Abstract
Most melanocytic lesions in children are considered ‘nonproblematic’ and are managed conservatively because of their invariable benignity. Congenital melanocytic nevi (CMN) and Spitz nevi are the most problematic pigmented lesions in childhood. Regarding CMN, the biggest risk of melanoma development occurs with increased nevus size, being particularly high in giant CMN, in children younger than 10 years. On the other hand, awareness should be related to new, rapidly growing lesions (the clinical hallmark of Spitz/Reed nevi and melanoma). The aim of this review is to present clinical and dermoscopic features of a large spectrum of pediatric melanocytic lesions with special attention to problematic lesions that may occur in childhood.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertdermatol; (4) view/print certificate.
Release date: 28 March 2013; Expiration date: 28 March 2014
Learning objectives
Upon completion of this activity, participants will be able to:
• Distinguish high-risk melanocytic skin lesions for melanoma among children
• Assess characteristics of benign melanocytic skin lesions among children
• Evaluate the diagnosis and management of giant congenital melanocytic nevi
• Evaluate the diagnosis and management of Spitz nevi
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles Vega
Associate Professor and Residency Director, Department of Family Medicine, University of California, CA, USA.
Disclosure: Charles P Vega, MD, FAAFP, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Vincenzo Piccolo, MD
Department of Dermatology, Second University of Naples, Naples, Italy.
Disclosure: Vincenzo Piccolo, MD, has disclosed no relevant financial relationships.
Elvira Moscarella, MD
Dermatology and Skin Cancer Unit, Arcispedale S.Maria Nuova, IRCCS, Reggio Emilia, Italy.
Disclosure: Elvira Moscarella, MD, has disclosed no relevant financial relationships.
Iris Zalaudek, MD
Department of Dermatology, Medical University of Graz, Graz, Austria.
Disclosure: Iris Zalaudek, MD, has disclosed no relevant financial relationships.
Gerardo Ferrara, MD
Anatomic Pathology Unit, Department of Oncology, Gaetano Rummo General Hospital, Benevento, Italy.
Disclosure: Gerardo Ferrara, MD, has disclosed no relevant financial relationships.
Rosalba Picciocchi, MD
Pediatric Dermatology Unit, A.O.R.N. Santobono-Pausillipon, Naples, Italy.
Disclosure: Rosalba Picciocchi, MD, has disclosed no relevant financial relationships.
Orsola Ametrano, MD
Pediatric Dermatology Unit, A.O.R.N. Santobono-Pausillipon, Naples, Italy.
Disclosure: Orsola Ametrano, MD, has disclosed no relevant financial relationships.
Giuseppe Argenziano, MD
Dermatology and Skin Cancer Unit, Arcispedale S.Maria Nuova, IRCCS, Reggio Emilia, Italy.
Disclosure: Giuseppe Argenziano, MD, has disclosed no relevant financial relationships.
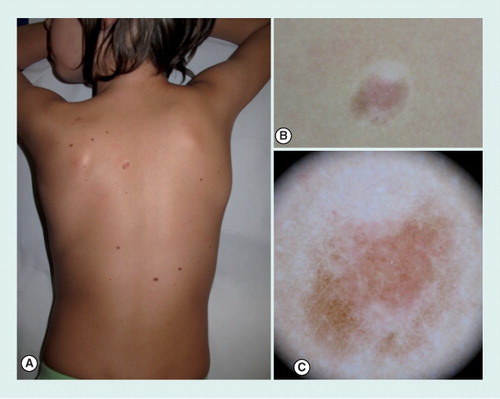
(A) Clinical overview. (B) Close-up clinical image showing a pigmented lesion surrounded by a whitish halo. (C) With dermoscopy, the lesion appears almost completely involuted, with a homogeneous pattern, surrounded by a rim of scar-like depigmentation.
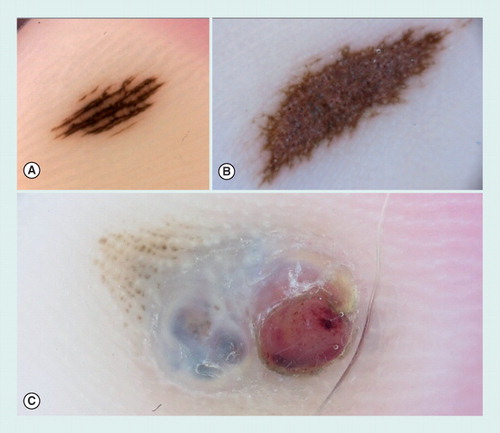
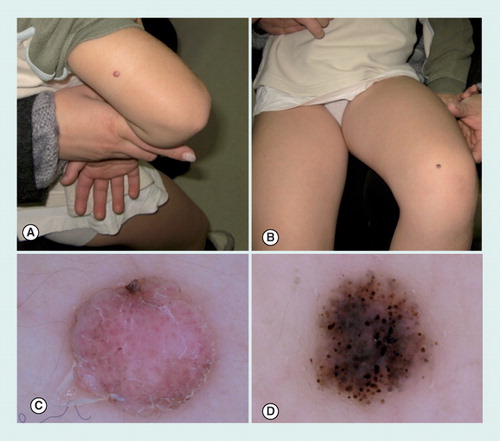
(A) A small acquired acral nevus showing a parallel furrow pattern. (B) Congenital acral nevus presenting a palpable area in the center of the lesion and displaying a grayish coloration by dermoscopy, corresponding to a dermal component of the nevus. At the periphery, a parallel furrow pattern can be detected. (C) Dermoscopy of an acral melanoma, arising on a pre-existing nevus. The melanoma was growing as a nodular component displaying a blue–white veil and an ulcerated red area.
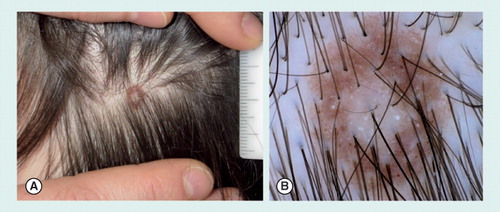
(A) Clinical view with presence of a white central area surrounded by a rim of brown pigmentation. (B) Under a dermoscope, the lesion shows a pigment network with perifollicular hypopigmentation, and scattered homogeneous brown globules. At the center, a hypopigmented, white area is detected.
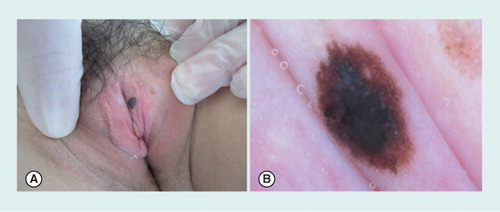
(A) Clinical image showing a dark brown macule on the labia. (B) On dermoscopy, the lesion shows a mixed pattern with a homogeneous brown pigmentation and a central blue–grey area.
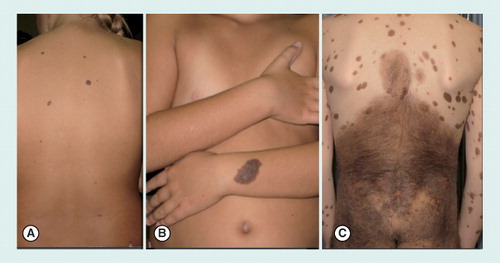
(A) Small (<1.5 cm diameter) congenital nevi on the back of an 8-year-old girl. (B) Medium size (1.5–20 cm) congenital nevus on the arm of an 8-year-old girl. (C) Giant congenital nevus (>20 cm), involving the area of the buttocks and the back, in a 9-year-old boy. Of note, the presence of multiple satellite nevi can be noticed.
(A) A Spitz and (B) a Reed nevus arising, respectively, as a pink and black papule on the upper and lower arm of a 3-year-old boy. (C) Dermoscopy of nonpigmented Spitz nevus with dotted vessels and pinkish background. (D) Dermoscopy of Reed nevus, showing a globular pattern, composed by heavily pigmented brown-to-black globules.
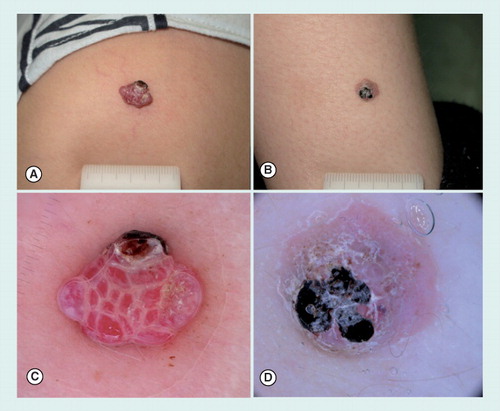
(A) A pyogenic granuloma and (B) an atypical Spitz tumor arising on the lower arm of two prepubertal children. Both lesions presented as pink to red nodules, partially covered by a blood crust. (C) Dermoscopy of pyogenic granuloma, displaying multiple red lacunes intersected by whitish lines. In the upper part of the lesion, a small ulcerated area is visible. (D) Aspecific dermoscopic pattern of an atypical Spitz tumor presenting a pink background with a few dotted vessels and a keratotic surface partially covered by a blood crust.
