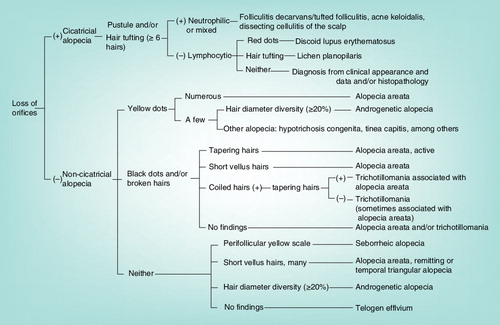
Abstract
Trichoscopy is hair and scalp dermoscopy using a handheld dermoscope or polarized light video microscope. This article shows the revised trichoscopic algorithm for common hair loss diseases. For the algorithm for cicatricial alopecia, loss of orifices, micropustules and/or hair tufting with six or more hairs should be carefully observed. When loss of hair orifices cannot be seen in the hair loss area, diagnosis as noncicatricial alopecia is established. Among noncicatricial alopecia, alopecia areata is most commonly encountered and should be mainly considered for differential diagnosis. Therefore, the first check point is yellow dots, black dots or broken hairs. The hallmark of androgenetic alopecia and tinea capitis is hair diameter diversity (≥20%) and comma hairs, respectively. Hair shaft abnormalities can be diagnosed from the characteristics of trichoscopy reported until now.
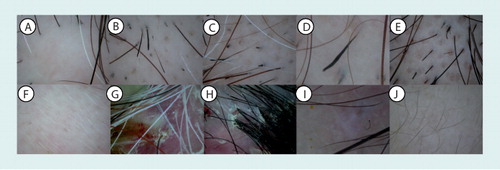
(A) loss of orifices in linear scleroderma (sclerodermie en coup de sabre), (B) broken hairs, (C) black dots, (D) tapering hairs (exclamation mark hairs), (E) yellow dots and (F) short vellus hairs in alopecia areata, (G) micropustules and (H) hair tufting with six or more hairs in folliculitis decalvans/tufted folliculitis, (I) comma hairs in tinea capitis, and (J) short vellus hairs in androgenetic alopecia.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertderm; (4) view/print certificate.
Release date: 15 November 2012; Expiration date: 15 November 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Distinguish an effective algorithm for generating a diagnosis from trichoscopy
• Assess the appearance of different hair diseases on trichoscopy
• Analyze findings of temporal triangular alopecia
• Evaluate trichoscopy findings in determining the prognosis of alopecia areata
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine
Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Shigeki Inui, MD, PhD
Department of Regenerative Dermatology, Osaka University Graduate School of Medicine, Osaka, Japan
Disclosure: Shigeki Inui, MD, PhD, has disclosed no relevant financial relationships.
Trichoscopy
Trichoscopy is hair and scalp dermoscopy using a handheld dermoscope or polarized light video microscope Citation[1,2]. Historically, in 1993, Kossard and Zagarella described spotted white dots in cicatricial alopecia in dark skin as the first dermoscopic finding in hair loss diseases Citation[3]. While some reports later showed the usefulness of dermoscopy for hair diseases Citation[4–9], this technique was still not so popular. However, after Ross et al. Citation[10] reported the usefulness of various dermoscopic features in common hair loss diseases and Rakowska et al. Citation[11] showed availability of the dermoscope in observing hair shaft abnormality, dermoscopy for hair diseases has become a hot topic and has been studied by many groups. Dermoscopy was originally used for observing and diagnosing pigmented skin lesions, such as melanocytic nevus and melanoma, and it was claimed that dermoscopy, especially without immersion gel, for hair diseases is a different methodology from the orthodox dermoscopy (personal communication). This technique was then termed as trichoscopy Citation[2]. Because of noninvasiveness and easy accessibility to the hair or scalp, it is very useful in the clinical practice. By trichoscopy, alopecic and hair shaft disorders can be observed and diagnosed, avoiding skin biopsy that is otherwise necessary to precisely diagnose. Indeed, yellow dots, a typical trichoscopic feature in alopecia areata (AA), are perceptible only by trichoscopy Citation[10,12,13]. The algorithmic method for trichoscopic diagnosis of hair loss disease was previously proposed Citation[14] and in this article the revised version is shown and discussed. Additionally, trichoscopy for hair shaft abnormalities is summarized based on the recent findings using trichoscopy.
