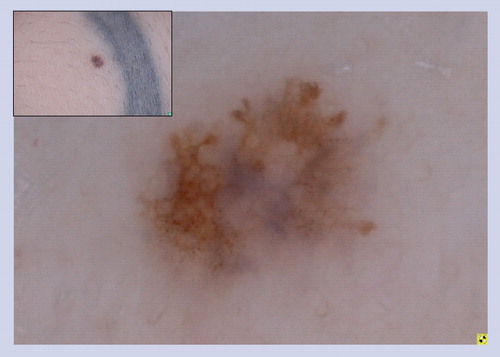
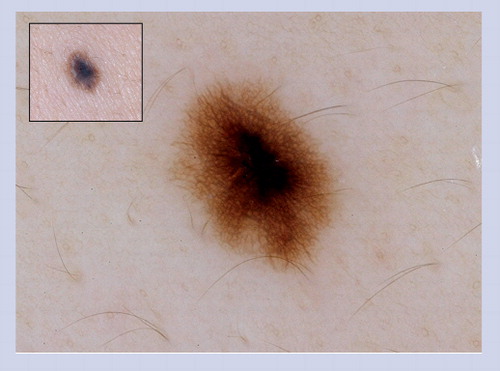
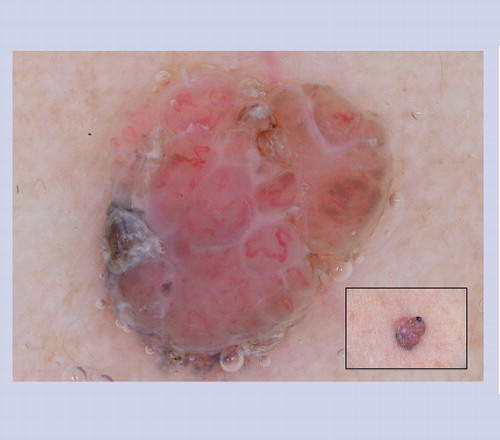
These lesions often manifest the clinical ABCD features of melanoma, and under dermoscopy they often display some of the structures listed in that heighten the suspicion of melanoma. This particular lesion has atypical dots and globules, irregular network with branched streaks and an irregular blotch. Superficial spreading melanomas are not commonly found in children.
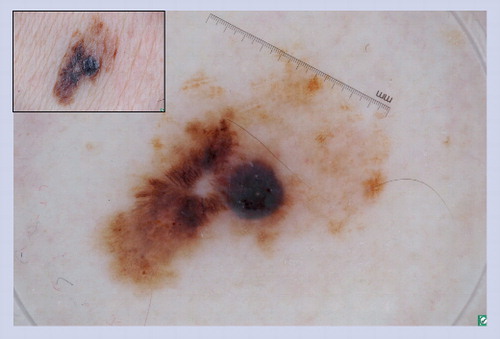
This lesion developed over a few months on the cheek of a 17-year-old. No ABCD features were present on clinical examination. However, under dermoscopy irregular blood vessels are visualized, which helps to correctly diagnose the lesion as a cutaneous malignancy.
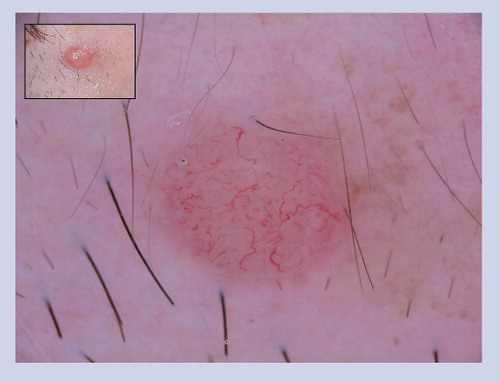
This lesion lacks clinical ABCD features; however, it does reveal irregular serpentine blood vessels, which are melanoma predictive structures, as noted in .
Melanocytic neoplasms of childhood belong to three distinct classes, including congenital nevi, acquired nevi and melanoma. Congenital melanocytic nevi (CMN) consist of nevi that are clinically evident at birth, as well as nevi manifesting congenital features that become clinically apparent shortly after birth (i.e., tardive CMN) Citation[1]. In addition, nevus spilus and segmental speckled-lentiginous nevus are also considered to be CMN. Melanocytic nevi that develop many months to years after birth are termed acquired melanocytic nevi (AMN) and include junctional nevi, compound nevi, dermal nevi, blue nevi (BN) and Spitz nevi (SN). Although rare, the incidence of melanoma in the pediatric population appears to be rising, and as such, it has become imperative that clinicians include melanoma in the differential diagnosis of atypical pigmented or amelanotic lesions in children Citation[2–4]. It is important to acknowledge that melanoma can develop in healthy children, as well as those with underlying genetic or immunologic disorders, such as xeroderma pigmentosum. Approximately half of all pediatric melanomas arise in a de novo fashion, while the other 50% emerge in association with pre-existing cutaneous lesions. Nearly 30% of childhood melanomas arise within giant CMN, and approximately 20% develop in association with AMN Citation[5]. Thus, any suspicious or evolving lesion in a child should not be dismissed as a benign entity, and a diagnosis of melanoma ought to enter the differential.
The clinical features of melanoma in children can be quite subtle, and can mimic SN and angiomas, often resulting in missed opportunities to perform a diagnostic biopsy in a timely fashion. Interestingly, retrospective studies suggest that at the time of diagnosis, up to 60% of pediatric melanomas are of intermediate thickness, which may in part be due to a delay in the clinical diagnosis and hesitation on the part of the clinician to perform a biopsy Citation[6,7]. On the other hand, many benign CMN and AMN can manifest clinical features resembling melanoma, prompting unnecessary biopsies. It goes without saying that any technique that increases the sensitivity for detecting melanoma, while at the same time improving specificity, would be highly valued by clinicians. One technique that can improve a physician’s ability to detect melanoma and avoid superfluous biopsies in both children and adults is dermoscopy.