Childhood melanoma is a rare occurrence Citation[1,2] and its annual incidence is estimated in 0.8 cases per million in the first decade of life Citation[3]. Nevertheless, accuracy in melanoma detection in children and adolescents still remains low. A recent study has tested accuracy in melanoma detection in childhood using the ‘number needed to excise’ value, obtained by dividing the total number of excised lesions by the number of melanomas Citation[4]. The overall ‘number needed to excise’ value in pediatric patients over the 10-year study period was 593.8, meaning that approximately 594 lesions were excised to find one melanoma. This value is 20-times higher than the rates usually found in adult patients, thus suggesting that clinicians should raise their threshold for malignancy when faced with melanocytic lesions in pediatric patients. An effective strategy to reduce unnecessary excision without missing melanoma would be to focus on problematic lesions. Congenital nevi and Spitz/Reed nevi are the most problematic lesions in differential diagnosis with melanoma in childhood Citation[1,4–7]. In particular, large congenital melanocytic nevi (CMN) represent the most important risk factor for melanoma among children younger than 12 years of age with a third of melanomas arising on giant nevi Citation[8–11]. On the other hand, Spitzoid lesions tend to share clinical, dermoscopic and even histopathologic features with melanoma, thus rendering differential diagnosis particularly challenging even for expert clinicians. In this scenario, awareness should be related to new rapidly growing lesions (the clinical hallmark of Spitz/Reed nevi and melanoma) and to congenital nevi with a recent history of changing size, color and/or shape.
Dermoscopy is currently a pivotal tool in the diagnosis of skin tumors, allowing a better sensitivity and specificity in the diagnosis of skin tumors, as compared with naked eye examination. The role of dermoscopy in a pediatric setting is to help differentiate melanocytic nevi and nonmelanocytic skin lesions from melanoma, thus allowing a better accuracy in childhood melanoma detection Citation[12–16]. The aim of this review is to present clinical and dermoscopic features of a large spectrum of pediatric melanocytic lesions with special attention to problematic lesions that may occur in childhood.
Nonproblematic melanocytic nevi
Common nevi
The total number of melanocytic nevi varies with age, following a dynamic course of evolution. Nevus counts and nevus density increase from youth to midlife and thereafter decrease Citation[17,18]. Moreover, upcoming evidence suggests that the morphological subtypes of nevi are also age-dependent. Traditionally, nevi present at birth or appearing in the first year of life are defined as congenital, whereas those appearing later after birth are defined as acquired. Common acquired nevi are small, flat to slightly elevated, brown lesions that can appear anywhere on the surface of the body. Histopathological examination usually shows that they are either compound or dermal nevi Citation[19].
Several studies have examined the prevalence of dermoscopic patterns in childhood nevi. By dermoscopy, the globular pattern seems to be the most characteristic in childhood, both small congenital and early acquired nevi display a dermoscopic globular appearance Citation[20–22]. Conversely, a reticular pattern usually typifies nevi developing after puberty Citation[20–22]. Zalaudek et al. found a prevalence of globular pattern (60%) followed by complex (21%), reticular (12%) and homogeneous (5%) pattern Citation[20]. Scope et al. found a homogeneous pattern in 44% of children, followed by globular (37%), reticular (13%) and complex (5%) pattern Citation[23]. Overall, the frequency of globular and reticular patterns increases with age and ‘homogeneous’ nevi may evolve into patterned nevi Citation[23]. Recently, Scope et al. introduced the concept of nevus volatility in childhood, finding that children with higher back nevus counts have greater nevus volatility, being more likely to both develop new nevi and have nevi that disappear during follow-up Citation[24]. Moreover, globular nevi, both congenital and acquired, seem to be more frequent on the upper back than lower back and limbs, thus also suggesting a site-dependent variability of the different dermoscopic patterns Citation[23].
Dermoscopic examination of common nevi in children usually shows no atypical features, and these lesions are managed conservatively. However, it is not rare to find nevi with an eccentric hyperpigmentation, the so-called ‘Bolognia’s sign’ Citation[25]. This feature is frequently found in melanomas, but nevi with eccentric hyperpigmentation show a brown to gray–black homogeneous pigmentation with no other dermoscopic features of melanoma. In addition, this ‘innocent’ hyperpigmentation may often disappear during follow-up.
Nevi with more pronounced atypical features generally arise after puberty and are present until 40–50 years of age Citation[26–28]. Clinical features of these nevi include: flat or moderately elevated surfaces, heterogeneous color and diameter more than 6 mm. The presence of multiple nevi with atypical features is a marker of increased risk for melanoma development. Therefore, a regular follow-up is recommended after puberty, especially in children presenting additional risk factors, such as high nevus count, positive family history of melanoma, fair skin type and history of sunburns. In this category, the appearance of melanoma is de novo and atypical nevi represent the only marker for risk of developing melanoma. The major difficulty consists in identification of incipient melanoma and differentiation from atypical nevi. Tsao et al. demonstrated that the possibility of transformation of a nevus into melanoma is quite low; it ranges from a minimum of one in 200,000 in women and men under 40 years of age to a maximum of one in 33,000 in women and men over 60 years of age Citation[29]. Bauer and Garbe estimated lower percentages of transformation in people younger than 20 years of age Citation[16,30,31].
Blue nevi
Blue nevi (BN) are a subset of melanocytic proliferations characterized by dermal dendritic melanocytic cells. Children and adolescents are more likely to develop BN, which have an incidence estimated at 1–2% in the white population. BN occur as two main different groups: common BN and cellular BN Citation[32]. BN usually appear as bluish, smooth surfaced papules, nodules and plaques. The main clinical difference between common and cellular BN is the diameter, with the latter being larger (up to 30 mm) than the former (<10 mm). Cellular BN may also have a polychromatic appearance (i.e., mixed blue, brown and white in color).
Dermoscopic examination of BN shows a typical steel-blue homogeneous coloration generated by the presence of heavily pigmented melanocytes in the dermis; different color variants are also possible Citation[33] always in the absence of any other dermoscopic structure. A mixed blue and white color is frequently seen in dermoscopy in BN of the extremities Citation[34]. Small and common BN do not need further management, while cellular BN, especially when they grow rapidly, can create problems in being differentiated from melanoma, and more so if they present with atypical dermoscopic features. Moreover, BN with a peripheral satellitosis mimicking malignant melanoma (MM) Citation[35–38] have been described. In these cases, excision is necessary to confirm the benign nature of the melanocytic proliferation.
Combined nevi
A combination of two different nevus cell populations at histopathological examination is the hallmark of combined nevi Citation[39]. The most frequent combination is between small congenital and a BN, but also other combinations are possible Citation[40]. Depending on the type of combination, dermoscopy can show different patterns: globules combined with blue homogeneous pattern or reticular pattern at the periphery surrounding a blue colored central area. Due to the doubtful clinical and dermoscopic presentation, these lesions are often excised in order to rule out melanoma.
Halo nevi
Halo nevi (HN; also called Sutton nevi) are congenital or acquired nevi surrounded by a rim of vitiligo characterized, over a variable period of time, by a progressive disappearance of central part of the nevus due to spontaneous regression in which T lymphocytes are considered to play a key role Citation[41–46]. HN show an increased frequency in children and young adults and their preferential location is the trunk, on which they can be multiple. HN are often associated with autoimmune diseases such as vitiligo and Hashimoto thyroiditis, but also with atopic dermatitis and Turner syndrome Citation[47,48].
Dermoscopic examination often shows, in the first phase before the disappearance of the nevus, a globular pattern surrounded by a variably sized rim of scar-like depigmentation. Later on, the central nevus completely involutes and a gray pigmentation due to melanophages and dermal vessels can be seen Citation[49].
Differential diagnosis should be made with halo melanoma and pseudo-halo nevus. Regressive melanoma can sometimes mimic a halo nevus. Clues to differentiate halo melanomas are the presence of an asymmetric rim of a white scar-like area, that is eccentric and is not well defined as in HN. In addition, the remnants of the melanocytic lesion show atypical dermoscopic features, suggesting the diagnosis of regressive melanoma.
Pseudo HN are artifactual HN produced by sunscreen application exclusively on a common melanocytic nevus Citation[50].
Nevi on special locations
Melanocytic skin lesions can develop anywhere on the surface of the body. The dermoscopic patterns of nevi essentially reflect the histopathologic distribution of the pigmented structures within the skin layers. As a consequence, when appearing on special body areas, namely, body areas with peculiar anatomical structures (face and acral sites), nevi display distinct dermoscopic patterns different from those usually found on the trunk. Scalp and mucosal areas are also considered special body areas, because melanocytic lesions developing on these areas frequently show concerning features by histopathology that may overlap with melanoma.
The most common patterns of nevi developing on acral sites (palms and soles), on the scalp and on mucosal areas, will be described in the following sections. Facial melanocytic lesions are usually considered in differential diagnosis with melanoma of the lentigo maligna type, a particular type of melanoma that usually develops in adults and elderly patients, thus facial lesion are not discussed in this review.