Algorithmic method for diagnosis of hair loss diseases by trichoscopy: cicatricial or noncicatricial?
The revised trichoscopic algorithm is shown in . At the first step, whether the loss of orifices exists or not should be observed. The loss of orifices corresponds to permanent destruction of hair follicles, resulting in fibrosis, and is therefore of great importance for predicting prognosis because it determines if the hair loss is irreversible cicatricial or reversible noncicatricial. However, it may be very difficult to define it in some cases. Indeed, ophiasis type AA shows similar appearance to the loss of orifices without other typical signs of AA on hair loss areas, mimicking frontal fibrosing alopecia (FFA) Citation[15]. These two entities are ‘look-alike’ also in clinical appearance because they show recalcitrant hairline hair loss and therefore can be a diagnostic dilemma Citation[16]. Characteristic trichoscopic images in FFA are loss of orifices, perifollicular scale and feeble perifollicular erythema Citation[17,18], while those of AA are broken hairs, black dots, tapering hairs (exclamation mark hairs), yellow dots and short vellus hairs – Citation[13]. As for pathologic process, tapering hairs correspond to rapid catagen transition from anagen, broken hairs and black dots to the resultant hair fragility Citation[19], and yellow dots to distention of the affected follicular infundibulum with keratinous material and sebum Citation[10]. Among the signs for AA, yellow dots appear during application of topical corticosteroid Citation[15], indicating that careful long-term follow-up and observation are important to precisely carry out differential diagnosis. Furthermore, cicatricial marginal alopecia (CMA) was described as a new distinctive pattern of alopecia Citation[20]. Trichoscopic features of this entity are low hair density with loss of follicular ostia, reduced diameter of the remaining hairs and absence of perifollicular hyperkeratosis or abnormal scalp vessels Citation[21]. CMA must be considered in the differential diagnosis of FFA and ophiasis type AA.
Cicatricial alopecia: neutrophilic or lymphocytic?
After cicatricial alopecia is indicated from the loss of orifices, the existence of micropustules and/or hair tufting with six or more hairs Citation[22] should be carefully observed. Either finding indicates neutrophil- or mixed cell-mediated cicatricial alopecia such as folliculitis decalvans/tufted folliculitis, acne keloidalis and dissecting cellulitis of the scalp according to the recently proposed classification of primary cicatricial alopecia Citation[23]. In the long-lasting folliculitis decalvans/tufted folliculitis, white and milky-red (strawberry ice cream color) areas without hair follicular opening predominate Citation[24]. For further distinct diagnosis among these, the clinical appearance must be taken into consideration. If there are no findings such as micropustules and hair tufting, lymphocytic cicatricial alopecia is highly suggested. However, when neutrophilic or mixed cell alopecia is not so active or well controlled, no pustules are detected and the number of tufting hairs is four or five, suggesting that neutrophilic or mixed cell cicatricial alopecia may not be completely excluded from the above-mentioned points. Because two or three hair shafts emerge from one hair follicle unit in normal subjects, hair tufting is defined as positive when the number is four or more. Regarding the pathomechanism of hair tufting, inflammation around the isthmus and infundibulum damages infundibular epithelium of several follicular units, followed by coalescence of follicular units due to perifollicular fibrosis Citation[25]. At the further step, follicular red dots are specific for discoid lupus erythematosus Citation[26] and hair tufting indicates lichen planopilaris Citation[27]. The red dots correspond pathologically to dilated infundibula containing keratotic material. Additionally, reduction in number and size of sebaceous glands and the presence of dilated vessels and red blood cell extravasation in perifollicular distribution around the isthmus make the color reddish Citation[26]. After the inflammatory stage of lichen planopilaris, violaceous-blue interfollicular area, corresponding to pigment incontinence, can be seen by trichoscopy Citation[24]. Moreover, in the fibrotic stage, large irregular white dots merge into milky-red (strawberry ice cream color) or white areas Citation[3,28]. Without these features, the diagnosis must be determined based on clinical appearance and/or histopathological findings. Although it is possible to histopathologically discriminate between neutrophilic/mixed cell and lymphocytic cicatricial alopecia, there are rarely significant histopathological differences among the diseases of each category Citation[29], indicating that clinical morphology is more stressed for this diagnostic step. White dots were originally described in a black patient of lichen planopilaris as a focal decrease in epidermal melanin pigment overlying the site of fibrous tracts Citation[3], but the recent report showed that eccrine pores are also detected as white dots on the scalp of people with pigmented skin Citation[30]. Therefore, white dots should be carefully observed whether they are fibrous spots or simply eccrine pores.