A dermatoscope is a noninvasive, hand-held tool that allows for the visualization of morphologic structures within pigmented skin lesions . The presence or absence of certain structures and colors, and their relative distribution within a lesion, can be used to guide management decisions, including whether or not to perform a biopsy. The utility of dermoscopy is exemplified by the ability to correctly identify benign SN, which often clinically resemble malignant melanoma. The diagnostic accuracy of SN increases from 56% with naked eye examination to 93% with the aid of dermoscopy Citation[8,9]. Given that surgical excision was previously the only option available to closely evaluate atypical cutaneous lesions, the benefit of noninvasive dermoscopy is incontrovertible. Examination of nevi with serial dermoscopic evaluations in addition to sequential clinical monitoring decreases the need for surgical removal of suspicious lesions from 15.6 to 9%, when compared with unaided visual assessment alone Citation[10].
Two dermoscopic devices and methods of examining skin lesions exist, and include nonpolarized and polarized dermoscopy. In the case of nonpolarized dermoscopy, oil, alcohol or water is applied to the skin to create a liquid interface, and the lens of the dermatoscope comes in direct contact with the patient’s skin. Polarized dermatoscopes, on the other hand, do not require instrument-to-skin contact or an immersion liquid for visualization of subsurface morphology. As such, polarized dermoscopy is the method of choice when examining apprehensive children who tend to resist the physical contact involved in nonpolarized dermoscopic examinations.
In this article, we discuss prominent clinical and dermoscopic features across the spectrum of melanocytic lesions in children, and underscore the value of dermoscopy in the diagnosis of nevi and melanoma in the pediatric population.
Melanocytic nevi & melanoma
Congenital melanocytic nevi
Congenital nevi are likely the result of aberrant melanocyte migration, differentiation and deposition in the dermis during the early stages of embryogenesis Citation[11]. CMN are classified by size relative to the greatest diameter that they are expected to attain during adulthood. They are categorized into small (<1.5 cm), medium (1.5–19.9 cm), large (>20 cm) and very large or giant ‘garment’ nevi (>40–60 cm) Citation[12]. CMN have the tendency to enlarge in proportion to the growth of the child and may additionally develop changes in color and surface topography over time Citation[13,14]. Although the absolute risk for developing melanoma in association with CMN is low, the relative risk is high, and is more significant for larger CMN than for smaller CMN Citation[15]. Thus, it is important to scrutinize these lesions for focal changes that may herald the development of melanoma.
On clinical examination, CMN appear as flat to elevated, light to dark brown lesions with variable shades of pigmentation and irregular borders. The surface topography ranges from smooth to mammillated to cribriform, and coarse terminal hairs may protrude from the lesion, which becomes most prominent during puberty Citation[16]. Nearly 75% of patients with large CMN have satellite nevi; however, these satellite nevi do not appear to carry an increased risk for developing cutaneous melanoma Citation[17]. Dermoscopic assessment of the subsurface morphology of CMN and associated satellite lesions with attention to recognizable benign patterns is beneficial in distinguishing CMN from melanoma, and in identifying a focus of melanoma arising along the dermo–epidermal junction within a CMN Citation[15,17]. Most CMN, especially smaller lesions, manifest a symmetric, organized pattern on clinical and dermoscopic evaluation. That being said, it is not uncommon to encounter CMN with a disorganized pattern under dermoscopy, and in such cases it may be difficult to exclude melanoma from the diagnosis without performing a biopsy to definitively rule out malignancy .
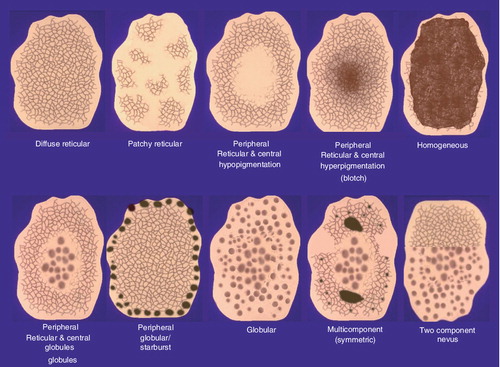
The predominant dermoscopic patterns in CMN are diffuse reticular, patchy reticular, globular, peripheral reticular with central globules (reticular-globular) and homogeneous & . The type of dermoscopic pattern manifested in a CMN is related in large part to the anatomical location of the nevus. Most CMN located on the lower extremities exhibit a reticular pattern, on the head and neck a globular pattern, and the majority of CMN on the torso manifest a globular or reticular-globular pattern Citation[18,19]. The reticular network in CMN are defined by their quality, distribution and some unique traits that are specific to CMN. The lines of the network can be thin or thick in quality. The distribution of the reticulation most commonly extends throughout the lesion , but may be focally present within the lesion or localized to the periphery . Furthermore, the pigment network can appear as thickened linear network fragments or branched streaks resembling fungal hyphae. Observing the aforementioned patterns under dermoscopy in the absence of dermoscopic features that are concerning for malignancy greatly increases the likelihood that a lesion is a CMN and not a melanoma.
As previously mentioned, most CMN on the head, neck and trunk have a globular component Citation[20]. The quality, distribution and specific types of globules within CMN are important to note. Globules are delineated as small or large in size, and when the globules are large in size, angulated in shape and crowded back-to-back within the lesion, they are described as having a ‘cobblestone’ pattern Citation[21]. In addition, it is common to see small globules centered within the hole of the network in CMN; this particular configuration of globules is called target network with globules or target globules Citation[22]. The relative distribution of globules within nevi is variable, although common patterns include sparse distribution throughout the lesion, dense aggregates throughout the lesion and central clustering surrounded by reticulation. Additional unique dermoscopic structures commonly visualized in CMN include small milia-like cysts, perifollicular pigment changes and various types of blood vessels Citation[23,24].
Congenital melanocytic nevi that deviate from the patterns described above, exhibit dermoscopic features that are commonly observed in melanoma , reveal an asymmetric or multicomponent pattern, or exhibit a rapid growth should be viewed with caution, and melanoma should enter the differential diagnosis . It is important to note that despite parental concerns regarding the increased relative risk of malignant transformation within a CMN, there is no data to suggest that the prophylactic removal of these lesions lowers the incidence of melanoma Citation[25]. With that being said, absence of evidence is not evidence of absence. It should be intuitively obvious that complete surgical removal of a CMN will prevent the development of melanomas that may have been destined to arise within that CMN. Furthermore, if psychosocial or cosmetic concerns become paramount, surgical excision, dermabrasion, curettage or laser therapy may be entertained to improve cosmetic and functional outcomes with varying levels of patient satisfaction Citation[26]. For patients at an increased risk for developing melanoma, periodic clinical monitoring with serial photographic and dermoscopic evaluation is essential to screen for changes that would reflect malignant transformation.