Acral nevi
Melanocytic lesions developing on palms and soles usually display a parallel pattern due to particular anatomical structures inherent to this location Citation[51,52]. The pigmentation may follow the furrows as well as the ridges of glabrous skin. In particular, the parallel furrow pattern, the lattice-like pattern and the fibrillar pattern are commonly found in acral melanocytic nevi, whereas the parallel ridge pattern is highly suggestive of melanomas on acral sites Citation[53]. Acral nevi can usually be managed conservatively. Of note, acral congenital nevi may present a palpable area in the center of the lesion, sometimes displaying a grayish coloration that can represent a matter of concern for clinicians, and corresponds to a dermal component of the nevus. In these cases, differential diagnosis between a parallel ridge and a parallel furrow pattern can be made easier by evaluating the distribution of the pigment at the periphery of the lesion .
Scalp nevi
Scalp nevi in children and teenagers are known indicators for a higher total nevus count, being considered as a marker of the so-called ‘moley child’ Citation[54,55]. Thus, teenagers with scalp nevi may benefit from regular whole body skin examinations. Moreover, a subset of scalp nevi may reveal worrisome features on histopathology, although they have no documented risk for malignant transformation Citation[56]. Common scalp nevi in children and teenagers typically reveal a pigmented network with perifollicular hypopigmentation that may give rise to some border irregularity of the nevus or a uniform globular pattern. Children and adolescents with fair skin types may present scalp nevi with central hypopigmentation, also called eclipse nevi. Dermoscopically, eclipse nevi show a central area of hypopigmentation, sometimes with a keratotic surface, which is surrounded by a brown pigmented network of different color intensity. These lesions can usually be managed conservatively .
Mucosal lesions
Melanocytic skin tumors of the mucosa, in particular the vulva and penis, reveal peculiar clinical and epidemiological characteristics different from those of other body sites. Only a few studies have examined specifically the dermoscopic features of melanocytic lesions in these body areas Citation[57–59].
All studies agree in indicating age as a strong predictor of the benignity or malignancy of the lesions. Nevi seem to be strongly associated with younger age compared with melanoma or melanosis. By dermoscopy, the combination of blue, gray or white color with structureless zones are the strongest indicators of malignant mucosal lesions. Nevi usually exhibit a globular/cobblestone pattern or mixed pattern. Of note, a small subset of benign vulvar nevi, known as atypical melanocytic nevi of the genital type, show concerning features by histopathology that may overlap with melanoma. These lesions may present a mixed pattern by dermoscopy, with a homogeneous brown–gray pigmentation or globules. Thus diagnosis and management should be based on a good clinical, dermoscopic and histopathologic correlation, taking into account the age of the patient, in order to avoid over-diagnosis of melanoma in this special body area .
Problematic melanocytic nevi
Congenital nevi
CMN are considered, by some authors, to be neural crest-derived hamartomas, which are visible at, or shortly after birth as pigmented tumors Citation[60]. The incidence of any size of CMN of neonates ranges from 0.2 to 2.1% incidence of CMN in neonates regardless of nevus size Citation[58]. They are categorized according to the maximum diameter of the nevus; small (<1.5 cm), medium (1.5–20 cm) and large (>20 cm) CMN Citation[60]. The latter group may be further classified as G1 (20–30 cm), G2 (30–40 cm) and G3 (>40 cm).
The risk of malignant transformation is a matter of ongoing debate. Evidence suggests that the risk increases with the size of the nevus; moreover, the age of onset and depth of origin of MM seem to differ in relation with the size of the nevus. In summary, the risk of MM development is higher for giant CMN than for small- and medium-size nevi; MM arising in CMN tends to develop at younger age and to originate deep within large CMN and superficially within small or medium sized CMN. However, more data are necessary before drawing conclusions. The authors report the most recent evidence about incidence and risk of MM development within large- and small- to medium-sized CMN in the following sections .
Giant CMN
Many studies have been conducted on the risk of melanoma associated with giant CMN Citation[60–62]. The magnitude of the risk for large CMN varies widely between studies (0–50%) Citation[60]. Two important meta-analyses have been conducted in recent years Citation[61,62]. A 2003 review of eight studies (432 giant CMN patients) found that 12 patients (2.8%) developed melanoma during the reported follow-up periods Citation[61]. Of the 12 patients who developed melanoma, ten developed it within their giant CMN, while data were unavailable/unknown for the other two patients. In 2006, Krengel et al. analyzed 14 studies with a total of 6571 CMN patients who were followed for a mean of 3.4–23.7 years and found that 46 (0.7%) developed a total of 49 melanomas (mean age at diagnosis: 15.5 years; median age: 7 years). The authors found a markedly increased relative risk (465) of developing melanoma during childhood and adolescence Citation[62].
Concerning the depth of origin of CMN-associated MM, many believe that MM developing within giant CMN arise from a greater depth Citation[63]. However, data on this hypothesis appear limited at this time. Prospective data are completely lacking, and there are no large series that compare origins of melanomas among nevi of different sizes.
Large CMN are known to sometimes be associated with systemic diseases, and in particular with neurocutaneous melanocytosis (NCM). NCM is a melanocytic proliferation involving the CNS, usually associated with multiple CMN Citation[64]. The presence of NCM could alter the prognosis of the affected patients due to an increase of intracranial pressure. A fatal outcome has been reported in more than half the patients affected by symptomatic NCM; death occurred within about 3 years after the appearance of symptoms, mostly in individuals younger than 10 years of age Citation[16,65].
Brain MRI is indicated for patients at risk for NCM. Although a positive result will be given in about 23% of patients with no neurologic symptoms; only a minor rate of patients will subsequently develop a symptomatic NCM Citation[16,66]. Conversely, a negative MRI does not permit to rule out the possibility of symptoms developing later. It has been suggested by some authors that periodical MRIs would be useful in patients at risk, although clinical examination could also be sufficient. MRI may be also useful in patients affected by symptomatic NCM that could be eligible for surgical treatment due to ameliorate symptoms. Moreover, in children with large- or medium-sized nevi affecting the lumbosacral region, MRI could be used to detect spinal abnormalities such as tethered cord syndrome Citation[16,67].
Originating from large CMN, nodules may appear in the context of nevus often clinically and dermoscopically mimicking melanomas. A wide variety in size, rapid growth, potential ulceration and difficulty to histologically distinguish them from melanoma are characteristic of these nodules Citation[16].
Nodular proliferations can show four different patterns of presentation Citation[16,68]: lesions mimicking superficial spreading melanomas, histologically characterized by the presence of large epithelioid melanocytes in the upper dermis and sometimes spreading to epidermis in a pagetoid way; lesions mimicking nodular melanomas, characterized by a nodular proliferation of large melanocytes with uniform nuclei in the dermis; proliferative neurocristic hamartomas with deep proliferation in dermis and subcutaneous tissue, characterized by a changeable degree of neural or mesenchymal differentiation; true melanomas, mostly showing small blast-like melanocytes, whose features are scan cytoplasm, hyperchromatic nuclei and high mitotic rate Citation[16].
Chromosomal abnormalities of atypical nodules compared with CMN and true melanomas arising on CMN were studied by Bastian et al. Citation[16,69]. The genomic differences among these three groups could become useful in histologically doubtful cases. Chromosomal aberrations were commonly found in atypical nodules, rather than classic CMN in which they were absent Citation[16]. Moreover, the authors noticed that there was a qualitative difference in chromosomal aberrations, with structural changes predominating in melanomas, and abnormalities in the number of chromosomes being mostly frequent in proliferative nodules Citation[16,69].
Small & medium CMN
There is a lack of consensus on the risk of developing melanoma in these groups of nevi; it has been estimated that up to 1% of patients with small and medium CMN will develop a melanoma over a lifetime Citation[69–77]. Other physicians consider the risk of melanoma development related with age; children younger than 10 years have an incidence of approximately 0.7 per million, while for children aged 15–19 years, the incidence increases up to 13.2 per million Citation[16,70]. When there are no abnormalities at clinical and dermoscopic examination, these lesions can be managed conservatively, if the patient is compliant to follow-up and no cosmetic alteration is present Citation[16]. Lesions should be followed up with clinical examination annually, and eventually with digital dermoscopy. Moreover, parents should be informed on the increasing size of the nevus proportional to the body growth of the affected child Citation[16].
Diagnosis & management
At clinical examination, diagnosis of medium and large CMN is simple, considering their size and appearance since birth Citation[16,78]. Dermoscopy will in most cases, show a globular or cobblestone pattern. Different patterns, such as reticular pattern, mixed reticular/globular pattern and diffuse pigmentation, can be present in medium CMN. Lesions appearing at lower limbs usually show a reticular pattern. Further dermoscopic features commonly seen in CMN are: small brown dots typically seen within the network meshes, comma-like vessels, milia-like cysts and hypertrichosis Citation[16]. The management of CMN needs individualization, taking into consideration the patient’s age, nevus size, location and depth, risk for malignant transformation, risk for NCM, ease of examining the nevus for suspicious changes, cosmetic and psychological impact associated with the presence of the CMN and/or the presence of aesthetically displeasing surgical scars Citation[78].
For large CMN, surgical excision could be performed to prevent melanoma development. When nevus size is too large and complete excision is not possible, a staged excision could be made. Some authors recently challenged the recommendation of surgically removing large CMN due to the scarce prevalence of melanoma developing from CMN Citation[78].