Noncicatricial alopecia: do yellow dots, black dots or broken hairs exist or not?
When loss of hair orifices cannot be seen in the hair loss area, diagnosis as noncicatricial alopecia is established. Among noncicatricial alopecia, AA is most commonly encountered in the clinical practice and should be mainly considered for differential diagnosis. The first check point is subsequently whether yellow dots, black dots or broken hairs are seen or not. When numerous yellow dots are observed, the diagnosis of AA is confirmed. However, the limited number of yellow dots are seen in other type of alopecia including androgenetic alopecia (AGA) Citation[10,31], hypotrichosis congenita and tinea capitis Citation[14]. When hair diameter diversity (≥20%) Citation[9], corresponding to hair follicle miniaturization, is seen, the diagnosis should be AGA. Moreover, comma hairs Citation[32] , corkscrew hairs Citation[33], zigzag hairs and interrupted (Morse code-like) hairs Citation[24] are characteristic for tinea capitis. When these are not detected, further clinical and/or histopathological investigation is needed. As mentioned above, yellow dots are a sensitive marker for AA but are not specific because this sign is reportedly detected even in one of cicatricial alopecia discoid lupus erythematosus Citation[24].
AA & trichotillomania
In noncicatricial alopecia, black dots and/or broken hairs strongly suggest AA but trichotillomania should be carefully ruled out for diagnosing AA. AA can be discriminated from trichotillomania based on the existence of tapering hairs Citation[34] and/or clustered short vellus hairs (≤10 mm). The activity of AA can be monitored by the ratio of pathognomic hairs such as broken hairs, black dots and tapering hairs and short vellus hairs. However, AA often shows various trichoscopic conditions depending on the lesional sites, suggesting the importance of careful observation of the entire hair loss area. In trichotillomania, tapering hairs and clustered short vellus hairs are not observed because the premature catagen induction is unlikely to occur. The regrowing hairs in trichotillomania are in anagen phase, providing terminal hair shaft growth. Another point for differential diagnosis is coiled hairs formed by hair pulling in trichotillomania Citation[34]. However, we should be careful about coexistence of AA and trichotillomania Citation[35,36]. When tapering hairs exist, association with AA is indicated but if there are no signs such as tapering hairs, short vellus hairs or coiled hairs, either AA or trichotillomania and their association have to be considered. Collectively, it is worth noting that we have to be cautious for trichoscopic differential diagnosis for these two entities and need to obtain comprehensively clinical and/or histopathological data in ambiguous cases.
Seborrheic alopecia
When there are no yellow dots, black dots or broken hairs, perifollicular yellow scale, possibly mixed with sebum, are a clue to diagnose seborrheic alopecia Citation[14]. Because psoriasis rarely causes hair loss Citation[37], there may be a problem about differential diagnosis between these two diseases. The most significant findings in scalp psoriasis are red dots and globules, twisted red loops and glomerular vessels, corresponding to dilated capillaries in the dermal papillae, while those in seborrheic alopecia are arborizing vessels and atypical red vessels Citation[38].