Nevus spilus (speckled lentiginous nevus)
Nevus spilus is purported to be a subtype of CMN that initially presents as a well-delineated hyperpigmented patch or café au lait macule that later develops a characteristic, superimposed speckling pattern Citation[27,28]. The lesions are clinically asymptomatic and expand in size in proportion to the growth of the affected child. In some cases, speckled lentiginous nevus (SPLN) have a relatively small diameter, but the nevus can in fact involve a large portion of the body surface area. Monitoring of SPLN in a clinical and dermoscopic manner provides information about any variations in morphology that would be indicative of melanoma.
Two subtypes of nevus spilus have been reported in the literature and include the macular variant, nevus spilus maculosus, and the papular variant, nevus spilus papulosus Citation[29,30]. The macular type is characterized by evenly distributed, flat, dark speckles within a large, well-circumscribed, light brown patch. The papular type, on the other hand, consists of a well-circumscribed lentiginous patch that is studded with multiple raised papules. Histologic evaluation of the speckled component in the papular form demonstrates dermal or compound melanocytic nests, whereas in the macular type the melanocytes are predominantly junctional. Both macular and papular SPLN are associated with an increased probability of developing SN, which must be distinguished from melanoma Citation[29]. Interestingly, the risk of developing cutaneous melanoma within a SPLN appears to be greater for those exhibiting the macular subtype (25 vs 10%) Citation[29].
Nevus spilus has been likened to a ‘melanocytic garden’ in which BN, SN, junctional nevi, compound nevi, intradermal nevi and melanoma can arise Citation[27,31]. Given the reported association of melanoma arising within a nevus spilus, periodic clinical monitoring and dermoscopic evaluation seems reasonable Citation[32]. Under dermoscopy, the background of a benign nevus spilus appears homogeneous tan in color and may exhibit a faint, delicate network. Hyperpigmented macules or papules within a SPLN generally show dermoscopic features that correlate with the type of nevus present (refer to the ‘Acquired melanocytic nevi’ section below) Citation[33]. Utilizing dermoscopy facilitates ongoing supervision of such lesions and minimizes unnecessary surgical procedures in children Citation[34].
Acquired melanocytic nevi
Acquired melanocytic nevi are benign proliferations of melanocytes, and both genetic and environmental factors have been associated with the development of acquired nevi Citation[35,36]. On clinical examination, AMN typically measure less than 6 mm in diameter and have a homogeneous tan to brown color, with a smooth surface topography Citation[37]. AMN are classified histologically into three groups: junctional nevi; compound nevi; and intradermal nevi.
Most acquired nevi exhibit one of ten symmetric and organized dermoscopic patterns . Typical patterns observed in acquired nevi include: diffuse or patchy network; peripheral reticulation with central hypo- or hyper-pigmentation; homogeneous tan/pink/brown or blue pigmentation; peripheral network with central globules; peripheral globular or starburst; globular; two-component; and symmetric multicomponent Citation[38,39]. In general, most individuals manifest a predominant nevus pattern, which is defined as the nevus pattern configuration in more than 30% of their nevi Citation[40]. The prevalent nevus pattern depends to some degree on the age and skin type of the individual. Children of less than 10 years of age classically have globular or homogeneous patterned nevi, whereas adults generally display reticulated nevi Citation[41]. Individuals with Fitzpatrick skin type I (fair complexion) have a predominant nevus pattern typified by light brown coloration with central hypopigmentation , types II and III have light to dark brown nevi with multifocal pigmentation, and type IV individuals (dark complexion) classically have black or dark brown nevi with central hyperpigmentation Citation[40]. Visualization of a predominant nevus pattern can help identify those individuals who are at an increased risk for developing melanoma, and aid in the detection of ‘ugly duckling’ outlier lesions that may represent malignancy Citation[42,43].
A reported dermoscopic finding in some AMN is the presence of peripheral globules around the entire perimeter of the nevus . This pattern signifies active growth of the nevus, and therefore it can be predicted that such lesions will symmetrically enlarge over time. Once these nevi enter senescence, they manifest a reticular or homogeneous pattern, and the peripheral globules tend to diminish in size until they eventually disappear from sight Citation[44]. From a management perspective, it is reassuring to know that symmetrically enlarging acquired nevi manifesting a peripheral rim of globules do not need to be subjected to biopsy and can be confidently monitored clinically and dermoscopically in children and young adults.
Although the ABCD mnemonic (asymmetry, border irregularity, color variation and diameter >6 mm) can assist in differentiating benign melanocytic nevi from melanoma, it is clear that the criteria lacks specificity since many melanocytic nevi, especially dysplastic nevi, can manifest the ABCDs or can enlarge over time Citation[45,46]. For that reason, one should not assume that all evolving melanocytic lesions in children represent melanoma. In the Framingham study, 76% of children were noted to develop new nevi, 29% had regressing nevi, 34% had enlarging nevi and as many as 50% of AMN had dermoscopic variation from baseline when viewed at a 3-year follow-up visit Citation[47]. It is essential to appreciate that changing lesions that maintain the benign patterns listed in and are not otherwise suspicious for malignancy do not require excision.
In the literature, the perception of a structureless blue area has been described on dermoscopic evaluation of some AMN, including BN, combined nevi and compound and intradermal nevi Citation[48]. AMN with melanin pigment in the dermis, either in melanophages or pigmented melanocytes, will exhibit a blue hue when viewed dermoscopically Citation[49]. A direct contrast must be made with the ‘blue–white veil’ that is associated with melanoma and consists of an irregular structureless area with blue pigment with an overlying ‘ground glass’ hazy appearance Citation[50,51]. The faint blue hue noted within some melanocytic nevi may simulate the blue–white veil perceived in melanoma, and such lesions should be closely monitored or biopsied to definitively rule out malignancy.