Treatments alternative to surgery include dermoabrasion, curettage, chemical peels and laser therapy Citation[78]. Dermoabrasion consists of removing the whole epidermis and upper dermis to eliminate the superficial melanocytic cells. It is preferentially performed during infancy. However, this technique leaves the skin more fragile, thinner, tender and with lower hair density Citation[16,79]. With curettage, superficial dermis, in which there is a high concentration of melanocytes, is separated from deep dermis through a natural cleavage plan, present only in the first weeks of life Citation[16,80]. After curettage, a dense and sclerotic connective tissue replaces the removed dermis. Chemical peels could produce a reduction of the melanocyte number and is indicated in light pigmentation CMN Citation[16,81]. Renal and cardiac toxicity must be listed among the side-effects of this treatment. Regarding laser therapy, Q-switched ruby laser is the most commonly used laser in treatment of CMN, because of its wavelength, it is selectively adsorbed by melanin Citation[16]. Scarring is an uncommon outcome of this treatment, although repigmentation may appear in most treated patients, producing a final depigmentation rate of about 50% Citation[16,82].
Alternatively, annual clinical and dermoscopic follow-up can be suggested; however, because of the greater depth of origin of MM arising within large CMN, early recognition of MM is virtually impossible in these cases. On the contrary, in small- and medium-sized CMN, assuming that melanoma develops superficially within the nevus, dermoscopy can be useful to detect early changes within the nevus before they appear clinically visible, thus allowing an early diagnosis and prompt surgical excision of suspicious lesions. Common dermoscopic features of early MM arising on congenital nevi are the development of a blue–white veil, regression structures and/or atypical vascular pattern.
Spitz/Reed nevi
The term ‘Spitz nevus’ derives from Sophie Spitz, who in 1948 first described pediatric melanocytic lesions that histologically resembled melanomas and yet lacked their aggressive behavior. Currently, the clinical histology of Spitz-type lesions has become tremendously complex. We recognize, from both clinical and histopathologic points of view, a spectrum of Spitzoid lesions. On one end we find common Spitz/Reed nevi, melanocytic proliferations that frequently occur in children and are histopathologically classified as benign. On the other end, we recognize ‘Spitzoid melanomas,’ a morphologic type of melanomas with Spitzoid features, which are readily identified as malignant on histopathologic examination. In between these two extremes, we place a series of Spitzoid lesions that present varying features of clinical and histopathologic atypia and unknown malignant potential. These intermediate forms of Spitzoid lesions have been referred to with a variety of terms such as ‘Spitz nevus with atypia’, ‘atypical Spitz nevus’, ‘atypical Spitz tumors’ (AST) and melanocytic tumors of uncertain malignant potential. Currently, some authors have suggested the term of AST as the more widely accepted; however, no adequate histologic criteria exist to clearly classify these lesions as benign or malignant, and even expert dermatopathologists are unable to reliably predict the outcome of this group of atypical Spitz lesions based on morphologic criteria. These uncertainties are the major reason why evidence-based management guidelines for Spitz tumors have not been established until now. The current state-of-the-art recommendation for management regarding clinical, dermatoscopic and histopathologic features of Spitzoid lesions are summarized in the following sections.
Classic Spitz/Reed nevi
Spitz nevus, in its classic definition, is considered as a pinkish or flesh-colored papular or nodular lesion characterized by a rapid growth, usually located at lower limbs or face, appearing in children or early adulthood Citation[16,83–86]; the main histopathologic feature is the presence of large spindle and/or epithelioid cells associated with poor or absent melanin Citation[16]. The eponym ‘Reed nevus’ refers to a benign melanocytic lesion originally described in 1975 as ‘pigmented spindle cell nevus’ Citation[16,87]. It is typical of young adults, presenting as a rapidly growing brown-to-black macular or papular lesion, often appearing on the lower limbs; at histopathologic examination, it presents characteristic interconnecting junctional fascicles of heavily pigmented spindle cells Citation[16]. Some authors have challenged the independence of Reed nevus from Spitz nevus due to the description of heavily pigmented spindle or epithelioid cells Citation[16,88], therefore also assigning Reed nevus to the larger spectrum of Spitz nevus Citation[16]. To date, authors are still considering Reed nevus as a different nosologic entity, which, in their opinion, is distinguishable from pigmented Spitz nevus Citation[16,85,89–95]; however, it still remains a debated question to distinguish, by histopathological examination, these two clinical entities although no relevant clinical and dermoscopic differences exist Citation[16,89]. On the basis of the aforementioned discussion, Spitz nevus can be divided into two categories: classical and the pigmented type (comprising Reed nevus) Citation[16].
Spitz/Reed nevi can present at dermoscopic examination with six main different patterns: globular, vascular, reticular, starburst, atypical and homogeneous Citation[96,97]. Spitz nevus classically presents as an amelanotic or hypopigmented lesion characterized by the presence of dotted vessels that constitute its vascular pattern Citation[98], responsible for its typical pink color. These vessels are typically monomorphic, homogeneously distributed in the context of the lesion, frequently grouped and encompassed by white lines that regularly intersect, forming the so-called ‘reticular depigmentation’. Additional features are a slight pigmented background with a diffuse brown-to-gray hue associated with gray–brown, small- to medium-sized globules, which are extensively and regularly spaced alongside each other.
Reed nevi appear very different dermatoscopically. Their initial pattern is globular or a starburst, the latter characterized by peripheral pigmented lines regularly arranged like an exploding star. During monitoring, the starburst pattern disappears and a homogeneous or reticular pattern is seen with streaks and pseudopods gradually less evident. The observed changes in dermoscopic patterns appear to represent different phases of the natural evolution of this type of nevus: the globular and starburst pattern are typical of the growth phase and the homogeneous or reticular pattern appears when the lesion becomes stable. In a recent study by Argenziano et al., authors followed a series of 64 lesions in pediatric patients (mean age: 10.4 years) for a mean follow-up period of 25 months. In this study, 79.7% (n = 51) of lesions showed an involution pattern and 20.3% (n = 13) showed a growing (n = 4) or stable pattern (n = 9). The great majority of growing lesions were pigmented or partially pigmented (92.3%), whereas 47.1% of lesions in involution were amelanotic (p = 0.005) Citation[99].
Histopathologically, classic Spitz nevi reveal neat organizational attributes such as symmetry, maturation, distinct margins, small size, and more often show epidermal hyperplasia, Kamino bodies and junctional clefting.
Although, there is lack of consensus regarding management of Spitz tumors, some authors recommend surgical excision of all types of Spitzoid lesions at any age. However, because younger age is associated with a lower predictive probability for true melanoma, a conservative management can be reserved in this age group for classic Spitz/Reed nevi. Regular dermoscopic monitoring every 4 months until stabilization or involution of the lesions has been recently proposed as a method to monitor common Spitz/Reed nevi before puberty Citation[100,101].
After puberty and in adulthood, excision of Spitzoid lesions is recommended, regardless of the atypical clinical and/or dermoscopic characteristics detectable.
Due to the lack of distinction criteria that permit distinguishing Spitz nevi from pyogenic granulomas, surgical excision and histopathologic examination are also recommended when lesions show features of pyogenic granuloma, independent of age Citation[102].
Atypical Spitz tumors
AST are melanocytic tumors characterized by ‘bland’ (low-grade) histopathologic features and a metastatic potential usually confined to regional lymph nodes Citation[100]. Different clinical parameters have been used to differentiate classic Spitz nevi from AST. First of all, younger age (<10 years) is associated with more probably benign Spitz nevus; conversely, AST usually affect older ages (10–20 years) Citation[100]. Location is another important parameter, with extremities being more likely affected by Spitz nevi, while the back is the main location of atypical Spitz nevi Citation[100]. Lesions <5–6 mm in diameter are usually common in classic Spitz nevus, while lesions >1 cm are likely to be atypical Citation[100]. Other features suggesting benignity are symmetry, well-defined borders, smooth surface and pink/reddish color Citation[100]. Conversely, AST often present as asymmetric with irregular borders, ulcerated or irregular surface and not uniformly colored Citation[100].
Upon dermoscopy, AST potentially show all the dermoscopic elements typical of melanoma. The presence of a blue-white veil, resulting from deep dermal pigmentation with overlying epidermal hyperplasia, can produce a further increase of dermoscopic atypia.
In clinical practice, childhood Spitz nevi must be excised if one of the following features is present: larger than 1 cm, nodular, ulcerated, rapidly changing or otherwise atypical Citation[100].
To date, no adequate clinical and histopathologic criteria exist to correctly classify these lesions. Ulceration, significant Breslow thickness, a high number of mitotic figures, even more if deep and atypical, may be associated with metastatic behavior Citation[100]. A recent work by Spatz et al. established several clinical and histopathological parameters (assigning to each one a score) to define three different risk categories of developing metastases: low, intermediate and high Citation[103]. However, most histopathological criteria are not useful when there is a doubtful overlap between AST and melanoma.
