Abstract
Diabetes is a chronic disease that manifests when insulin production by the pancreas is insufficient or when the body cannot effectively utilize the secreted insulin. The onset of diabetes often goes undetected until the later stages where subsequent glucose accumulation in the system (hyperglycemia) is observed. Over time, it leads to serious multi-organ damage, especially to the nerves and blood vessels. The WHO reports that approximately 346 million people worldwide are diagnosed with diabetes. With no cure available, long-term medical care for diabetes has become a global economic challenge globally. Hence, there is a need to explore novel early biomarkers and therapeutics for diabetes. One such potential molecule is the miRNAs. miRNAs are endogenous, noncoding RNAs that predominantly inhibit gene expression. Compelling evidence showed that altered miRNA expressions are linked to pathological conditions, including diabetes manifestation. This review focuses on the implications of miRNAs in diabetes and their related complications.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertendo; (4) view/print certificate.
Release date: 18 May 2012; Expiration date: 18 May 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Describe the structure and function of miRNAs
• Evaluate miRNAs associated with pancreatic function
• Evaluate the role of miRNAs in tissues important in the pathogenesis of diabetes
• Assess the current clinical usefulness of miRNAs
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME AUTHOR
Charles P Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA.
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
AUTHORS AND CREDENTIALS
Dwi Setyowati Karolina, BSc Hons
Department of Biochemistry, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University Health System, National University of Singapore, Singapore.
Disclosure: Karolina Dwi Setyowati, BSc Hons, has disclosed the following relevant financial relationships: this work was supported by National Medical Research Council Grant IRG: R-183-000-290-213 and EDG: R-183-000-230-275.
Arunmozhiarasi Armugam, PhD
Department of Biochemistry, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University Health System, National University of Singapore, Singapore.
Disclosure: Arunmozhiarasi Armugam, PhD, has disclosed the following relevant financial relationships: this work was supported by National Medical Research Council Grant IRG: R-183-000-290-213 and EDG: R-183-000-230-275.
Sugunavathi Sepramaniam, PhD
Department of Biochemistry, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University Health System, National University of Singapore, Singapore.
Disclosure: Sugunavathi Sepramaniam, PhD, has disclosed the following relevant financial relationships: this work was supported by National Medical Research Council Grant IRG: R-183-000-290-213 and EDG: R-183-000-230-275.
Kandiah Jeyaseelan, PhD, DSc
Department of Biochemistry, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University Health System, National University of Singapore, Singapore.
Disclosure: Kandiah Jeyaseelan, PhD, DSc, has disclosed the following relevant financial relationships: this work was supported by National Medical Research Council Grant IRG: R-183-000-290-213 and EDG: R-183-000-230-275.
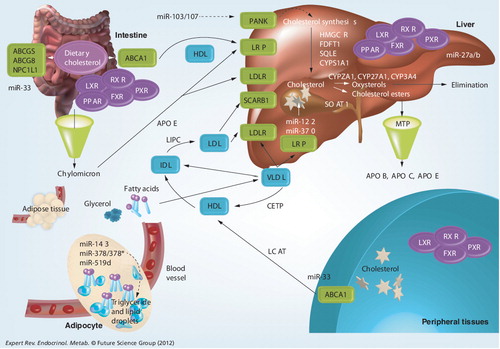
ABC: ATP-binding cassette transporter; CETP: Cholesterylester transfer protein; FDFT1: Farnesyl-diphosphate farnesyltransferase 1; FXR: Farnesoid X receptor; HDL: High-density lipoprotein; HMGCR: HMC-CoA receptor; IDL: Intermediate-density lipoprotein; LCAT: Lecithin:cholesterol acyltransferase; LDL: Low-density lipoprotein; LDLR: Low-density lipoprotein receptor; LRP: Low-density lipoprotein receptor-related protein; LXR: Liver X receptor; MTP: Microsomal triaglyceride transfer protein; NPC1L1, Niemann-Pick disease, type C1, gene-like 1; PANK: Pantothenate kinase; PPAR: Peroxisome proliferator-activated receptor; PXR: Pregnane X receptor; RXR: Retinoid X receptor; SCARB1: Scavenger receptor class B, member 1; SOAT1: Sterol O-acyltransferase 1; SQLE: Squalene epoxidase; VLDL: Very-low-density lipoprotein.
Diabetes mellitus (diabetes) is a complex metabolic disorder characterized by abnormal levels of glucose (fasting glucose >7 mmol/l) in the blood. During the fed state, the pancreas releases insulin to induce glucose uptake by the recipient cells, thus reducing the high blood glucose levels. However, during low sugar levels, it secretes glucagon to induce liver cells to release the stored glucose, thus increasing the blood glucose concentration. Hence, blood glucose homeostasis is maintained by balancing the pancreatic endocrine hormones, insulin and glucagon production. Diabetes is characterized by two subtypes: Type 1 diabetes (T1D), which is insulin dependent, and Type 2 diabetes (T2D), which is insulin independent. In T1D, the β-cells in the pancreas produce little or no insulin, resulting in the build-up of glucose. Individuals with T1D resort to daily insulin administration to manage their glucose levels (as T1D is insulin dependent), while on the other hand, individuals with T2D are nonresponsive to insulin produced by the pancreas (as T2D is insulin independent). Although as simple as it may sound, the molecular mechanisms underlying the progression of pathogenesis of diabetes and its role in multi-organ damage are far from clear.