Blue nevi
Blue nevi are rarely present at birth, and tend to emerge during childhood and adolescence. BN represent benign proliferations of dermal dendritic melanocytes; likely an aberrant, premature arrest of migrating melanocytes in the dermis during fetal development. A BN appears clinically as a small, smooth-surfaced, blue papule on the head, neck, distal extremities or buttocks. Various classes of BN have been described, and include black–blue nevi, cellular BN, sclerosing BN and polychromatic BN Citation[52]. Cellular BN are benign neoplasms that are large, blue–black lesions measuring 1–3 cm in diameter Citation[53]. Epitheliod BN are a histologic subtype of nevi that are associated with the Carney complex in children, encompassing a triad of cardiac myxomas, skin hyperpigmentation and endocrine overactivity Citation[54]. As a general rule, BN are benign in children; however, on exceedingly rare ocassions, a large, evolving, blue–black plaque may represent a primary or metastatic melanoma (‘malignant blue nevus’) Citation[55–57].
The dermoscopic features of BN vary when lesions are observed with nonpolarized and polarized light dermoscopy. In general, nonpolarized dermoscopy allows clinicians to diagnose a blue nevus with greater confidence than polarized dermoscopy, owning to the more homogeneous blue hue visualized under a nonpolarized light source Citation[58]. Dermoscopically, BN appear as symmetrical, sharply defined lesions displaying a uniformly homogenous steel-blue color Citation[59]. Arising as solitary lesions, BN may be concerning on visual inspection; however, the absence of any melanoma-associated structures and the presence of homogeneity on dermoscopic evaluation are reassuring characteristics of a BN Citation[60]. Any evidence of dermoscopic heterogeneity or the presence of a network, globules, vessels or chrysalis structures should raise suspicion for melanoma; however, even these lesions usually prove to be sclerosing BN or combined nevi (congenital nevus with several populations of melanocytes) on histopathologic evaluation.
Halo nevi (Sutton’s nevi, leukoderma acquisitum centrifugum)
Halo nevi (HN) are benign melanocytic nevi, mainly compound or dermal type, that are encircled by a zone of depigmentation Citation[61]. These nevi are common in the pediatric population, and it is not uncommon for affected individuals to have multiple HN on the body Citation[62]. It has been hypothesized that HN undergo spontaneous involution by a T-cell-mediated immune response over time, and may even fully disappear by adulthood Citation[63]. Once the regression of the central nevus is complete, the area of previous depigmentation tends to repigment to the color of the surrounding skin. Although HN are benign, on very rare occasions regressing melanomas may mimic HN Citation[64]. In addition, although infrequently, a regressing melanoma can lead to the sudden transformation of existing nevi into HN Citation[65–67]. Thus, patients presenting with many eruptive HN should be examined carefully to ensure that they do not have an undiagnosed melanoma somewhere on their skin.
Dermoscopic evaluation of a HN typically reveals a globular or homogeneous pattern in the central pigmented portion of the lesion, delimited by a halo of depigmentation Citation[68]. The melanocytic nevus within the halo reduces in size during the regression process and eventually disappears Citation[69]. Following regression, a tan to pink central region with dotted vessels may be observed in place of the nevus. Continued monitoring of HN usually reveals a decrease in halo diameter over time, eventuating in the replacement of the depigmented area with normal-appearing skin color. Excision of HN in children should only be contemplated if the dermoscopic features vary from the aforementioned benign patterns , if the halo is irregular (i.e., asymmetrical) or if the depigmented area reveals melanoma concerning structures, such as extensive peppering or a blue–white veil .
Spitz nevus
Spitz nevi are dome-shaped, pink to brown papules that often arise on the head, neck and extremities in children Citation[70]. The lesions generally measure less than 1 cm in diameter, but may grow over time and change color, raising concern for melanoma Citation[71]. In the past, SN were histopathologically mistaken for melanoma due to overlapping microscopic features, but are now accepted as a benign entity when appropriately diagnosed Citation[72,73]. The differential diagnosis of SN is broad, and includes Clark’s nevus, pyogenic granuloma, hemangioma, juvenile xanthogranuloma and melanoma.
The diagnosic accuracy of SN has increased from 56 to 93% with the use of dermoscopy Citation[8]. Differentiating melanoma from SN is less complicated when dermoscopic patterns are recognized and appreciated during clinical examination. The most common patterns seen in SN are starburst, thick reticular network, globular, homogeneous pink, homogeneous black lamella, negative pigment network and atypical or multicomponent pattern Citation[74]. In addition, it is not uncommon to see chrysalis structures within SN Citation[75,76]. Of the abovementioned patterns, the most commonly encountered type is the starburst pattern, which appears in over 50% of SN Citation[77]. SN with a starburst pattern often have a blue-gray to brown-black center that is surrounded by multiple rows of globules or streaks/pseudopods arranged in a radial pattern at the periphery. When this characteristic starburst pattern is encountered, there is a diagnostic sensitivity of 96% for SN Citation[78]. Sequential dermoscopic monitoring of a classic starburst patterned SN will likely reveal symmetric growth of the lesion followed by the transformation of the starburst pattern into a homogeneous pattern Citation[79,80].
A negative network pattern, in the presence or absence of chrysalis-like structures, is another frequently observed characteristic of SN Citation[81]. The negative network, which can be visualized with both polarized and nonpolarized dermoscopy, appears as light areas delineating the grid and dark regions representing the holes. Another way to envision a negative network is that the darker structures are irregular, elongated, curvilinear globules surrounded by relative hypopigmentation . In counter distinction, a chrysalis structure consists of shiny, bright white lines that likely correspond to dermal collagen, which due to its birefringent properties, is easily visible using polarized light dermoscopy . Negative network and chrysalis structures are not commonly encountered in melanocytic lesions, but when both are visualized, a diagnosis of SN versus melanoma is favored Citation[75].
Besides the prototypes mentioned above, SN can also present with a homogeneous dark and thickened network pattern Citation[82]. Globular patterned SN are noted in as many as 22% of lesions, and consist of regularly distributed globules or irregular globular aggregates of varying size and color Citation[74]. In addition, peripherally located globules, giving a starburst-like appearance, can also be detected Citation[83]. Dermoscopic structures are rarely visualized in homogeneous black-lamella type SN; however, in the homogeneous pink variant, it is common to observe dotted blood vessels within the lesion. These pink, vascular lesions can resemble amelanotic melanoma on clinical and dermoscopic examination Citation[84]. SN can also present with an asymmetric or disorganized pattern. Such lesions may exhibit a multicomponent pattern or eccentric hyperpigmentation and, unfortunately, are impossible to differentiate from melanoma on clinical examination Citation[85,86].