As a consequence, the management of AST is a matter of ongoing discussion. It was suggested by Kelley and Cockerell Citation[104] in 2000 that sentinel lymph node (SLN) biopsy should be performed along with wide excision for patients with AST. Lymph node positivity for metastatic tumor deposits would be the supporting element in favor of the malignant nature of the primary tumor Citation[104]. The full biologic and prognostic significance of a positive SLN discovered with AST is unclear. Nearly all of the reported SLN-positive cases yielded negative nodes after lymphadenectomy Citation[101]. In addition, almost none led to death in the subsequent 1- to 3-year follow-up period Citation[105,106]. There is no consensus on this matter and no outcome data exist with AST documenting a survival benefit with SLN biopsy. These data, moreover, also indicate that the prognostic value of SLN biopsy in AST in children is very limited Citation[101].
The main problem related to positive SLN in AST is that it induces a chain of events, including extensive lymphadenectomy and eventual adjuvant treatments. Both procedures are associated with many adverse effects and do not benefit patient survival. Luo et al. defined this condition in a highly provocative way: ‘benign metastasis’ Citation[101]. The question still remains open and AST represent a unique avenue in cancer research.
Genetic differences between Spitz nevus and melanoma have been studied through several molecular-biology techniques such as comparative genomic hybridization, loss of heterozygosity analysis, multiplex ligation-dependent probe amplification and DNA sequencing Citation[107–114].
A subset of Spitz tumors has been found to show, with comparative genomic hybridization, amplifications in chromosome 11p (containing the HRAS gene), which represent a unique chromosomal aberration not commonly detectable in melanoma Citation[115]. These Spitz tumors with 11p amplifications share histological features with melanomas.
Initial reports showed that Spitz nevi do not typically contain BRAF and NRAS mutations, commonly found in other types of melanocytic nevi and melanoma, including Spitzoid melanoma (SM) Citation[109,116–119]. The thrilling result has been recently challenged when mutations in BRAF (5–20%) and NRAS (0–5%) were also demonstrated in classic Spitz nevi Citation[120–122].
Melanoma
Melanoma, although relatively rare in children, accounts for 1–3% of childhood malignancies Citation[1]. Children and adolescents (0–17 years of age) have accounted for only 1.3% of the cases of cutaneous melanoma in the USA during the past two decades Citation[123], 79% occurring in adolescents Citation[124] and only 0.3–0.4% during the first decade of life Citation[125]. The estimated annual incidence of disease in children and adolescents younger than 15 years is approximately one per million Citation[126,127].
An increase of incidence in childhood melanoma has been indicated by recent reports Citation[126,127], but lack of agreement still exists, because of different age groups considered in the different studies Citation[3]. If considering postpubertal age, an actual rise in incidence of melanoma is registered, whereas no increase has been reported for children younger than 10 years Citation[3]. In fact, childhood melanoma incidence increased 2.9% per year in the period from 1973 to 2001 Citation[128] and, it is seven-times more frequent in children aged >10 years than in first decade of life.
In both children and adults, risk factors for melanoma development are: intermittent and intense sun exposure, history of sunburns, tendency for freckling, fair skin, blue or green eyes and blond or red hair Citation[128]. In childhood, one can consider further additional risk factors including xeroderma pigmentosum, giant CMN, high nevus count, presence of atypical nevi or many acquired melanocytic nevi, family history of melanoma and immunosuppression Citation[128].
Giant CMN, among the aforementioned risk factors, represent the most important Citation[16]. In fact, approximately a third of all melanomas before the age of 12 years derive from pre-existing giant CMN Citation[10,16] and they often have a bad prognosis (70% of deaths). Nevertheless, the possibility of melanoma development from CMN is low (0.7%) Citation[16].
Recently, an extensive review on melanoma occurring in patients younger than 20 years showed that about 18 deaths per year were detected in the USA in the period from 1969 to 2004 with a total of 643 deaths Citation[16,129]. Calculating the overall age-adjusted mortality rate of childhood melanoma, it resulted in 2.25 deaths per year Citation[16]. It is interesting to observe how the mortality was age-related, being from eight- to 18-times higher after puberty with about 20% of deaths occurring before 14 years of age Citation[16]. Mortality rate for melanoma in childhood has progressively decreased between 1968 and 2004 Citation[16,130]. Childhood melanoma shows a higher thickness than adult type Citation[130], a higher prevalence of positive regional lymph nodes, but a better prognosis if considering the overall survival in adult melanoma with the same features Citation[6,130,131]. Some argue that the different behaviors of melanoma in childhood could also be related to misdiagnosis, as in several histopathologic studies, lesions initially diagnosed as melanoma were reclassified as Spitz nevi/tumors when reviewed retrospectively Citation[1,4–6]. Paradela et al. have compared the prognosis of SM and non-SM in pediatric patients (under the age of 18 years) Citation[132]. Although the authors found that SM have poorer prognostic factors (higher Breslow thickness and mitotic rate, vertical growth phase, nodal metastases), they found a lower mortality rate in SM group (5.9%) than non-SM group (12%). This less aggressive behavior could be due to lower potential for widespread distant metastases of SM as compared with conventional melanomas or to the younger age of children with SM. Of note, authors did not include all the lesions for which a definite diagnosis between AST and SM was not possible Citation[132].
Because of the exceptional occurrence of melanoma in childhood, there are no accurate and detailed clinical or dermoscopic criteria to define it. Pediatric melanoma usually lacks features of the classical pigmented melanoma; it often presents as an amelanotic and nodular lesion, more similar to a pyogenic granuloma or nonpigmented Spitz nevus. In these cases, the clinical EFG (E = elevation; F = firm on palpation; G = growing progressively for more than a month) rule may be helpful in summarizing the clinical symptoms of such melanomas. The most frequent dermoscopic pattern observed in amelanotic melanoma is a polymorphous pattern composed of a combination of dotted vessels and linear, irregular vessels often associated with remnants of pigmentation. Less frequent features, but highly specific for nodular amelanotic melanoma, are milky-red globules characterized by a red–white color, irregular size and blurred borders. The most frequent dermoscopic pattern observed in pigmented melanoma is a multicomponent pattern.
Melanoma appearing in the context of a CMN presents as a growing nodular lesion within the pre-existing nevus. Early detection of changes, and also before the appearance of nodules, is possible with periodic dermoscopic examination of CMN. As a general rule, a biopsy should be performed either on a growing amelanotic nodule or when a recent and sudden change is detected by clinical or dermoscopic examination Citation[16].
The management of childhood melanoma does not differ from that in adults. Early detection remains the mainstay in the treatment to assure a favorable prognosis. If a suspected lesion is detected, narrow margin excision with a request for expert histopathologic diagnosis is recommended. If confirmed, re-excision with adequate margins is warranted. The role of SLN biopsy as a prognostic method in childhood melanoma requires future clarification and should be considered critically. After surgical removal, regular follow-up visits according to established protocols should be performed.
Expert commentary
Childhood melanoma is extremely rare, and in the great majority of cases, melanocytic lesions in childhood are benign and can be managed conservatively. Nevertheless, accuracy in melanoma detection in children and adolescents still remains low, and a high proportion of benign lesions are excised in order to rule out melanoma. An effective strategy to reduce unnecessary excision without overlooking melanoma would be to focus on problematic lesions. Congenital nevi and Spitz/Reed nevi are the most problematic lesions in differential diagnosis with melanoma in childhood. The risk of melanoma associated with CMN is related to the nevus size and particular attention should be paid to giant CMN, especially in younger age. However, since the risk of melanoma development within CMN seems to be less than 1%, the question is raised of which the best and most reasonable management of giant CMN is: excision in young age or, alternatively, periodic clinical and dermoscopic monitoring. One more cause of concern in very young children with CMN is the risk of developing NCM, a melanocytic proliferation of the CNS that may provoke intracranial hypertension. Children affected with symptomatic NCM usually have a scarce prognosis, therefore a neurological consultation could be very helpful for early detection of precocious disease manifestations.
Spitz/Reed nevi appearing before puberty are simply recognized and managed conservatively when <1 cm sized and showing no clinical and dermoscopic atypia. Conversely, for Spitzoid lesions of childhood and adolescence presenting as larger than 1 cm, nodular, ulcerated or rapidly changing, excision must be considered. Due to the lack of identification criteria that permit the distinguishing of Spitz nevi from pyogenic granulomas, surgical excision and histopathologic examination are also recommended.
Overlapping features with melanoma may be occasionally shown by Spitz nevi. Although rare, it is conceivable to find Spitzoid lesions diagnosed as malignant only retrospectively (i.e., after the appearance of metastases). Conversely, it has been demonstrated that some childhood melanomas were re-evaluated and retrospectively diagnosed as Spitz nevi. Wide excision is mandatory in Spitzoid lesions with atypical histopathologic features and the decision of performing SLN biopsy should be individually considered. The location of the lesion has to be taken into account and parents should be aware that this procedure has not been proven to give a survival benefit and it may carry a risk of iatrogenic morbidity.
In conclusion, as a general rule to exclude childhood melanoma, a biopsy should be always performed when, within a CMN, a growing amelanotic nodule appears or a change is discovered clinically, and especially dermoscopically.