For many decades, several laboratories have embarked on identifying the key component(s) that play crucial roles in the onset of diabetes and its progression Citation[1]. Both protein and gene (mRNA) expression studies have been or are being used to uncover the processes and signaling pathways in the development of disease. In recent years, research focusing on elucidating the molecular mechanisms underlying the pathogenesis of diabetes has discovered the potential of miRNAs not only in delineating the disease manifestation but also as novel early biomarkers for diagnosis and probably as therapeutic agents. miRNAs are a class of small, endogenous, noncoding RNAs approximately 22 nucleotides in length that modulate gene expression. Generally, miRNAs exert inhibitory effects on gene expression by binding to the 3′-UTR of their mRNA targets. The first insulin-related miRNA to be identified was miR-375, which is also an islet-specific miRNA Citation[2]. miR-375 was found to target the 3′-UTR of the myotrophin (Mtpn) gene, which encodes the myotrophin protein, a key component of insulin exocytosis machinery Citation[2]. Following that, there has been an increasing number of glucose metabolism-related miRNAs being identified in both in vitro and in vivo models of diabetes Citation[3–7]. Paralleling these studies, miRNA-based clinical research has also provided evidence for these molecules to be implicated in the development and progression of diabetes Citation[8–10], including prediabetic stages as well as diabetic complications Citation[11–13]. Collectively, these data suggest that miRNAs can be developed as a promising new tool in understanding diabetes mellitus and possibly in treating this metabolic disorder. In this review, the implications of miRNAs in diabetes mellitus are discussed by outlining the mechanisms of these small noncoding RNA molecules in regulating the insulin secretory/signaling pathway, as well as glucose and lipid metabolism.
Pathogenesis of diabetes mellitus: T1D & T2D
T1D is an autoimmune condition in which the insulin-producing β-cells of the pancreas are destroyed and hence the body loses its ability to produce insulin Citation[14]. Previously known as juvenile diabetes, T1D is usually diagnosed in children and young adults, affecting only approximately 5% of the overall diabetes population Citation[15]. T1D is believed to be an immunological disease where little is known about its risk factors. Nevertheless, epigenetic factors are possible contributors to the development of the disorder. Researchers have also suggested that viral-mediated autoimmunity may also trigger self-destruction or infection of the pancreatic islet cells Citation[16]. In summary, a malfunction/dysfunction of the pancreatic islets is known to result in T1D. Without the help of insulin therapy, T1D is generally fatal. As such, T1D patients are entirely dependent on daily multiple insulin administration to sustain life. Alternatively, individuals with T1D may opt for procedures such as pancreatic or pancreatic islet-cell transplantation to replace the non functional insulin-producing cells. Nevertheless, such procedures can cause complications that would outweigh the inconveniences of living with insulin administration on a daily basis.
The second category of diabetes, T2D, is the most common form, which accounts for 90–95% of all diabetic cases Citation[17]. Besides family history and genetic factors, risk factors for T2D include obesity, high blood pressure and high cholesterol levels Citation[18]. T2D occurs when the sensitivity of the peripheral tissues is compromised such that they can no longer respond appropriately to insulin, a condition also known as insulin resistance. Defects in insulin sensitivity and insulin secretion cause glucose to accumulate in the blood Citation[19]. Over time, the response of the peripheral tissues to insulin becomes progressively blunted and the balance between insulin output and demand is disrupted, thus aggravating the condition. On the other hand, it has also been suggested that β-cell dysfunction observed in T1D patients can also occur in T2D. It is considered to be an early event in the cascade leading to T2D Citation[20].
Thus, to distinguish T1D from T2D, in a clinical setting, C-peptide assay is commonly used. C-peptide is a protein that is produced along with insulin. Insulin synthesis begins from the production of the preproinsulin, which comprises an A-chain, C-peptide, a B-chain and a signal peptide. Cleavage of the signal peptide produces proinsulin. Subsequently, the C-peptide is cleaved, leaving the A-chain and B-chain to form the insulin molecule. Hence, C-peptide levels in the body represent the production of endogenous insulin. In individuals with T1D, the C-peptide readings are typically low or undetectable (at fasting: <0.4 ng/ml), as these individuals lose the ability to produce insulin, while those with T2D showed readings that are usually above average (average fasting values between 0.4 and 2.1 ng/ml) but are hyperglycemic, indicating a state of insulin resistance.