Remitting AA, androgenetic alopecia & temporal triangular alopecia
When only short vellus hairs are seen without any other findings, differential diagnosis becomes problematic. Typically, this sign is observed in remitting AA, showing the regrowth activity of hairs, but some diseases should be included in the differential diagnosis. This feature can be seen in the most advanced area of AGA Citation[39] but the diagnosis of AGA is relatively easy by searching hair diameter diversity in the premature sites. Incomplete hair loss of the temporal scalp at childhood is sometimes difficult to specifically diagnose with two possibilities of remitting AA and temporal triangular alopecia (TTA). Although TTA is also referred as congenital triangular alopecia, the hair loss starts at birth only in 36.5% of patients and therefore the nomenclature of TTA is preferred Citation[40]. Hair loss in TTA is persistent while single type AA showing only short vellus hairs without pathognomic hairs apparently remits within 6 months Citation[41]. From this fact and the previous report of trichoscopy in TTA Citation[42], the diagnostic criteria including trichoscopic findings for TTA was proposed as follows Citation[41]:
• Triangular or lancet-shaped patch of alopecia involving front-temporal scalp;
• Trichoscopically normal follicular openings with vellus hairs surrounded by normal terminal hair area;
• Trichoscopically no broken hairs, tapering hairs, black dots, yellow dots and orifice loss;
• Persistent without significant hair regrowth for 6 months after clinically or trichoscopically confirming the existence of vellus hairs.
In addition, TTA can be discriminated from CMA by the absence of loss of hair orifices and low hair density. The differential diagnosis from aplasia cutis and nevus sebaceous is possible based on a complete lack of skin appendages and a translucent appearance in aplasia cutis and bright yellow dots not associated with hair follicles in nevus sebaceous Citation[43].
Telogen effluvium
In telogen effluvium, one report showed that yellow dots and short vellus hairs are seen in some cases of telogen effluvium Citation[39] and another indicated presence of upright regrowing hairs and predominance of hair follicle openings with only one emerging hair shaft as clues to diagnose telogen effluvium Citation[24]. However, specific diagnostic findings are rarely observed and therefore basically the diagnosis of telogen effluvium is based on exclusion of AGA, diffuse-type AA and other types of alopecia.
Limitation of trichoscopy & algorithm
Taken together, trichoscopy is very powerful tool to diagnose hair loss diseases but it must be kept in mind that the final diagnosis should be carried out from comprehensive consideration of trichoscopic and clinical images. When there are unsolved discrepancies, careful follow-up and histopathological investigation are recommended. Moreover, the algorithm shown here covers only limited common hair loss diseases and therefore requires expansion to the wider range of diseases.
Trichoscopic clues for diagnosing other hair loss diseases
Another pathway for diagnosing hair loss diseases is trichoscopic clues by characteristic features. In lipedematous alopecia of the scalp, the linear area of teleangiectasia within the scalp creases, possibly caused by compression of the superficial blood capillaries, can be trichoscopically seen Citation[44]. The orange spots seen by trichoscopy suggest scalp sarcoidosis and dystrophic hairs may indicate granulomatous activity Citation[45]. The orange spots correspond to the round, well-formed granulomas in the superficial dermis. Hair casts are trichoscopically detected as white to brown cylindrical structures encircling the proximal hair shafts, providing a diagnostic clue for traction alopecia Citation[46]. In the moth-eaten alopecia of secondary syphilis, the histopathology is similar to that of AA Citation[47,48] and therefore the trichoscopy may be mimicking. However, so far there is no report of trichoscopy in syphilitic alopecia.
Trichoscopic findings as clinical markers in AA
Trichoscopy is useful for not only diagnosis but also management of AA Citation[13,49]. In AA, the black dots, yellow dots and short vellus hairs correlated with the severity of disease. In addition the black dots, tapering hairs, and broken hairs correlate positively with disease activity but short vellus hairs correlate negatively Citation[13]. The yellow dots or short vellus hairs in the hair loss area were detected by trichoscopy in 63.7 or 72.7% of the 300 Asian patients with AA, respectively, and either yellow dots or short vellus hairs in the hair loss areas seen in 94.0% of the patients, suggesting that this combination enhances the sensitivity of diagnosis of AA Citation[13]. Additionally, because yellow dots predominate in long-lasting AA Citation[24], disease duration of the enrolled patients may make a difference in the incidence of yellow dots. In the African–American patients with AA, a diffuse honeycomb-like pigmented network and white dots are observed by trichoscopy, suggesting that skin color affects trichoscopy images Citation[50]. Moreover, normal-looking hairs with tapering toward the orifices are found in the perilesional hair-bearing scalp of AA patients Citation[51]. Because similar features were previously described as ‘coudability’ by Shuster Citation[52,53], this type of hair was designated as coudability hair. These were seen in 38% of AA patients and correlated with disease activity in a relatively early stage of AA Citation[51]. During contact immunotherapy for AA, diffuse slate-colored pigmentations appear in 5.91% of patients (11 out of 186) Citation[54]. Using trichoscopy, the reticular and granular pigmentations were observed, sparing the orifices and eccrine pores. Histopathology of the pigmentations showed lichenoid or vacuolar interface dermatitis with necrotic keratinocytes and dermal melanophages, consistent with pigmented contact dermatitis. Furthermore this sign represents a clinical indicator of poor responsiveness to treatment Citation[54].