Five-year view
Future studies investigating the natural evolution of melanocytic nevi in children are needed. A better knowledge of the natural history of nevi in this age group would probably allow a better management of pediatric patients, leading to a decrease in the number of unnecessary excisions of benign lesions. In this field, a better understanding of the biology of Spitzoid lesions is mandatory in improving the clinical management of these neoplasms. Spitzoid lesions seem to be a particular subgroup of melanocytic proliferations that show a malignant morphology but a relatively benign biological behavior. They can sometimes be morphologically indistinguishable from melanoma but follow a benign clinical course. To date, there is still a spectrum of Spitzoid neoplasm cases for which morphology cannot predict biology of the tumor. Further research is needed that will probably include new findings from molecular biology, in order to better classify and finally manage these lesions.
Key issues
• Melanocytic lesions in children are managed conservatively in the great majority of cases.
• Large congenital melanocytic nevi (CMN) and Spitzoid lesions are the main problematic lesions in children.
• The risk of melanoma associated with CMN is proportional to the nevus size; however, less than 1% of children with congenital nevi will develop melanoma, thus regular clinical and dermoscopic follow-up can represent a reasonable management option.
• The risk of neurocutaneous melanocytosis should be taken into account in children with giant CMN, and a high number of satellite lesions. This condition represents a proliferation of melanocytes within the CNS and may have a very poor prognosis due to the risk of increased intracranial pressure.
• Classical or pigmented Spitz nevi of prepubertal age can be easily recognized and managed conservatively with periodic clinical and dermoscopic evaluation.
• Large (>1 cm), nodular, ulcerated, rapidly changing, atypical Spitz lesions in childhood must be excised.
References
- Han D, Zager JS, Han G et al. The unique clinical characteristics of melanoma diagnosed in children. Ann. Surg. Oncol. 19(12), 3888–3895 (2012).
- Fraitag S. Melanocytic nevi in children. Ann. Pathol. 24(6), 587–604 (2004).
- Ferrari A, Bono A, Baldi M et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics 115(3), 649–654 (2005).
- Moscarella E, Zalaudek I, Cerroni L et al. Excised melanocytic lesions in children and adolescents – a 10-year survey. Br. J. Dermatol. 167(2), 368–373 (2012).
- Wechsler J, Bastuji-Garin S, Spatz A et al.; French Cutaneous Cancerology Group. Reliability of the histopathologic diagnosis of malignant melanoma in childhood. Arch. Dermatol. 138(5), 625–628 (2002).
- Paredes B, Hardmeier T. Spitz nevus and Reed nevus: simulating melanoma in adults. Pathologe 19(6), 403–411 (1998).
- Leman JA, Evans A, Mooi W, MacKie RM. Outcomes and pathological review of a cohort of children with melanoma. Br. J. Dermatol. 152(6), 1321–1323 (2005).
- Huynh PM, Grant-Kels JM, Grin CM. Childhood melanoma: update and treatment. Int. J. Dermatol. 44(9), 715–723 (2005).
- Richardson SK, Tannous ZS, Mihm MC Jr. Congenital and infantile melanoma: review of the literature and report of an uncommon variant, pigment-synthesizing melanoma. J. Am. Acad. Dermatol. 47(1), 77–90 (2002).
- Kanzler MH, Mraz-Gernhard S. Primary cutaneous malignant melanoma and its precursor lesions: diagnostic and therapeutic overview. J. Am. Acad. Dermatol. 45(2), 260–276 (2001).
- Bonifazi E, Bilancia M, Berloco A, Ciampo L, De Roma MR. Malignant melanoma in children aged 0–12. Review of 289 cases of the literature. Eur. J. Pediat. Dermatol. 11, 157–175 (2001).
- Argenziano G, Soyer HP, Chimenti S et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J. Am. Acad. Dermatol. 48(5), 679–693 (2003).
- Argenziano G, Soyer HP. Dermoscopy of pigmented skin lesions – a valuable tool for early diagnosis of melanoma. Lancet Oncol. 2(7), 443–449 (2001).
- Soyer HP, Argenziano G, Talamini R, Chimenti S. Is dermoscopy useful for the diagnosis of melanoma? Arch. Dermatol. 137(10), 1361–1363 (2001).
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J. Am. Acad. Dermatol. 17(4), 571–583 (1987).
- Moscarella E, Zalaudek I, Ferrara G, Manzo M, Savarese I, Argenziano G. Problematic melanocytic lesions in children. Expert Rev. Dermatol. 4(3), 249–261 (2009).
- English DR, Armstrong BK. Melanocytic nevi in children. I. Anatomic sites and demographic and host factors. Am. J. Epidemiol. 139(4), 390–401 (1994).
- Naldi L, Adamoli L, Fraschini D et al. Number and distribution of melanocytic nevi in individuals with a history of childhood leukemia. Cancer 77(7), 1402–1408 (1996).
- Worret WI, Burgdorf WH. Which direction do nevus cells move? Abtropfung reexamined. Am. J. Dermatopathol. 20(2), 135–139 (1998).
- Zalaudek I, Grinschgl S, Argenziano G et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br. J. Dermatol. 154(2), 299–304 (2006).
- Aguilera P, Puig S, Guilabert A et al. Prevalence study of nevi in children from Barcelona. Dermoscopy, constitutional and environmental factors. Dermatology (Basel) 218(3), 203–214 (2009).
- Westhafer J, Gildea J, Klepeiss S, Clarke L, Helm K. Age distribution of biopsied junctional nevi. J. Am. Acad. Dermatol. 56(5), 825–827 (2007).
- Scope A, Marghoob AA, Dusza SW et al. Dermoscopic patterns of naevi in fifth grade children of the Framingham school system. Br. J. Dermatol. 158(5), 1041–1049 (2008).
- Scope A, Dusza SW, Marghoob AA et al. Clinical and dermoscopic stability and volatility of melanocytic nevi in a population-based cohort of children in Framingham school system. J. Invest. Dermatol. 131(8), 1615–1621 (2011).
- Pizzichetta MA, Massone C, Grandi G, Pelizzo G, Soyer HP. Morphologic changes of acquired melanocytic nevi with eccentric foci of hyperpigmentation (‘Bolognia sign’) assessed by dermoscopy. Arch. Dermatol. 142(4), 479–483 (2006).
- Halpern AC, Guerry D 4th, Elder DE, Trock B, Synnestvedt M, Humphreys T. Natural history of dysplastic nevi. J. Am. Acad. Dermatol. 29(1), 51–57 (1993).
- Haley JC, Hood AF, Chuang TY, Rasmussen J. The frequency of histologically dysplastic nevi in 199 pediatric patients. Pediatr. Dermatol. 17(4), 266–269 (2000).
- Schaffer JV. Pigmented lesions in children: when to worry. Curr. Opin. Pediatr. 19(4), 430–440 (2007).
- Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch. Dermatol. 139(3), 282–288 (2003).
- Bauer J, Garbe C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 16(3), 297–306 (2003).
- Bauer J, Garbe C. Risk estimation for malignant transformation of melanocytic nevi. Arch. Dermatol. 140(1), 127; author reply 127 (2004).
- González-Cámpora R, Galera-Davidson H, Vázquez-Ramírez FJ, Díaz-Cano S. Blue nevus: classical types and new related entities. A differential diagnostic review. Pathol. Res. Pract. 190(6), 627–635 (1994).
- Ferrara G, Soyer HP, Malvehy J et al. The many faces of blue nevus: a clinicopathologic study. J. Cutan. Pathol. 34(7), 543–551 (2007).
- Di Cesare A, Sera F, Gulia A et al. The spectrum of dermatoscopic patterns in blue nevi. J. Am. Acad. Dermatol. 67(2), 199–205 (2012).
- Lourari S, Lamant L, Viraben R, Paul C, Meyer N. Photoletter to the editor: blue nevus with satellitosis mimicking melanoma. Contribution of dermoscopy and reflectance confocal microscopy. J. Dermatol. Case Rep. 6(2), 54–56 (2012).
- Piana S, Grenzi L, Albertini G. Cellular blue nevus with satellitosis: a possible diagnostic pitfall. Am. J. Dermatopathol. 31(4), 401–402 (2009).
- Sahin MT, Demir MA, Yoleri L, Can M, Oztürkcan S. Blue naevus with satellitosis mimicking malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 15(6), 570–573 (2001).
- del Río E, Vázquez Veiga HA, Suárez Peñaranda JM. Blue nevus with satellitosis mimicking malignant melanoma. Cutis. 65(5), 301–302 (2000).
- Gartmann H, Müller HD. Combined occurrence of blue nevus and nevus cell nevus in one and the same tumor (‘combined nevus’). Z. Hautkr. 52(7), 389–398 (1977).
- de Giorgi V, Massi D, Salvini C, Trez E, Mannone F, Carli P. Dermoscopic features of combined melanocytic nevi. J. Cutan. Pathol. 31(9), 600–604 (2004).
- Sutton RL. An unusual variety of vitiligo (leucoderma acquisitum centrifugum). J. Cut. Dis. 34, 797–800 (1926).
- Kopf AW, Morrill SD, Silberberg I. Broad spectrum of leukoderma acquisitum centrifugum. Arch. Dermatol. 92(1), 14–33; discussion 33 (1965).