Unlike T1D, the risk of developing T2D can be reduced by practicing a healthy lifestyle. More than 346 million people worldwide have diabetes and so far no corrective measures have been found Citation[17,21]. Recently, it was discovered that bariatric surgery, a weight-loss procedure designed for obese patients, has antidiabetic secondary effects. Overall, 77% of the obese patients had their diabetes resolved almost immediately or within days after this surgery Citation[22,23]. Nonetheless, only individuals with a BMI >40 kg/m2 or those with a BMI between 35 and 40 kg/m2 with serious metabolic conditions such as diabetes and high blood pressure are eligible for such a procedure. This leaves the remaining 45% of the diabetic patients who are not overtly obese (BMI <40 kg/m2) with no available remedy Citation[24]. A recent study by Lim et al. demonstrated that diabetes progression could be reversed by reducing calorie intake Citation[25]. Although this approach has great potential, the practice of limiting calorie intake to 600 kcal daily might not be feasible in the long run. Therefore, there is still a need to search for novel therapeutics in combating diabetes mellitus.
miRNAs: biogenesis & mode of action
miRNAs are small, highly conserved RNA molecules approximately 22 nucleotides in length generated from endogenous hairpin-shaped transcripts. miRNAs are widely accepted to be gene regulators. The biogenesis of miRNAs is a multistep event catalyzed by specific RNA polymerases Citation[26,27]. The miRNA genes are located in clusters and often transcribed as polycistronic primary miRNAs under strict developmental stage and tissue-specific regulation. Production of primary miRNAs (∼1 kb) is mediated by RNA polymerase II and in some cases RNA polymerase III Citation[26]. Primary miRNAs are then processed by Drosha (RNase III enzyme) to liberate precursor miRNAs approximately 70 nucleotides long with 2-nucleotide 3′-overhang. The 2-nucleotide 3′-overhang end structure of the precursor miRNA is then recognized by exportin-5, a Ran-GTP-dependent nuclear export factor and exported into the cytoplasm Citation[26]. After this, the precursor miRNA is further processed by Dicer (RNase III enzyme) to generate an approximately 22-nucleotide mature miRNA–miRNA duplex Citation[28]. The mature miRNA duplex has one guide strand, which is selected by the argonaute protein (endonuclease) to be integrated into an RNA-induced silencing complex (RISC) to form the miRNA–RISC assembly, which executes the regulatory function of miRNA Citation[29]. The other strand, known as the ‘passenger strand’, is degraded as a RISC complex substrate Citation[30].
The binding of miRNAs to their mRNA targets does not require exact complementarity; hence, each miRNA displays a promiscuous character targeting more than 100 mRNAs. This characteristic enables miRNAs to regulate up to 60% of the human genome and hence they are often implicated in various biological processes – both physiological and pathophysiological conditions Citation[31,32]. miRNAs generally act on their target mRNA by binding to the 3′-UTR and affecting post-transcriptional processes. Besides being known as translational inhibitors, miRNAs have also been reported to participate in gene activation Citation[33–36]. Inhibitory effects of miRNAs are mediated via two mechanisms. The first mechanism is when perfect binding occurs between the miRNA and its target mRNA causing the latter to be degraded through cleavage. The other mechanism takes place when the binding is imperfect, leading to translation repression whereby the miRNA-repressed mRNAs are engulfed into the P-bodies for storage Citation[37]. Post-transcriptional regulation in P-bodies is complicated. The P-bodies consist of several protein factors, including XRN1 (cytoplasmic 5′→3′ exoribonuclease), the decapping enzyme DCP2 and its cofactors, which colocalize together with their mRNA targets to regulate gene expression Citation[38]. Identification of the several proteins present in the P-bodies suggests that they not only serve as mRNA degradation sites but also participate in a broad range of post-transcriptional processes such as mRNA surveillance, RNA interference and translational repression or initiation Citation[38].
miRNAs in insulin-secreting cells (pancreatic β-cells)
Pancreatic β-cells play the initiating role in the complex mechanism of glucose homeostasis. These cells are involved in the production of insulin. The involvement of miRNAs in the insulin pathway begins as early as in the development and maintenance of the pancreatic islets. Lynn et al. demonstrated that ablation of Dicer 1 in mice blocked miRNA processing, which led to defects in the development of endocrine pancreatic lineages, especially the insulin-producing β-cells Citation[39]. A more recent study by Melkman-Zehavi et al. reported that Dicer-1-deficient β-cells showed a dramatic decrease in insulin at both the mRNA and protein levels, which renders the mice diabetic Citation[40]. The team demonstrated that silencing miR-24, miR-26, miR-148 or miR-182 independently in β-cells or primary islets was associated with increased levels of transcriptional repressors, namely Bhlhe22 and Sox6. This, in turn, led to the downregulation of insulin promoter activity and its corresponding mRNA levels. These studies highlight the importance of miRNAs in the maintenance of β-cells, which are, in turn, crucial for normal insulin production. Identification of miRNAs that were found to affect insulin transcription renders these miRNAs as potential therapeutic targets whereby insulin production can be regulated by modulating their expression accordingly.