Trichoscopy in diffuse-type AA
There are some reports regarding trichoscopy in diffuse-type AA or mimicking cases. Diffuse-type AA on acute progression corresponds to acute diffuse and total alopecia of the female scalp (ADTAFS) characterized as AA showing rapid progression of diffuse alopecia of female scalp and preferable prognosis Citation[55]. Previous trichoscopy study showed that black dots, tapering hairs and broken hairs are the specific diagnostic markers for ADTAFS to rule out female pattern hair loss (FPHL) and telogen effluvium. Additionally, some of these features were seen in 95% of cases, indicating them as a sensitive marker. Regarding the yellow dots, while numerous yellow dots were found in ADTAFS, a limited number of yellow dots were found on the hair loss scalp of the FPHL patients, suggesting that a differential diagnosis is possible from the number of yellow dots Citation[56]. Conversely, in AA incognita (AAI), which is known as another type of diffuse-type AA, dystrophic hairs, exclamation point hairs (tapering hairs) and cadaverized hair were present in only 28.6% of patients while yellow dots and short vellus hairs were seen in all cases Citation[57]. In this study of AAI, the histopathology showed increased vellus hairs and only subtle peribulbar lymphocytic infiltrate, suggesting that these AAI cases were at remitting phase, not active and inflammatory phase. Moreover, there is one expert opinion that AAI is not in the spectrum of AA and the trichoscopic description of AAI Citation[57] fits with FPHL or acute telogen effluvium Citation[58], indicating some overlap among these conditions.
Quantitative analysis using trichoscopy
Trichoscopy can be used for quantitative analysis for hair loss diseases. Rakowska et al. quantitatively evaluated trichoscopy images of normal female subjects and patients of FPHL and telogen effluvium Citation[59] and found that FPHL may be differentiated from chronic telogen effluvium based on trichoscopy criteria as follows. Major criteria are:
• Ratio of more than four yellow dots in four images (70-fold magnification) in the frontal area
• Lower than average hair thickness in the frontal area compared to the occiput
• More than 10% of thin hairs (below 0.03 mm) in the frontal area
Minor criteria encompass increased frontal to occipital ratio of:
• Single-hair pilosebaceous units
• Vellus hairs
Perifollicular discoloration
Fulfillment of two major criteria or one major and two minor criteria allows to diagnose FPHL with a 98% specificity.
Hair shaft abnormalities
Hair shaft abnormalities can be diagnosed from the characteristics of trichoscopy summarized in Citation[11,60–62]. In the case of woolly hair syndrome, differential diagnosis from curly hairs of normal subjects is easy using trichoscopy because they do not differ significantly from straight hairs Citation[60]. However, there are some limitations; the characteristic sign of ‘tiger tail’ in trichothiodystrophy can not be observed by trichoscopy and light microscopy is more useful Citation[62]. In addition, when pediculosis capitis can be suspected, trichoscopy should not be performed because direct touch to hair shafts may cause contagions.
Expert commentary & five-year view
Because of increasing trichoscopic findings in hair diseases, trichoscopy has become a very important technique and allowed us to avoid scalp biopsy for difficult cases. In the next 5 years, more observations will be reported and subsequently how we understand the accumulated discoveries should be pivotal. Here, one strategy for this purpose based on an algorithmic method was proposed but there could be other approaches, which are expected to improve our knowledge about hair diseases. Furthermore, trichoscopy can be used for quantitative analysis as mentioned above and additional criteria for other diseases will be developed in the future. However, summarizing and standardizing trichoscopic nomenclature will be needed as various terminologies were and will be introduced. Moreover, recently UV-enhanced trichoscopy has been introduced by Rudnicka et al. Citation[24], and by using this new equipment, tinea capitis, pityrosporum folliculitis and porphyria are more precisely and easily diagnosed because the UV light covers the spectrum of a Wood’s lamp. Furthermore, sophisticated methods for trichoscopy are expected to be developed in the future. As a new technique, reflectance confocal laser scanning microscopy has been reported to be helpful for observing hair follicle structures in hair loss diseases Citation[63–65] and is expected to be investigated in more detail.