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? A study of 142 cases. J. Cutan. Pathol. 22(4), 342–348 (1995).
- Akasu R, From L, Kahn HJ. Characterization of the mononuclear infiltrate involved in regression of halo nevi. J. Cutan. Pathol. 21(4), 302–311 (1994).
- Harvell JD, Meehan SA, LeBoit PE. Spitz’s nevi with halo reaction: a histopathologic study of 17 cases. J. Cutan. Pathol. 24(10), 611–619 (1997).
- Zeff RA, Freitag A, Grin CM, Grant-Kels JM. The immune response in halo nevi. J. Am. Acad. Dermatol. 37(4), 620–624 (1997).
- Leow LJ, Goh BK. Halo congenital naevus in a middle-aged patient with vitiligo. Australas. J. Dermatol. 49(4), 229–232 (2008).
- Brazzelli V, Larizza D, Martinetti M et al. Halo nevus, rather than vitiligo, is a typical dermatologic finding of turner’s syndrome: clinical, genetic, and immunogenetic study in 72 patients. J. Am. Acad. Dermatol. 51(3), 354–358 (2004).
- Kolm I, Di Stefani A, Hofmann-Wellenhof R et al. Dermoscopy patterns of halo nevi. Arch. Dermatol. 142(12), 1627–1632 (2006).
- Zalaudek I, Moscarella E, Argenziano G. Artifactual ‘pseudo-halo nevi’ secondary to sunscreen application. J. Am. Acad. Dermatol. 54(6), 1106–1107 (2006).
- Saida T, Oguchi S, Ishihara Y. In vivo observation of magnified features of pigmented lesions on volar skin using video macroscope. Usefulness of epiluminescence techniques in clinical diagnosis. Arch. Dermatol. 131(3), 298–304 (1995).
- Akasu R, Sugiyama H, Araki M, Ohtake N, Furue M, Tamaki K. Dermatoscopic and videomicroscopic features of melanocytic plantar nevi. Am. J. Dermatopathol. 18(1), 10–18 (1996).
- Oguchi S, Saida T, Koganehira Y, Ohkubo S, Ishihara Y, Kawachi S. Characteristic epiluminescent microscopic features of early malignant melanoma on glabrous skin. A videomicroscopic analysis. Arch. Dermatol. 134(5), 563–568 (1998).
- Fabrizi G, Pagliarello C, Parente P, Massi G. Atypical nevi of the scalp in adolescents. J. Cutan. Pathol. 34(5), 365–369 (2007).
- De Giorgi V, Sestini S, Grazzini M, Janowska A, Boddi V, Lotti T. Prevalence and distribution of melanocytic naevi on the scalp: a prospective study. Br. J. Dermatol. 162(2), 345–349 (2010).
- Kessides MC, Puttgen KB, Cohen BA. No biopsy needed for eclipse and cockade nevi found on the scalps of children. Arch. Dermatol. 145(11), 1334–1336 (2009).
- Blum A, Simionescu O, Argenziano G et al. Dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction: results of a multicenter study by the International Dermoscopy Society (IDS). Arch. Dermatol. 147(10), 1181–1187 (2011).
- Lin J, Koga H, Takata M, Saida T. Dermoscopy of pigmented lesions on mucocutaneous junction and mucous membrane. Br. J. Dermatol. 161(6), 1255–1261 (2009).
- Ferrari A, Zalaudek I, Argenziano G et al. Dermoscopy of pigmented lesions of the vulva: a retrospective morphological study. Dermatology (Basel) 222(2), 157–166 (2011).
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J. Am. Acad. Dermatol. 67(4), 495.e1–17; quiz 512 (2012).
- Watt AJ, Kotsis SV, Chung KC. Risk of melanoma arising in large congenital melanocytic nevi: a systematic review. Plast. Reconstr. Surg. 113(7), 1968–1974 (2004).
- Krengel S, Hauschild A, Schäfer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br. J. Dermatol. 155(1), 1–8 (2006).
- Marghoob AA. Congenital melanocytic nevi. Evaluation and management. Dermatol. Clin. 20(4), 607–16, viii (2002).
- Bett BJ. Large or multiple congenital melanocytic nevi: occurrence of neurocutaneous melanocytosis in 1008 persons. J. Am. Acad. Dermatol. 54(5), 767–777 (2006).
- Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J. Am. Acad. Dermatol. 24(5 Pt 1), 747–755 (1991).
- Foster RD, Williams ML, Barkovich AJ, Hoffman WY, Mathes SJ, Frieden IJ. Giant congenital melanocytic nevi: the significance of neurocutaneous melanosis in neurologically asymptomatic children. Plast. Reconstr. Surg. 107(4), 933–941 (2001).
- Humphreys RP. Clinical evaluation of cutaneous lesions of the back: spinal signatures that do not go away. Clin. Neurosurg. 43, 175–187 (1996).
- Clark WH, Elder DE, Guerry D. Displastic nevi and malignant melanoma. In: Pathology of the Skin (1st Edition). Farmer ER, Hood AF (Eds). Mc GrawHill, OH, USA, 729–735 (1996).
- Bastian BC, Xiong J, Frieden IJ et al. Genetic changes in neoplasms arising in congenital melanocytic nevi: differences between nodular proliferations and melanomas. Am. J. Pathol. 161(4), 1163–1169 (2002).
- Rhodes AR, Melski JW. Small congenital nevocellular nevi and the risk of cutaneous melanoma. J. Pediatr. 100(2), 219–224 (1982).
- Betti R, Inselvini E, Vergani R, Crosti C. Small congenital nevi associated with melanoma: case reports and considerations. J. Dermatol. 27(9), 583–590 (2000).
- Williams ML, Pennella R. Melanoma, melanocytic nevi, and other melanoma risk factors in children. J. Pediatr. 124(6), 833–845 (1994).
- Rhodes AR. The risk of malignant melanoma arising in congenital melanocytic nevi. An argument against the assignment of risk based on size alone. Am. J. Dermatopathol. 6, 184–188 (1984).
- Sahin S, Levin L, Kopf AW et al. Risk of melanoma in medium-sized congenital melanocytic nevi: a follow-up study. J. Am. Acad. Dermatol. 39(3), 428–433 (1998).
- Scalzo DA, Hida CA, Toth G, Sober AJ, Mihm MC Jr. Childhood melanoma: a clinicopathological study of 22 cases. Melanoma Res. 7(1), 63–68 (1997).
- Berg P, Lindelöf B. Congenital melanocytic naevi and cutaneous melanoma. Melanoma Res. 13(5), 441–445 (2003).
- Zaal LH, Mooi WJ, Klip H, van der Horst CM. Risk of malignant transformation of congenital melanocytic nevi: a retrospective nationwide study from The Netherlands. Plast. Reconstr. Surg. 116(7), 1902–1909 (2005).
- Ibrahimi OA, Alikhan A, Eisen DB. Congenital melanocytic nevi: where are we now? Part II. Treatment options and approach to treatment. J. Am. Acad. Dermatol. 67(4), 515.e1–13; quiz 528 (2012).
- Bohn J, Svensson H, Aberg M. Dermabrasion of large congenital melanocytic naevi in neonates. Scand. J. Plast. Reconstr. Surg. Hand Surg. 34(4), 321–326 (2000).
- De Raeve LE, Roseeuw DI. Curettage of giant congenital melanocytic nevi in neonates: a decade later. Arch. Dermatol. 138(7), 943–947 (2002).
- Hopkins JD, Smith AW, Jackson IT. Adjunctive treatment of congenital pigmented nevi with phenol chemical peel. Plast. Reconstr. Surg. 105(1), 1–11 (2000).
- Waldorf HA, Kauvar AN, Geronemus RG. Treatment of small and medium congenital nevi with the Q-switched ruby laser. Arch. Dermatol. 132(3), 301–304 (1996).
- Kopf AW, Andrade R. Benign juvenile melanoma. In: Year Book of Dermatology. Kopf AW, Andrade R (Eds). Year Book Medical Publisher Inc., Chicago, IL, USA, 7–52 (1966).
- Weedon D, Little JH. Spindle and epithelioid cell nevi in children and adults. A review of 211 cases of the Spitz nevus. Cancer 40(1), 217–225 (1977).
- Gartmann H, Ganser M. The Spitz nevus. Spindle cell and/or epithelioid cell nevus – clinical analysis of 652 tumors. Z. Hautkr. 60(1–2), 22–28 (1985).
- Weedon D. Lentigines, nevi and melanomas. In: Skin Pathology (2nd Edition). Weedon D (Ed.). Churchill Livingstone, Edinburgh, UK, 803–858 (2002).
- Reed RJ, Ichinose H, Clark WH Jr, Mihm MC Jr. Common and uncommon melanocytic nevi and borderline melanomas. Semin. Oncol. 2(2), 119–147 (1975).
- Paniago-Pereira C, Maize JC, Ackerman AB. Nevus of large spindle and/or epithelioid cells (Spitz’s nevus). Arch. Dermatol. 114(12), 1811–1823 (1978).