The management of SN remains challenging given the risk of failure to differentiate a benign SN from a malignant neoplasm Citation[87]. The decision to excise a presumed SN in lieu of clinical monitoring is dependent on a host of factors, such as the clinical findings, dermoscopic findings, history and anxiety of the physician and/or patient/family, just to mention a few.
Melanoma
Approximately 2% of melanomas appear in individuals less than 20 years of age, and as such, the diagnosis of melanoma during childhood is not often entertained in the differential diagnosis Citation[4,88,89]. Although the symptoms and risk factors associated with melanoma in children parallel those in adults, a higher percentage of amelanotic and nodular melanomas are diagnosed in the pediatric population when compared with adults Citation[6]. These melanomas often lack the ABCD features commonly associated with superficial spreading melanoma, rendering the early detection of such lesions challenging. In other words, physicians often fail to include melanoma in the differential diagnosis of atypical lesions in children, resulting in significant delays in arriving at an accurate diagnosis. Confounding the problem of diagnostic delay is the reluctance by many physicians to subject children to a skin biopsy Citation[90].
Melanomas usually manifest an asymmetric dermoscopic pattern, or a pattern that deviates from the benign prototypes shown in Citation[91,92]. In addition, melanomas tend to contain at least one of the ten dermoscopic structures listed in Citation[93]. In other words, melanocytic lesions with organized and/or symmetric dermoscopic structures that display the benign dermoscopic patterns outlined in have an exceedingly low probability of being diagnosed as cutaneous melanoma. Any lesion that exhibits global patterns, specific structures or colors that deviate from the benign classifications noted in should be closely evaluated for attributes of malignancy .
The most common histopathologic subtypes of melanoma diagnosed in children are superficial spreading melanoma and nodular melanoma Citation[94]. Superficial spreading melanomas generally grow slowly and manifest one or more of the structures outlined in & . In comparison, nodular melanomas are often amelanotic and tend to grow rapidly, and the appreciation of atypical vascular structures on dermoscopy is of great value in diagnosing these melanomas . Malignant melanoma should enter the differential diagnosis of any nodular pink lesion exhibiting dotted vessels, irregular linear vessels or serpentine vessels, especially if the lesion is firm and rapidly growing Citation[60,95]. The fact that a patient is young should never preclude a physician from obtaining a biopsy if there is an index of suspicion for melanoma.
Expert commentary
The evaluation of melanocytic neoplasms in children is important given the rising incidence of melanoma in the pediatric population. The use of dermoscopy can facilitate in diagnosing melanomas while these tumors are still thin and treatment modalities generally have positive outcomes. A multifaceted approach increases the sensitivity and specificity for diagnosing melanoma, and includes a thorough clinical history, visual examination and routine dermoscopic evaluation of suspicious skin lesions. A recent meta-analysis demonstrated that the diagnostic accuracy of melanoma improved 49% with the use of dermoscopy Citation[96]. Although there is overwhelming evidence for the benefits of using dermoscopy, it is only utilized by approximately 20–25% of dermatologists in the USA Citation[96]. Thus, there remains a great need to encourage the diffusion of dermoscopy into the clinical practice.
Dermoscopy gives clinicians the ability to visualize subsurface colors, structures and patterns in skin neoplasms, which in turn leads to more accurate clinical diagnoses of cutaneous lesions and the implementation of appropriate management techniques. In the case of nevi that simulate melanoma on naked-eye visual examination, dermoscopy often provides a view of reassuring morphologic features that call for continued clinical monitoring, rather than surgical excision. Serial dermoscopy is especially beneficial in the monitoring of patients with CMN or in patients with familial atypical multiple mole-melanoma syndrome or atypical mole syndrome. The decision of whether to observe a clinically atypical lesion or to perform a biopsy can often be made with improved confidence based on the presence or absence of various features observed on dermoscopic evaluation. In addition, use of dermoscopy also has the potential to enhance patient and parental confidence in the diagnosis and management recommendations provided by the physician.
Five-year view
Increased efforts to improve physician awareness regarding the benefits of using dermoscopy remain paramount. Towards that end, continued dissemination of information regarding the benign and malignant dermoscopic features of lesions should prove to be a valuable endeavor. In addition, evidence-based literature suggests that dermoscopy is most beneficial in the hands of well-trained physicians, and as such, much still needs to be done to facilitate the integration of dermoscopy training into medical practice, residency programs and perhaps even medical school teachings. Although dermoscopy was only utilized by dermatologists in the early phases of its development, many family physicians are starting to eagerly engage in learning and implementing its use in clinical practice. We have previously published on the potential utility of dermoscopy for the pathologist Citation[97] and are currently investigating the feasibility of introducing dermoscopy to medical students who are starting their clinical skills curriculum. Another intriguing development is the recent observation that some high-risk patients have started purchasing their own dermatoscopes to assist them in their self-skin examination efforts. Thus, it is plausible that the dermatoscope will be in the hands of not just dermatologists, but the hands of many different types of healthcare providers and motivated patients over the next 5 years.
A better understanding of the utility of dermoscopy combined with improved training programs is likely to transform dermoscopy into a widely used tool, akin to the stethoscope, among primary care physicians, medical specialists and those in medical training. The dissemination of dermoscopy among medical professionals will likely have great impact on the early detection of melanoma, translating into lives saved from this potentially fatal malignancy. Dermoscopy seeks to gain a special place in the detection and monitoring of melanocytic neoplasms in children, as the technique assuredly helps guide physicians as to whether or not surgical interventions are necessary. Without a doubt, the ease with which nevoid lesions can be evaluated is enhanced with dermoscopy.
Table 1. Dermoscopic structures and their histopathological correlates.
Table 2. Benign dermoscopic patterns.
Table 3. Histopathologic correlates of dermoscopic structures indicative of melanoma.
Key issues
• Children present to pediatricians and dermatologists with a variety of melanocytic lesions, ranging from congenital nevi to acquired nevi to melanoma.
• Dermoscopy is a tool that improves the physician’s ability to diagnose melanoma and to differentiate benign nevi from melanoma.
• Knowledge of the dermoscopic colors, patterns and structures of benign versus malignant lesions is imperative for the physician using dermoscopy.
• Dermoscopy can guide management decisions regarding whether or not to biopsy or excise a clinically atypical or suspicious pigmented lesion.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Rhodes AR, Albert LS, Weinstock MA. Congenital nevomelanocytic nevi: proportionate area expansion during infancy and early childhood. J. Am. Acad. Dermatol.34(1), 51–62 (1996).