Among the handful of miRNAs identified to be preferentially expressed in the pancreas, miR-375 is the most studied. miR-375 is abundantly expressed in the islet cells and has functional control over glucose-stimulated insulin release. Overexpression of miR-375 was found to inhibit insulin secretion, while administration of its antagomirs reverted insulin release back to normalcy Citation[2]. Bioinformatics search predicted Mtpn, t-SNAREs yeast homolog 1A, p38 MAPK, Max-interacting protein 1 and monocarboxylic acid transporter member 8 as miR-375 targets Citation[2]. These molecules participate in insulin production and secretion, as well as β-cell differentiation and function. Increasing miR-375 expression reduced Mtpn and t-SNAREs yeast homolog 1A to a significant level, although transfection with its antagomirs was only effective in upregulating Mtpn expression Citation[2]. Functionally, Mtpn regulates the actin network in membrane docking and fusion for insulin exocytosis Citation[41,42]. In addition, siRNA silencing of Mtpn has also been shown to attenuate insulin release, supporting the hypothesis that negative regulation of Mtpn by miR-375 is associated with insulin release, which ultimately determines glucose homeostasis in the circulation Citation[2].
miR-375-knockout mice exhibited diabetic phenotypes such as hyperglycemia and glucose intolerance due to reduced β-cell mass but increased α-cell number Citation[43]. Despite showing normal insulin secretion and clearance, decreased β-cell mass affected the insulin expression and adaptation to insulin resistance. Furthermore, increased number of α-cells consequently led to enhanced gluconeogenesis and hepatic glucose output due to a raised plasma glucagon level Citation[43]. These reports have highlighted the significance of miR-375 in maintaining β-cell function as well as the critical regulation of insulin expression and release for glucose homeostasis. Hence, dysregulation of miR-375 level is potentially detrimental to the metabolic equilibrium of the organism.
Appropriate insulin release from pancreatic β-cells is essential in blood glucose homeostasis and miRNAs have been identified to be involved in the regulation of insulin exocytosis. Elevated levels of miR-9 exert a negative effect on insulin secretion by inhibiting the transcription factor onecut-2, which subsequently increase granuphilin/Slp4, a negative regulator of insulin release Citation[44]. miR-96 has shown similar effects on granuphilin independent of onecut-2 Citation[45]. Instead, it targets Noc2, which is required for insulin exocytosis. miR-124a was also implicated in the insulin exocytotic machinery by directly targeting Foxa2 Citation[46]. Negative regulation of miR-124a on Foxa2 brings about inhibition of the downstream factors such as Kir6.2 and Sur-1, as well as Pdx-1, which are important for insulin secretion Citation[46]. Overexpression of miR-124a was also found to modulate other insulin exocytosis-related proteins, including Mtpn, Rab27a, Noc2, SNAP25, Rab3A and synapsin-1A. These data suggest that several of these miRNAs work in a cooperative manner to achieve optimal insulin secretory capacity.
The initial phase of T1D is marked by infiltration of immune cells in the pancreatic islets such that the β-cells are exposed to proinflammatory cytokines such as TNF-α, IFN-γ and IL-1β. Pancreatic β-cells that are chronically exposed to these proinflammatory mediators not only showed reduced insulin content and secretion but also became sensitized to apoptosis. Global miRNA profiling of these treated cells revealed a significant increase in miR-21, miR-34a and miR-146 expression Citation[5]. In addition, the islets of nonobese diabetic mice also showed enhanced expression of these miRNAs upon development of prediabetic insulitis. Antisense treatment against these miRNAs reduced apoptosis and improved glucose-stimulated insulin release. These findings were in conjunction with a later study by Lovis et al. who demonstrated the significance of miR-34a in β-cell function Citation[7]. Increased expression of miR-34a was found in a MIN6B1 cell line subjected to palmitate to induce β-cell dysfunction. miR-34a suppressed glucose-stimulated insulin secretion by repressing VAMP2 expression, an important vesicle protein in β-cell exocytosis Citation[7]. The same study also reported high levels of miR-34a and miR-146 in the islets of diabetic db/db mice. Antagomir administration against miR-34a or miR-146 was able to attenuate the apoptotic rate of palmitate-subjected cells. However, it was not able to restore insulin secretion.