Table 1. Characteristic trichoscopic features of hair shaft abnormalities.
Key issues
• The loss of orifices corresponds to permanent destruction of hair follicles, resulting in fibrosis, indicating the diagnosis of cicatricial alopecia.
• The existence of micropustules and/or hair tufting with six or more hairs indicates neutrophil- or mixed cell-mediated cicatricial alopecia such as folliculitis decalvans/tufted folliculitis, acne keloidalis and dissecting cellulitis of the scalp.
• The characteristic features of alopecia areata are broken hairs, black dots, tapering hairs (exclamation mark hairs), yellow dots and short vellus hairs.
• The activity of alopecia areata can be monitored by the ratio of pathognomic hairs such as broken hairs, black dots and tapering hairs and short vellus hairs.
• Among lymphocytic cicatricial alopecia, follicular red dots are specific for discoid lupus erythematosus and hair tufting indicates lichen planopilaris.
• The hallmark of androgenetic alopecia and tinea capitis is hair diameter diversity (≥20%) and comma hairs, respectively.
References
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J. Drugs Dermatol. 7(7), 651–654 (2008).
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch. Dermatol. 144(8), 1007 (2008).
- Kossard S, Zagarella S. Spotted cicatricial alopecia in dark skin. A dermoscopic clue to fibrous tracts. Australas. J. Dermatol. 34(2), 49–51 (1993).
- D’Amico D, Vaccaro M, Guarneri F, Borgia F, Cannavo S, Guarneri B. Phototrichogram using videomicroscopy: a useful technique in the evaluation of scalp hair. Eur. J. Dermatol. 11(1), 17–20 (2001).
- de Lacharrière O, Deloche C, Misciali C et al. Hair diameter diversity: a clinical sign reflecting the follicle miniaturization. Arch. Dermatol. 137(5), 641–646 (2001).
- Micali G, Lacarrubba F. Possible applications of videodermatoscopy beyond pigmented lesions. Int. J. Dermatol. 42(6), 430–433 (2003).
- Deloche C, de Lacharrière O, Misciali C et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch. Dermatol. Res. 295(10), 422–428 (2004).
- Lacarrubba F, Dall’Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am. J. Clin. Dermatol. 5(3), 205–208 (2004).
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br. J. Dermatol. 152(3), 556–559 (2005).
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J. Am. Acad. Dermatol. 55(5), 799–806 (2006).
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J. Drugs Dermatol. 6(2), 222–224 (2007).
- Inui S, Nakajima T, Itami S. Dry dermoscopy in clinical treatment of alopecia areata. J. Dermatol. 34(9), 635–639 (2007).
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int. J. Dermatol. 47(7), 688–693 (2008).
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J. Dermatol. 38(1), 71–75 (2011).
- Inui S, Itami S. Emergence of trichoscopic yellow dots by topical corticosteroid in alopecia areata mimicking frontal fibrosing alopecia: a case report. J. Dermatol. 39(1), 39–41 (2012).
- Kwong RA, Kossard S. Alopecia areata masquerading as frontal fibrosing alopecia. Australas. J. Dermatol. 47(1), 63–66 (2006).
- Inui S, Nakajima T, Shono F, Itami S. Dermoscopic findings in frontal fibrosing alopecia: report of four cases. Int. J. Dermatol. 47(8), 796–799 (2008).
- Rubegni P, Mandato F, Fimiani M. Frontal fibrosing alopecia: role of dermoscopy in differential diagnosis. Case Rep. Dermatol. 2(1), 40–45 (2010).
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch. Dermatol. 139(12), 1555–1559 (2003).
- Goldberg LJ. Cicatricial marginal alopecia: is it all traction? Br. J. Dermatol. 160(1), 62–68 (2009).
- Tosti A, Torres F, Misciali C, Vincenzi C, Duque-Estrada B. The role of dermoscopy in the diagnosis of cicatricial marginal alopecia. Br. J. Dermatol. 161(1), 213–215 (2009).