- Hamre MR, Chuba P, Bakhshi S, Thomas R, Severson RK. Cutaneous melanoma in childhood and adolescence. Pediatr. Hematol. Oncol.19(5), 309–317 (2002).
- Karlsson P, Boeryd B, Sander B, Westermark P, Rosdahl I. Increasing incidence of cutaneous malignant melanoma in children and adolescents 12–19 years of age in Sweden 1973–1992. Acta Derm. Venereol.78(4), 289–292 (1998).
- Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J. Clin. Oncol.23(21), 4735–4741 (2005).
- Huynh PM, Grant-Kels JM, Grin CM. Childhood melanoma: update and treatment. Int. J. Dermatol.44(9), 715–723 (2005).
- Ferrari A, Bono A, Baldi M et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics115(3), 649–654 (2005).
- Zuckerman R, Maier JP, Guiney WB Jr, Huntsman WT, Mooney EK. Pediatric melanoma: confirming the diagnosis with sentinel node biopsy. Ann. Plast. Surg.46(4), 394–399 (2001).
- Steiner A, Pehamberger H, Binder M, Wolff K. Pigmented Spitz nevi: improvement of the diagnostic accuracy by epiluminescence microscopy. J. Am. Acad. Dermatol.27(5 Pt 1), 697–701 (1992).
- Binder M, Puespoeck-Schwarz M, Steiner A et al. Epiluminescence microscopy of small pigmented skin lesions: short-term formal training improves the diagnostic performance of dermatologists. J. Am. Acad. Dermatol.36(2 Pt 1), 197–202 (1997).
- Carli P, de Giorgi V, Chiarugi A et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J. Am. Acad. Dermatol.50(5), 683–689 (2004).
- Cramer SF. The melanocytic differentiation pathway in congenital melanocytic nevi: theoretical considerations. Pediatr. Pathol.8(3), 253–265 (1988).
- Arndt KA. Precursors to malignant melanoma: congenital and dysplastic nevi. JAMA251(14), 1882–1883 (1984).
- Arneja JS, Gosain AK. Giant congenital melanocytic nevi. Plast. Reconstr. Surg.120(2), 26e–40e (2007).
- Tannous ZS, Mihm MC Jr, Sober AJ, Duncan LM. Congenital melanocytic nevi: clinical and histopathologic features, risk of melanoma, and clinical management. J. Am. Acad. Dermatol.52(2), 197–203 (2005).
- Marghoob AA, Schoenbach SP, Kopf AW, Orlow SJ, Nossa R, Bart RS. Large congenital melanocytic nevi and the risk for the development of malignant melanoma. A prospective study. Arch. Dermatol.132(2), 170–175 (1996).
- Soyer HP, Argenziano G, Chimenti S, Ruocco V. Dermoscopy of pigmented skin lesions. Eur. J. Dermatol.11(3), 270–276; quiz 277 (2001).
- DeDavid M, Orlow SJ, Provost N et al. A study of large congenital melanocytic nevi and associated malignant melanomas: review of cases in the New York University Registry and the world literature. J. Am. Acad. Dermatol.36(3 Pt 1), 409–416 (1997).
- Changchien L, Dusza SW, Agero AL et al. Age- and site-specific variation in the dermoscopic patterns of congenital melanocytic nevi: an aid to accurate classification and assessment of melanocytic nevi. Arch. Dermatol.143(8), 1007–1014 (2007).
- Argenziano G, Soyer HP, Chimenti S et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J. Am. Acad. Dermatol.48(5), 679–693 (2003).
- Zalaudek I, Manzo M, Savarese I, Docimo G, Ferrara G, Argenziano G. The morphologic universe of melanocytic nevi. Semin. Cutan. Med. Surg.28(3), 149–156 (2009).
- Braun RP, Rabinovitz HS, Oliviero M, Kopf AW, Saurat JH. Pattern analysis: a two-step procedure for the dermoscopic diagnosis of melanoma. Clin. Dermatol.20(3), 236–239 (2002).
- Zalaudek I, Manzo M, Ferrara G, Argenziano G. New classification of melanocytic nevi based on dermoscopy. Expert Rev. Dermatol.3(4), 477–489 (2008).
- Zalaudek I, Manzo M, Ferrara G, Manzo M, Savarese I, Argenziano G. Problematic melanocytic lesions in children. Expert Rev. Dermatol.4(3), 249–261 (2009).
- Ingordo V, Iannazzone SS, Cusano F, Naldi L. Reproducibility of dermoscopic features of congenital melanocytic nevi. Dermatology217(3), 231–234 (2008).
- Makkar HS, Frieden IJ. Congenital melanocytic nevi: an update for the pediatrician. Curr. Opin. Pediatr.14(4), 397–403 (2002).
- Marghoob AA, Borrego JP, Halpern AC. Congenital melanocytic nevi: treatment modalities and management options. Semin. Cutan. Med. Surg.22(1), 21–32 (2003).
- Schaffer JV, Orlow SJ, Lazova R, Bolognia JL. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch. Dermatol.137(2), 172–178 (2001).
- Stewart DM, Altman J, Mehregan AH. Speckled lentiginous nevus. Arch. Dermatol.114(6), 895–896 (1978).
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology212(1), 53–58 (2006).
- Happle R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin. Exp. Dermatol.34(2), 133–135 (2009).
- Cramer SF. Speckled lentiginous nevus (nevus spilus): the ‘roots’ of the ‘melanocytic garden’. Arch. Dermatol.137(12), 1654–1655 (2001).
- Johr RH, Binder M. Management of nevus spilus-a better way. Pediatr. Dermatol.17(6), 491–492 (2000).
- Haenssle HA, Kaune KM, Buhl T et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J. Am. Acad. Dermatol.61(2), 337–341 (2009).
- Zalaudek I, Sgambato A, Ferrara G, Argenziano G. Diagnosis and management of melanocytic skin lesion in the pediatric praxis. A review of the literature. Minerva Pediatr.60(3), 291–312 (2008).
- Valiukeviciene S, Miseviciene I, Gollnick H. The prevalence of common acquired melanocytic nevi and the relationship with skin type characteristics and sun exposure among children in Lithuania. Arch. Dermatol.141(5), 579–586 (2005).