miRNA profiling in glucose-responsive as well as nonresponsive MIN6 cells identified a panel of ten miRNAs (miR-27a, miR-130a, miR-192, miR-200a, miR-320, miR-337, miR-369-5p, miR-379, miR-410 and miR-532), which were downregulated in the latter group Citation[47]. Among these, knockdown of miR-410, miR-200a and miR-130a independently showed a reduction in the glucose-stimulated insulin secretion. Pre-miR transfections suggested that increasing miR-410 levels could enhance glucose-induced insulin release in MIN6 cells. An independent study in MIN6 cells, which were chronically subjected to high glucose exposure, identified 61 differentially expressed miRNAs compared with those cultured in normal glucose level Citation[48]. Follow-up validation work confirmed the upregulation of miR-124a, miR-107 and miR-30d, while miR-690, miR-484 and miR-296 showed significant reduction in expression upon prolonged high glucose treatment. Among these glucose-related miRNAs, Tang et al. focused on investigating the role of miR-30d in pancreatic β-cell function and subsequently discovered that miR-30d mediated glucose-dependent insulin transcription but not secretion Citation[48]. The group demonstrated that introduction of miR-30d could increase insulin gene transcription while inhibition of its activity completely abolished the glucose-stimulated insulin gene transcription. Pdx-1 and NeuroD1 are two β-cell-specific transcription factors of insulin gene transcription that harbor miR-30d binding sites. However, modulation of miR-30d expression did not show significant effects on any of the transcription factors, suggesting that miR-30d could work via inhibition of unknown insulin gene suppressors. Collectively, these studies revealed the coordinated regulatory network of miRNAs in controlling an extensive range of mRNAs involved in maintaining insulin synthesis and secretion and hence dysregulation of these miRNAs could potentially contribute to the manifestation of diabetes.
miRNAs in insulin-responsive tissues (liver, adipose tissue & skeletal muscle)
Blood glucose homeostasis also requires the insulin target cells to respond effectively to the hormone. The peripheral tissues, namely the liver, adipose and skeletal muscles, are the three main target tissues in the body that engage in insulin action and hence glucose uptake from the blood. There are several physiological and pathological factors, such as diet, pregnancy, stress and obesity that can affect the sensitivity of these tissues toward insulin. When the sensitivity of the peripheral tissues is compromised such that they can no longer respond appropriately to insulin, a condition called ‘insulin resistance’ occurs, leading to glucose accumulation in the blood. The failure to maintain glucose homeostasis accelerates the progression of T2D. Although the actual mechanisms underlying insulin resistance are yet to be elucidated, it has been proven that an miRNA network is implicated in the regulation of insulin signaling in the peripheral tissues.
Liver
The liver plays a crucial role in glucose homeostasis and hence any disturbance in the equilibrium of the liver will affect the glucose balance in the body. miRNAs participate in hepatic pathophysiology upon the development of diabetes. The importance of miRNA in liver physiology is evident in a study by Sekine et al. where selective deletion of Dicer in the liver at early birth led to mild hyperglycemia in the fed state, while in the fasting state it caused severe hypoglycemia due to glycogen deficiency Citation[49]. Herrera et al. compared the global miRNA expression in the liver and adipose tissues of spontaneously diabetic Goto-Kakizaki (GK) rats with those in normoglycemic Brown Norway rats Citation[50]. Out of 170 miRNAs detected in both insulin target tissues, miR-125a showed the most significant upregulation in the liver and a lower fold-change in the adipose tissues. In silico analysis of miR-125a targets revealed genes that are involved in glucose and lipid metabolism. A later study by the same group included another normoglycemic control – Wistar–Kyoto rats – that are more genetically related to the GK rats in comparison to Brown Norway rats Citation[50]. To get an overall picture of the miRNA expression, all three insulin target tissues were included in the study. The team identified a group of 18 miRNAs that were differentially expressed. Among these 18 miRNAs, miR-191, miR-193 and miR-195 showed significant upregulation while miR-200a expression was reduced when compared with either of the normoglycemic controls. miR-222, miR-27a, miR-195, miR-103 and miR-10b were found to have expression patterns consistent with glycemic status, suggesting their implication in the primary events of T2D pathogenesis. Interestingly, miR-125a, which showed the most significant change in their previous study, was not reported.
There are other studies using different models to investigate the implication of miRNAs in the pathogenesis of diabetes. In the livers of the streptozotocin (STZ)-induced T1D mouse model, miR-34a and miR-122 expression were found to show significant up- and down-regulation, respectively Citation[6]. miR-34a has previously been discussed for its important role in β-cell function, whereas miR-122 is a liver-specific miRNA that is also most abundantly expressed in the liver. Being the most abundant miRNA in the liver, miR-122 is implicated in several hepatic functions. The dysregulation of these two miRNAs, miR-34a and miR-122, in diabetic livers further strengthens their implications in hepatic abnormalities and hyperglycemia.
Several other miRNAs that have been associated with hepatic physiology and/or glucose metabolism are summarized in . Besides being a regulator of glucose homeostasis, the liver also participates in lipid metabolism, and many miRNAs that have been associated with hepatic pathogenesis are also related to obesity, another hallmark of diabetes. These miRNAs will be discussed in relation to lipid metabolism in the later sections of this article.