- Pincus LB, Price VH, McCalmont TH. The amount counts: distinguishing neutrophil-mediated and lymphocyte-mediated cicatricial alopecia by compound follicles. J. Cutan. Pathol. 38(1), 1–4 (2011).
- Price V, Mirmirani P. How are the cicatricial alopecias classified? In: Cicatricial Alopecia: An Approach To Diagnosis And Management. Springer, NY, USA (2011).
- Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J. Dermatol. Case Rep. 5(4), 82–88 (2011).
- Miura M, Dekio I, Yamasaki Y, Ohyama M. Sparing of the bulge area could preserve intact lower portion of hair follicles in a case of tufted folliculitis. J. Eur. Acad. Dermatol. Venereol. 23(1), 87–89 (2009).
- Tosti A, Torres F, Misciali C et al. Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch. Dermatol. 145(12), 1406–1409 (2009).
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin. Cutan. Med. Surg. 28(1), 3–10 (2009).
- Duque-Estrada B, Estrada BD, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An. Bras. Dermatol. 85(2), 179–183 (2010).
- Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont TH, Price VH. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J. Am. Acad. Dermatol. 52(4), 637–643 (2005).
- Abraham LS, Piñeiro-Maceira J, Duque-Estrada B, Barcaui CB, Sodré CT. Pinpoint white dots in the scalp: dermoscopic and histopathologic correlation. J. Am. Acad. Dermatol. 63(4), 721–722 (2010).
- Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J. Dermatol. 36(2), 82–85 (2009).
- Slowinska M, Rudnicka L, Schwartz RA et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J. Am. Acad. Dermatol. 59(Suppl. 5), S77–S79 (2008).
- Hughes R, Chiaverini C, Bahadoran P, Lacour JP. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch. Dermatol. 147(3), 355–356 (2011).
- Abraham LS, Torres FN, Azulay-Abulafia L. Dermoscopic clues to distinguish trichotillomania from patchy alopecia areata. An. Bras. Dermatol. 85(5), 723–726 (2010).
- Trüeb RM, Cavegn B. Trichotillomania in connection with alopecia areata. Cutis. 58(1), 67–70 (1996).
- Wilkin JK. Trichotillomania associated with alopecia areata. Cutis. 31(1), 65–66 (1983).
- Runne U, Kroneisen-Wiersma P. Psoriatic alopecia: acute and chronic hair loss in 47 patients with scalp psoriasis. Dermatology (Basel). 185(2), 82–87 (1992).
- Kim GW, Jung HJ, Ko HC et al. Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. Br. J. Dermatol. 164(3), 652–656 (2011).
- Karadag Köse O, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J. Am. Acad. Dermatol. 67(2), 206–214 (2012).
- Yamazaki M, Irisawa R, Tsuboi R. Temporal triangular alopecia and a review of 52 past cases. J. Dermatol. 37(4), 360–362 (2010).
- Inui S, Nakajima T, Itami S. Temporal triangular alopecia: trichoscopic diagnosis. J. Dermatol. 39(6), 572–574(2011).
- Iorizzo M, Pazzaglia M, Starace M, Militello G, Tosti A. Videodermoscopy: a useful tool for diagnosing congenital triangular alopecia. Pediatr. Dermatol. 25(6), 652–654 (2008).
- Neri I, Savoia F, Giacomini F, Raone B, Aprile S, Patrizi A. Usefulness of dermatoscopy for the early diagnosis of sebaceous naevus and differentiation from aplasia cutis congenita. Clin. Exp. Dermatol. 34(5), e50–e52 (2009).
- Piraccini BM, Voudouris S, Pazzaglia M, Rech G, Vicenzi C, Tosti A. Lipedematous alopecia of the scalp. Dermatol. Online J. 12(2), 6 (2006).
- Torres F, Tosti A, Misciali C, Lorenzi S. Trichoscopy as a clue to the diagnosis of scalp sarcoidosis. Int. J. Dermatol. 50(3), 358–361 (2011).
- Tosti A, Miteva M, Torres F, Vincenzi C, Romanelli P. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br. J. Dermatol. 163(6), 1353–1355 (2010).
- Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. J. Cutan. Pathol. 18(2), 87–92 (1991).
- Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. A histopathological study of 12 patients. Am. J. Dermatopathol. 17(2), 158–162 (1995).
- Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J. Dermatol. 56(4), 407–411 (2011).
- de Moura LH, Duque-Estrada B, Abraham LS, Barcaui CB, Sodre CT. Dermoscopy findings of alopecia areata in an African-American patient. J. Dermatol. Case Rep. 2(4), 52–54 (2008).
- Inui S, Nakajima T, Itami S. Coudability hairs: a revisited sign of alopecia areata assessed by trichoscopy. Clin. Exp. Dermatol. 35(4), 361–365 (2010).
- Shuster S. ‘Coudability’: a new physical sign of alopecia areata. Br. J. Dermatol. 111(5), 629 (1984).
- Shuster S. The coudability sign of alopecia areata: the real story. Clin. Exp. Dermatol. 36(5), 554–555 (2011).
- Inui S, Nakajima T, Toda N, Itami S. Pigmented contact dermatitis due to therapeutic sensitizer as complication of contact immunotherapy in alopecia areata. J. Dermatol. 37(10), 888–893 (2010).
- Sato-Kawamura M, Aiba S, Tagami H. Acute diffuse and total alopecia of the female scalp. A new subtype of diffuse alopecia areata that has a favorable prognosis. Dermatology (Basel) 205(4), 367–373 (2002).
- Inui S, Nakajima T, Itami S. Significance of dermoscopy in acute diffuse and total alopecia of the female scalp: review of twenty cases. Dermatology (Basel). 217(4), 333–336 (2008).
- Tosti A, Whiting D, Iorizzo M et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J. Am. Acad. Dermatol. 59(1), 64–67 (2008).
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Czuwara J, Rudnicka L. Alopecia areata incognita: true or false? J. Am. Acad. Dermatol. 60(1), 162–163 (2009).
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int. J. Trichology 1(2), 123–130 (2009).
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J. Dermatol. Case Rep. 2(2), 14–20 (2008).
- Rakowska A, Kowalska-Oledzka E, Slowinska M, Rosinska D, Rudnicka L. Hair shaft videodermoscopy in netherton syndrome. Pediatr. Dermatol. 26(3), 320–322 (2009).
- Wallace MP, de Berker DA. Hair diagnoses and signs: the use of dermatoscopy. Clin. Exp. Dermatol. 35(1), 41–46 (2010).
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J. Dermatol. Case Rep. 2(4), 55–59 (2008).
- Agozzino M, Tosti A, Barbieri L et al. Confocal microscopic features of scarring alopecia: preliminary report. Br. J. Dermatol. 165(3), 534–540 (2011).
- Ardigò M, Tosti A, Cameli N, Vincenzi C, Misciali C, Berardesca E. Reflectance confocal microscopy of the yellow dot pattern in alopecia areata. Arch. Dermatol. 147(1), 61–64 (2011).
Trichoscopy: a new frontier for the diagnosis of hair diseases
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertderm. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 25-year-old woman for patchy hair loss on her scalp, and you decide to perform trichoscopy. According to the algorithm in the current review, what is the first question to be answered as you examine this patient?
□ A Is the hair loss neutrophilic or lymphocytic?
□ B Are yellow dots present?
□ C Are black dots present?
□ D Is the hair loss cicatricial or non-cicatricial?
2. Trichoscopy reveals broken hairs, black dots, yellow dots, and short vellus hairs. What is this patient's probable diagnosis?
□ A Alopecia areata (AA)
□ B Frontal fibrosing alopecia (FFA)
□ C Cicatricial marginal alopecia
□ D Tinea capitis
3. You suspect that this patient might have temporal triangular alopecia. Which of the following choices is characteristic of this condition?
□ A Involvement of the temporal scalp only
□ B Normal follicular openings with vellus hairs surrounded by normal terminal hair areas
□ C Tapering hairs
□ D Regrowth of hair within 6 months
4. The patient is ultimately diagnosed with AA. Which of the following findings on trichoscopy correlates with a good prognosis for AA?
□ A Short vellus hairs
□ B Black dots
□ C Tapering hairs
□ D Broken hairs