- Kolde G, Vakilzadeh F. Pigmented spindle cell tumor. Hautarzt. 38(12), 743–745 (1987).
- Smith NP. The pigmented spindle cell tumor of Reed: an underdiagnosed lesion. Semin. Diagn. Pathol. 4(1), 75–87 (1987).
- Barnhill RL, Mihm MC Jr. Pigmented spindle cell naevus and its variants: distinction from melanoma. Br. J. Dermatol. 121(6), 717–725 (1989).
- Barnhill RL, Barnhill MA, Berwick M, Mihm MC Jr. The histologic spectrum of pigmented spindle cell nevus: a review of 120 cases with emphasis on atypical variants. Hum. Pathol. 22(1), 52–58 (1991).
- Barnhill RL, Mihm MC Jr, Magro CM. Plexiform spindle cell naevus: a distinctive variant of plexiform melanocytic naevus. Histopathology 18(3), 243–247 (1991).
- Grossin M. Reed’s nevus. Ann. Dermatol. Venereol. 119(2), 145 (1992).
- Sau P, Graham JH, Helwig EB. Pigmented spindle cell nevus: a clinicopathologic analysis of ninety-five cases. J. Am. Acad. Dermatol. 28(4), 565–571 (1993).
- Ferrara G, Argenziano G, Soyer HP et al. The spectrum of Spitz nevi: a clinicopathologic study of 83 cases. Arch. Dermatol. 141(11), 1381–1387 (2005).
- Peris K, Ferrari A, Argenziano G, Soyer HP, Chimenti S. Dermoscopic classification of Spitz/Reed nevi. Clin. Dermatol. 20(3), 259–262 (2002).
- Argenziano G, Zalaudek I, Corona R et al. Vascular structures in skin tumors: a dermoscopy study. Arch. Dermatol. 140(12), 1485–1489 (2004).
- Argenziano G, Agozzino M, Bonifazi E et al. Natural evolution of Spitz nevi. Dermatology (Basel) 222(3), 256–260 (2011).
- Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part I. Background and diagnoses. J. Am. Acad. Dermatol. 65(6), 1073–1084 (2011).
- Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part II. Natural history and management. J. Am. Acad. Dermatol. 65(6), 1087–1092 (2011).
- Zalaudek I, Sgambato A, Ferrara G, Argenziano G. Diagnosis and management of melanocytic skin lesion in the pediatric praxis. A review of the literature. Minerva Pediatr. 60(3), 291–312 (2008).
- Spatz A, Calonje E, Handfield-Jones S, Barnhill RL. Spitz tumors in children: a grading system for risk stratification. Arch. Dermatol. 135(3), 282–285 (1999).
- Kelley SW, Cockerell CJ. Sentinel lymph node biopsy as an adjunct to management of histologically difficult to diagnose melanocytic lesions: a proposal. J. Am. Acad. Dermatol. 42(3), 527–530 (2000).
- Ludgate MW, Fullen DR, Lee J et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer 115(3), 631–641 (2009).
- Barnhill RL, Flotte TJ, Fleischli M, Perez-Atayde A. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer 76(10), 1833–1845 (1995).
- Bastian BC, LeBoit PE, Hamm H, Bröcker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 58(10), 2170–2175 (1998).
- Bastian BC, Wesselmann U, Pinkel D, Leboit PE. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J. Invest. Dermatol. 113(6), 1065–1069 (1999).
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am. J. Pathol. 157(3), 967–972 (2000).
- Curtin JA, Fridlyand J, Kageshita T et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353(20), 2135–2147 (2005).
- van Dijk MC, Rombout PD, Mooi WJ et al. Allelic imbalance in the diagnosis of benign, atypical and malignant Spitz tumours. J. Pathol. 197(2), 170–178 (2002).
- van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and Spitzoid melanoma. Am. J. Surg. Pathol. 29(9), 1145–1151 (2005).
- Healy E, Belgaid CE, Takata M et al. Allelotypes of primary cutaneous melanoma and benign melanocytic nevi. Cancer Res. 56(3), 589–593 (1996).
- Takata M, Suzuki T, Ansai S et al. Genome profiling of melanocytic tumors using multiplex ligation-dependent probe amplification (MLPA): its usefulness as an adjunctive diagnostic tool for melanocytic tumors. J. Dermatol. Sci. 40(1), 51–57 (2005).
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors. Pathologe 28(6), 464–473 (2007).
- Davies H, Bignell GR, Cox C et al. Mutations of the BRAF gene in human cancer. Nature 417(6892), 949–954 (2002).
- Pollock PM, Harper UL, Hansen KS et al. High frequency of BRAF mutations in nevi. Nat. Genet. 33(1), 19–20 (2003).
- Palmedo G, Hantschke M, Rütten A et al. The T1796A mutation of the BRAF gene is absent in Spitz nevi. J. Cutan. Pathol. 31(3), 266–270 (2004).
- Mihic-Probst D, Perren A, Schmid S, Saremaslani P, Komminoth P, Heitz PU. Absence of BRAF gene mutations differentiates Spitz nevi from malignant melanoma. Anticancer Res. 24(4), 2415–2418 (2004).
- Saldanha G, Purnell D, Fletcher A, Potter L, Gillies A, Pringle JH. High BRAF mutation frequency does not characterize all melanocytic tumor types. Int. J. Cancer 111(5), 705–710 (2004).
- Fullen DR, Poynter JN, Lowe L et al. BRAF and NRAS mutations in Spitzoid melanocytic lesions. Mod. Pathol. 19(10), 1324–1332 (2006).
- Emley A, Yang S, Wajapeyee N, Green MR, Mahalingam M. Oncogenic BRAF and the tumor suppressor IGFBP7 in the genesis of atypical Spitzoid nevomelanocytic proliferations. J. Cutan. Pathol. 37(3), 344–349 (2010).
- Hamre MR, Chuba P, Bakhshi S, Thomas R, Severson RK. Cutaneous melanoma in childhood and adolescence. Pediatr. Hematol. Oncol. 19(5), 309–317 (2002).
- de Vries E, Steliarova-Foucher E, Spatz A, Ardanaz E, Eggermont AM, Coebergh JW. Skin cancer incidence and survival in European children and adolescents (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 42(13), 2170–2182 (2006).
- Ceballos PI, Ruiz-Maldonado R, Mihm MC Jr. Melanoma in children. N. Engl. J. Med. 332(10), 656–662 (1995).
- Karlsson P, Boeryd B, Sander B, Westermark P, Rosdahl I. Increasing incidence of cutaneous malignant melanoma in children and adolescents 12–19 years of age in Sweden 1973–92. Acta Derm. Venereol. 78(4), 289–292 (1998).
- Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J. Clin. Oncol. 23(21), 4735–4741 (2005).
- Jen M, Murphy M, Grant-Kels JM. Childhood melanoma. Clin. Dermatol. 27(6), 529–536 (2009).
- Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol. Surg. 34(2), 152–159 (2008).
- Livestro DP, Kaine EM, Michaelson JS et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer 110(3), 614–624 (2007).
- Bütter A, Hui T, Chapdelaine J, Beaunoyer M, Flageole H, Bouchard S. Melanoma in children and the use of biopsy. J. Pediatr. Surg. 40(5), 797–800 (2005).
- Paradela S, Fonseca E, Pita-Fernández S, Prieto VG. Spitzoid and non-Spitzoid melanoma in children. A prognostic comparative study. J. Eur. Acad. Dermatol. Venereol. doi:10.1111/j.1468-3083.2012.04686.x (2012) (Epub ahead of print).
Analysis of Clinical and Dermoscopic Features in Melanocytic Lesions With Special Emphasis on Problematic Lesions in Children
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertdermatol. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 12-year-old healthy girl with several melanocytic skin lesions that her parents have noticed for either months or years. They are concerned regarding her risk of skin cancer. Which of the following types of skin lesions are most associated with the diagnosis of melanoma among children?
□ A Blue nevi and combined nevi
□ B Congenital melanocytic nevi (CMN) and Spitz nevi
□ C Halo nevi and combined nevi
□ D CMN and blue nevi
2. The patient has several benign-appearing skin lesions. Which of the following statements regarding low-risk melanocytic nevi among children is most accurate?
□ A The globular pattern is most common on dermoscopy
□ B Blue nevi are generally flat
□ C Halo nevi are usually found on the arms and legs
□ D Even benign-appearing nevi on the palms and soles should be excised
3. The patient also has a CMN on her back with a diameter of 22 cm. What should you consider regarding the clinical features and management of this lesion?
□ A The risk of developing melanoma in this giant CMN is less than 0.01%
□ B Nodules originating from large CMN are typically easy to differentiate from melanoma
□ C She is a good candidate for dermoabrasion
□ D Early recognition of malignant melanoma is very difficult in cases of giant CMN
4. The patient also has a flesh-colored nodule on her thigh, which appears to be a Spitz nevus. What should you consider regarding these nevi?
□ A They are rich in melanin
□ B Atypical Spitz tumors (AST) are more frequent in children less than 10 years of age vs adolescents
□ C AST can demonstrate the same features as melanoma on dermoscopy
□ D Excision of spitzoid lesions is unnecessary among most adolescents