Adipose tissue
The adipose tissue is another critical regulator of glucose metabolism. An excessively abnormal amount of adipose tissue had been associated with the pathogenesis of diabetes. It is believed that imbalance in glucose homeostasis partially relies on the insensitivity or resistance of the adipose tissue to the action of insulin. Insulin induces lipogenesis, whereby blood glucose is converted into fatty acids for energy storage. In recent years, several miRNAs have been identified to be preferentially expressed in the adipose tissue and to regulate several biological processes in the tissue, including adipogenesis and adipocyte differentiation, as well as insulin action and fat metabolism Citation[51].
miR-143 is known to regulate adipocyte differentiation and inhibiting it leads to reduced adipogenesis Citation[52]. Interestingly, expression of miR-143 is downregulated in the adipose tissue of obese mice Citation[52,53]. miR-103 and miR-107 are two miRNAs that function in a similar way to miR-143 in accelerating adipogenesis. These two miRNAs target genes that are involved in the regulation of cellular acetyl-CoA and lipid levels Citation[54]. Zaragosi et al. performed deep sequencing in the search of miRNAs that show differential expression during adipogenesis of adipose tissue-derived stem cells Citation[55]. The team identified miR-642a-3p as an adipocyte-specific miRNA and the miR-30 family as an important regulator of human adipogenesis. Silencing the expression of the miR-30 family was found to block adipogenesis, whereas overexpressing miR-30a induced this process. A separate study by Karbiener et al. discovered that miR-27b levels decreased with adipogenesis Citation[56]. Overexpression of miR-27b was shown to inhibit the expression of PPAR-γ and C/EBP-α in the early stage of adipogenesis. Nevertheless, miR-27b induced the suppression of the adipogenic marker gene, as well as triglyceride accumulation at later stages. In support of these findings, the 3′-UTR of PPAR-γ was found to harbor a binding site for miR-27b. Another miRNA that has been identified to target PPAR family members, particularly PPAR-α, is miR-519d Citation[57].
Diabetic studies in adipose tissue identified enhanced expression of the miR-29 family during hyperglycemia and hyperinsulinemia Citation[50]. Furthermore, upregulation of miR-29a was also observed in the 3T3-L1 adipocyte cell line upon high glucose exposure Citation[4]. In a similar study by He et al., the miR-29 family members were all upregulated in the adipose tissue of diabetic GK rats, and also in vitro in 3T3-L1 adipocytes that were subjected to high glucose Citation[58]. Introduction of miR-29 in 3T3-L1 adipocytes blunted insulin-stimulated glucose uptake by inhibiting insulin signaling via the AKT signaling pathway. Likewise, elevated levels of miR-320 were observed in insulin-resistant 3T3-L1 adipocytes and antagomir treatment was able to improve insulin sensitivity via regulation of the insulin–PI3K signaling pathways Citation[59]. Interestingly, these therapeutic effects were only observed in the insulin-resistant adipocytes and not the normal cells. Collectively, these results suggest the important roles of miRNAs in regulating the insulin signaling pathway in the adipose tissues and hence modulation of these miRNAs individually and/or collectively could serve as a promising therapeutic approach against diabetes. Other miRNAs that have been reported to be involved in certain diabetes-related biological pathways in the adipose tissue are listed in .
Skeletal muscle
Skeletal muscle accounts for approximately 75% of insulin-stimulated glucose uptake, serving as the primary site for glucose uptake post-prandially. Insulin resistance in the skeletal muscle is also a hallmark of early onset of T2D. Many studies in both diabetic animal models and patients have revealed a list of miRNAs that showed dysregulation in the skeletal muscles . The impact of insulin on the global miRNA expression in skeletal muscle was investigated by Granjon et al. by comparing the miRNA expression in muscle biopsies of healthy subjects and after 3-h euglycemic–hyperinsulinemic clamp Citation[60]. miR-1, miR-133a, miR-206 and miR-29a/c are among the downregulated miRNAs, which are also known to be involved in muscle development and are highly expressed in insulin-sensitive tissues. Interestingly, there was no significant difference in the expression of both miR-1 and miR-133a in the skeletal muscle of diabetic patients compared wtih healthy controls. Gallagher et al. observed a similar reduction in miR-133a levels in T2D patients, which correlated with higher fasting glucose levels and other clinical parameters Citation[61]. Nevertheless, downregulation of miR-1, miR-133a and miR-206 after 3-h euglycemic–hyperinsulinemic clamp was not observed in a similar study by Nielsen et al. Citation[62].
The role of muscle-specific miR-1 and miR-133 in glucose homeostasis was also studied in vitro using cardiomyocytes. miR-133a/b was found to suppress glucose transporter 4 (GLUT4) expression, resulting in reduced insulin-stimulated glucose uptake, and this effect was postulated to be mediated by inhibition of Kruppel-like transcription factor KLF15, a direct target of miR133a/b Citation[63]. Other identified targets of miR-133a/b include human ether-a-go-go and KCNQ1, which are involved in the formation of K+ current channel in the heart and could be implicated in the long QT syndrome of diabetic patients Citation[64,65]. On the other hand, apoptosis in cardiomyocytes exposed to high glucose is associated with elevated miR-1 levels Citation[66]. IGF-1 and IGF-1 receptor are two known targets of miR-1 that could be involved in this process Citation[67].