- Harth Y, Friedman-Birnbaum R, Linn S. Influence of cumulative sun exposure on the prevalence of common acquired nevi. J. Am. Acad. Dermatol.27(1), 21–24 (1992).
- Rao BK, Wang SQ, Murphy FP. Typical dermoscopic patterns of benign melanocytic nevi. Dermatol. Clin.19(2), 269–284 (2001).
- Zalaudek I, Grinschgl S, Argenziano G et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br. J. Dermatol.154(2), 299–304 (2006).
- Zalaudek I, Hofmann-Wellenhof R, Kittler H et al. A dual concept of nevogenesis: theoretical considerations based on dermoscopic features of melanocytic nevi. J. Dtsch. Dermatol. Ges.5(11), 985–992 (2007).
- Zalaudek I, Docimo G, Argenziano G. Using dermoscopic criteria and patient-related factors for the management of pigmented melanocytic nevi. Arch. Dermatol.145(7), 816–826 (2009).
- Zalaudek I, Leinweber B, Hofmann-Wellenhof R et al. The epidermal and dermal origin of melanocytic tumors: theoretical considerations based on epidemiologic, clinical, and histopathologic findings. Am. J. Dermatopathol.30(4), 403–406 (2008).
- Lipoff JB, Scope A, Dusza SW, Marghoob AA, Oliveria SA, Halpern AC. Complex dermoscopic pattern: a potential risk marker for melanoma. Br. J. Dermatol.158(4), 821–824 (2008).
- Scope A, Dusza SW, Halpern AC et al. The ‘ugly duckling’ sign: agreement between observers. Arch. Dermatol.144(1), 58–64 (2008).
- Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Frequency and characteristics of enlarging common melanocytic nevi. Arch. Dermatol.136(3), 316–320 (2000).
- Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch. Dermatol.141(8), 998–1006 (2005).
- Abbasi NR, Shaw HM, Rigel DS et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA292(22), 2771–2776 (2004).
- LaVigne EA, Oliveria SA, Dusza SW, Geller AC, Halpern AC, Marghoob AA. Clinical and dermoscopic changes in common melanocytic nevi in school children: the Framingham school nevus study. Dermatology211(3), 234–239 (2005).
- Pellacani G, Bassoli S, Longo C, Cesinaro AM, Seidenari S. Diving into the blue: in vivo microscopic characterization of the dermoscopic blue hue. J. Am. Acad. Dermatol.57(1), 96–104 (2007).
- Massi D, De Giorgi V, Carli P, Santucci M. Diagnostic significance of the blue hue in dermoscopy of melanocytic lesions: a dermoscopic-pathologic study. Am. J. Dermatopathol.23(5), 463–469 (2001).
- Celebi ME, Iyatomi H, Stoecker WV et al. Automatic detection of blue–white veil and related structures in dermoscopy images. Comput. Med. Imaging Graph.32(8), 670–677 (2008).
- Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch. Dermatol.132(10), 1178–1182 (1996).
- Gonzalez-Campora R, Galera-Davidson H, Vazquez-Ramirez FJ, Diaz-Cano S. Blue nevus: classical types and new related entities. A differential diagnostic review. Pathol. Res. Pract.190(6), 627–635 (1994).
- Bhawan J, Chang WH, Edelstein LM. Cellular blue nevus. An ultrastructural study. J. Cutan. Pathol.7(2), 109–122 (1980).
- Boikos SA, Stratakis CA. Carney complex: the first 20 years. Curr. Opin. Oncol.19(1), 24–29 (2007).
- Schaffer JV. Pigmented lesions in children: when to worry. Curr. Opin. Pediatr.19(4), 430–440 (2007).
- Hocevar M, Kitanovski L, Frkovic Grazio S. Malignant blue nevus with lymph node metastases in five-year-old girl. Croat. Med. J.46(3), 463–466 (2005).
- Goldenhersh MA, Savin RC, Barnhill RL, Stenn KS. Malignant blue nevus. Case report and literature review. J. Am. Acad. Dermatol.19(4), 712–722 (1988).
- Pan Y, Gareau DS, Scope A, Rajadhyaksha M, Mullani NA, Marghoob AA. Polarized and nonpolarized dermoscopy: the explanation for the observed differences. Arch. Dermatol.144(6), 828–829 (2008).
- Ferrara G, Soyer HP, Malvehy J et al. The many faces of blue nevus: a clinicopathologic study. J. Cutan. Pathol.34(7), 543–551 (2007).
- Marghoob AA, Braun RP, Koph AW. Atlas of Dermoscopy. Taylor and Francis, London, UK (2005).
- Frank SB, Cohen HJ. The halo nevus. Arch. Dermatol.89, 367–373 (1964).
- Wayte DM, Helwig EB. Halo nevi. Cancer22(1), 69–90 (1968).
- Tokura Y, Yamanaka K, Wakita H et al. Halo congenital nevus undergoing spontaneous regression. Involvement of T-cell immunity in involution and presence of circulating anti-nevus cell IgM antibodies. Arch. Dermatol.130(8), 1036–1041 (1994).
- Arpaia N, Cassano N, Vena GA. Regressing cutaneous malignant melanoma and vitiligo-like depigmentation. Int. J. Dermatol.45(8), 952–956 (2006).
- Epstein WL, Sagebeil R, Spitler L, Wybran J, Reed WB, Blois MS. Halo nevi and melanoma. JAMA225(4), 373–377 (1973).
- Fishman HC. Letter: malignant melanoma arising with two halo nevi. Arch. Dermatol.112(3), 407–408 (1976).
- Whimster IW. Letter: the halo naevus and cutaneous malignant melanoma. Br. J. Dermatol.90(1), 111–113 (1974).
- Kolm I, Di Stefani A, Hofmann-Wellenhof R et al. Dermoscopy patterns of halo nevi. Arch. Dermatol.142(12), 1627–1632 (2006).
- Terushkin V, Scope A, Halpern AC, Marghoob AA. Pathways to involution of nevi: insights from dermoscopic follow-up. Arch. Dermatol.146(4), 459–460 (2010).
- Weedon D, Little JH. Spindle and epithelioid cell nevi in children and adults. A review of 211 cases of the Spitz nevus. Cancer40(1), 217–225 (1977).