Reduced expression of miR-24 in diabetic rat muscle was found to be inversely correlated with p38 MAPK expression Citation[45]. p38 MAPK promotes the activation of myocyte-enhancer factor 2, a muscle-specific transcription factor that regulates the transcription of insulin-responsive GLUT4 Citation[68]. Downregulation of miR-24 with a simultaneous increase of p38 MAPK activity in GK rat muscle suggested an adaptation mechanism for skeletal muscle in response to higher glucose concentration. Similarly, downregulation of miR-126 could also be a contributor to such an adaptive response. A validated target of miR-126 is p85β, an isoform of the regulatory subunit of PI3K, which is important in PI3K/AKT signaling for subsequent GLUT4 translocation to the membrane in skeletal muscle Citation[69]. As such, downregulation of miR-126 could be a way of promoting GLUT4 translocation for more glucose uptake.
In addition to these published reports, miR-144 has also been identified as a direct regulator of insulin receptor substrate-1 (IRS1) Citation[8]. IRS1 is a key component of the insulin signaling cascade, which was observed to be downregulated in T2D. Also the downregulation of IRS1 was found to be in conjunction with the increased miR-144 levels in T2D. Upregulation of miR-144 was found in all three insulin target tissues (liver, adipose and skeletal muscle), which were also reflected in the whole blood and exosomes of the T2D rat model. In vitro studies showed that miR-144 could indeed regulate IRS1 level and also confirmed that inhibition of miR-144 on IRS1 expression is direct in nature.
In general, these studies investigated the roles of miRNAs in diabetes by first identifying dysregulated miRNAs upon the initiation of the metabolic disorder, followed by in silico analysis of the possible mRNA targets. Although such methodology could churn out an extensive amount of data, its theoretical value is almost purely speculative. More intensive molecular work, such as modulation of the miRNAs of interest is necessary to identify specific miRNA–mRNA interactions. Nevertheless comparing results from different groups, which used different approaches to identify these miRNAs, could reveal the consistency in certain miRNA expression and hence facilitate the accurate identification of the crucial miRNAs that are involved in the pathogenesis of diabetes. Another interesting observation to note is the current trend in profiling miRNA expression in different insulin target tissues. Such approaches compare the dysregulated miRNAs across different tissues and hence allow the identification of miRNAs that are unique or similar in the different tissues. This would prove to be useful in future therapeutic applications whereby miRNA modulators could be delivered specifically to one or a few targeted tissues effectively.
miRNAs in diabetic complications
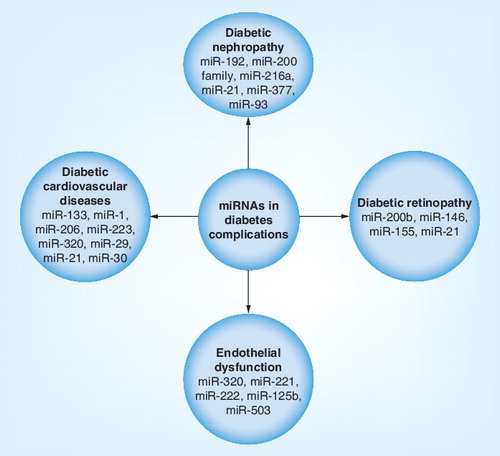
Most cases of diabetes are accompanied by specific pathological complications in the renal glomerulus (diabetic nephropathy [DN]) and the retina (diabetic retinopathy [DR]) as well as increasing the risk of cardiovascular diseases. Just as miRNAs contribute to the pathogenesis of diabetes, increasing reports have also associated dysregulation of miRNA expression with the progression of these diabetic complications .
Diabetic nephropathy
The role of miRNA in renal disease is evident in Dicer-knockout experiments, which highlighted the importance of miRNAs in renal development Citation[70]. DN is a kidney disease characterized by albuminuria due to kidney scarring. miRNAs that have been identified to have a role in the development and progression of DN include miR-192, the miR-200 family, miR-29, miR-216a, miR-21, miR-377 and miR-93. Upregulation of miR-192 in the kidneys was observed by Kato et al. in STZ-induced diabetes as well as in the db/db mouse Citation[71]. The group postulated that increased miR-192 levels were associated with downregulation of ZEB2, which subsequently led to increased collagen deposition in vivo. This observation correlates with matrix accumulation in DN. The team also proposed miR-216 as another important contributor of DN, which was also upregulated by TGF-β1, leading to increased col1a2 expression Citation[72]. miR-216 repression on YB-1, a potential target, may potentially contribute to fibrogenesis via TGF-β1 increase. On another note, the precise role of miR-192 became a controversy when Krupa et al. reported decreased miR-192 expression in patients with advanced DN Citation[73]. They showed that TGF-β1 decreased miR-192 expression in vitro and concluded that downregulation of miR-192 correlated with increased renal fibrosis in vivo Citation[71]. Of note, Wang et al. further confirmed that loss of miR-192 is associated with renal fibrosis and that TGF-β1 treatment decreased the level of miR-192 as well as miR-215, which also targets ZEB2 Citation[74]. On the contrary, connective tissue growth factor-induced fibrogenesis resulted in induction of miR-192 and miR-215. As expected, ZEB2, a target of both miRNAs, was downregulated. These findings indicated that miR-192 and miR-215 regulated ZEB2 expression independent of fibrosis. At the same time, these studies also highlight the complexity in the behavior of miRNAs.