- Barnhill RL, Argenyi ZB, From L et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum. Pathol.30(5), 513–520 (1999).
- Peters MS, Goellner JR. Spitz naevi and malignant melanomas of childhood and adolescence. Histopathology10(12), 1289–1302 (1986).
- Shimek CM, Golitz LE. The golden anniversary of the Spitz nevus. Arch. Dermatol.135(3), 333–335 (1999).
- Ferrara G, Argenziano G, Soyer HP et al. The spectrum of Spitz nevi: a clinicopathologic study of 83 cases. Arch. Dermatol.141(11), 1381–1387 (2005).
- Marghoob AA, Cowell L, Kopf AW, Scope A. Observation of chrysalis structures with polarized dermoscopy. Arch. Dermatol.145(5), 618 (2009).
- Scope A, Zalaudek I, Ferrara G, Argenziano G, Braun RP, Marghoob AA. Remodeling of the dermoepidermal junction in superficial spreading melanoma: insights gained from correlation of dermoscopy, reflectance confocal microscopy, and histopathologic analysis. Arch. Dermatol.144(12), 1644–1649 (2008).
- Peris K, Ferrari A, Argenziano G, Soyer HP, Chimenti S. Dermoscopic classification of Spitz/Reed nevi. Clin. Dermatol.20(3), 259–262 (2002).
- Yadav S, Vossaert KA, Kopf AW, Silverman M, Grin-Jorgensen C. Histopathologic correlates of structures seen on dermoscopy (epiluminescence microscopy). Am. J. Dermatopathol.15(4), 297–305 (1993).
- Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J. Am. Acad. Dermatol.43(3), 467–476 (2000).
- Argenziano G, Zalaudek I, Ferrara G, Lorenzoni A, Soyer HP. Involution: the natural evolution of pigmented Spitz and Reed nevi? Arch. Dermatol.143(4), 549–551 (2007).
- Pellacani G, Longo C, Ferrara G et al. Spitz nevi: in vivo confocal microscopic features, dermatoscopic aspects, histopathologic correlates, and diagnostic significance. J. Am. Acad. Dermatol.60(2), 236–247 (2009).
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J. Am. Acad. Dermatol.17(4), 571–583 (1987).
- Argenziano G, Zalaudek I, Corona R et al. Vascular structures in skin tumors: a dermoscopy study. Arch. Dermatol.140(12), 1485–1489 (2004).
- Menzies SW, Kreusch J, Byth K et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch. Dermatol.144(9), 1120–1127 (2008).
- Argenziano G, Scalvenzi M, Staibano S et al. Dermatoscopic pitfalls in differentiating pigmented Spitz naevi from cutaneous melanomas. Br. J. Dermatol.141(5), 788–793 (1999).
- Johr R, Stolz W. Dermoscopy: An Illustrated Self Assessment Guide. McGraw-Hill Medical, NY, USA (2010).
- Gelbard SN, Tripp JM, Marghoob AA et al. Management of Spitz nevi: a survey of dermatologists in the United States. J. Am. Acad. Dermatol.47(2), 224–230 (2002).
- Boddie AW Jr, Smith JL Jr, McBride CM. Malignant melanoma in children and young adults: effect of diagnostic criteria on staging and end results. South. Med. J.71(9), 1074–1078 (1978).
- Scope A, Halpern AC. Melanoma of childhood and adolescence. Cutis77(1), 13–14 (2006).
- Chamlin SL, Williams ML. Moles and melanoma. Curr. Opin. Pediatr.10(4), 398–404 (1998).
- Benelli C, Roscetti E, Pozzo VD, Gasparini G, Cavicchini S. The dermoscopic versus the clinical diagnosis of melanoma. Eur. J. Dermatol.9(6), 470–476 (1999).
- Lorentzen HF, Weismann K, Larsen FG. Structural asymmetry as a dermatoscopic indicator of malignant melanoma – a latent class analysis of sensitivity and classification errors. Melanoma Res.11(5), 495–501 (2001).
- Zalaudek I, Argenziano G, Ferrara G et al. Clinically equivocal melanocytic skin lesions with features of regression: a dermoscopic-pathological study. Br. J. Dermatol.150(1), 64–71 (2004).
- Ceballos PI, Ruiz-Maldonado R, Mihm MC Jr. Melanoma in children. N. Engl. J. Med.332(10), 656–662 (1995).
- Kreusch JF. Vascular patterns in skin tumors. Clin. Dermatol.20(3), 248–254 (2002).
- Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol.3(3), 159–165 (2002).
- Scope A, Busam KJ, Malvehy J et al.Ex vivo dermoscopy of melanocytic tumors: time for dermatopathologists to learn dermoscopy. Arch. Dermatol.143(12), 1548–1552 (2007).
Dermoscopy of nevi and melanoma in childhood
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/expertderm. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Which of the following statements about the potential advantages and disadvantages of dermoscopy of melanocytic lesions in children is correct?
□ A Dermoscopy is invasive
□ B Dermoscopy allows for the visualization of subsurface colors and structures within pigmented melanocytic skin lesions
□ C Dermoscopy is not helpful in diagnosing melanomas until the tumors have advanced thickness
□ D Dermoscopy is used by most US dermatologists
2. Your patient is a 3-year-old, white girl with a suspicious melanocytic lesion on her forearm. Which of the following features seen on dermoscopy is most likely to indicate that the lesion is benign?
□ A Reticular diffuse global pattern
□ B Negative pigment network
□ C Streaks
□ D Blue–white structures over flat areas
3. Dermoscopy on the patient in question 2 suggests that the lesion is a melanoma. Which of the following statements is most likely to apply to her work-up and management?
□ A Dermoscopy improves diagnostic accuracy for melanoma by 15% over clinical evaluation alone
□ B Chrysalis can be visualized without polarized light
□ C Observation or serial dermoscopy is preferred over biopsy
□ D Superficial spreading melanoma and nodular melanoma are the most common histopathologic subtypes of melanoma diagnosed in children
4. Which of the following statements about melanocytic neoplasms of childhood is correct?
□ A There are 5 distinct classes of melanocytic neoplasms of childhood
□ B Nevus spilus is an acquired melanocytic nevus (AMN)
□ C Spitz nevi are congenital melanocytic nevi (CMN)
□ D Melanoma should be included in the differential diagnosis of atypical pigmented or amelanotic lesions in healthy children