The role of miR-200 family is linked to epithelial–mesenchymal transition. Gregory et al. demonstrated that miR-200 family regulates the epithelial phenotype by targeting ZEB1/2, which are known repressors of E-cadherin Citation[75]. This provided the link between the miR-200 family, the prosclerotic TGF-β pathways and epithelial–mesenchymal transition in fibrosis. Expression of the miR-200 family in renal cells showed significant downregulation in the presence of TGF-β1 and TGF-β2 Citation[74,76]. As miR-200a binds to the 3′-UTR of TGF-β2, decreased miR-200 family levels provided a feedback loop to relieve the repression on TGF-β2, thereby promoting fibrosis. miR-29 was also linked to fibrosis in renal disease as it was found to be downregulated in the fibrotic kidney of obstructive nephropathy Citation[77]. More importantly, ultrasound microbubble-mediated gene transfer of miR-29b was found to significantly inhibit renal fibrosis progression in obstructive nephropathy, suggesting a plausible therapeutic agent for renal fibrosis. miR-21, which targets phosphate and tensin homolog, was found to be downregulated in response to the early stage of DN in both in vivo and in vitro conditions Citation[78]. In vitro transfection of miR-21 mimic blocked mesangial cell proliferation and at the same time reduced albuminuria in diabetic db/db mice. By contrast, miR-377 was excessively expressed in spontaneous and STZ-induced models of DN Citation[79]. Treatment of TGF-β1 in mesangial cells subjected to high-glucose conditions also increased miR-377 expression. Upregulation of VEGF is often observed in DN and blocking its expression has shown beneficial effects in improving kidney function in diabetic animals. miR-93 was identified in hyperglycemic conditions both in vitro and in vivo as ‘signature miRNA’ that targets VEGF-A Citation[80]. miR-93 was underexpressed in hyperglycemic conditions due to decreased expression of the promoter of MCM7 gene in which miR-93 resides. By overexpressing miR-93, Long et al. were able to inhibit hyperglycemia-induced expression of VEGF, which could be beneficial for DN therapeutic application Citation[80].
Diabetic retinopathy
High blood sugar in diabetes results in swelling of the eye lens that eventually leads to blurred vision. The three major eye problems related to diabetes are cataracts, glaucoma and retinopathy. DR is one of the leading causes of blindness in most developed countries. It develops due to deregulation in the expression of certain growth factors upon sustained hyperglycemic conditions, which eventually affects the physiology of the retina. Compared with DN, not many studies have been performed in investigating the implication of miRNAs in DR pathogenesis. Two published work in this area were reported by McArthur et al. and Kovacs et al. Citation[81,82]. Using an STZ-induced T1D rat model, McArthur et al. reported downregulation of miR-200b in the retinas of the diabetic rats Citation[81]. In addition, human umbilical vein endothelial cells (ECs) and bovine retinal capillary ECs exposed to hyperglycemia resulted in reduced miR-200b expression and simultaneously increased its target VEGF-A expression. Introducing miR-200b antagomir into human umbilical vein ECs cultured in normal conditions was found to bring about the glucomimetic effect of VEGF-A upregulation. Importantly, injection of miR-200b mimic into the vitreous humor of one eye of diabetic mice decreased VEGF-A expression while intravitreal administration of miR-200b antagomir increased VEGF-A levels. To obtain global retinal miRNA expression, Kovacs et al. performed miRNA profiling in the retina and ECs of STZ-induced T1D rats Citation[82]. Three miRNAs, miR-146, miR-155 and miR-21, showed elevated expression in the retinal ECs of diabetic rats. These three miRNAs were identified to be regulated by NF-κB, a regulator of immune response, which plays an important role in early DR. Moreover, overexpression of miR-146 could inhibit IL-1β-induced NF-κB activation in retinal ECs, suggesting a regulatory feedback loop that controls both NF-κB and miR-146 expression.
Cardiovascular diseases
Sustained hyperglycemia, such as in the case of diabetes, could lead to hypertension and heart valve defects. These conditions eventually manifest as cardiac hypertrophy, which is the thickening of the myocardial wall and hence reduction in the ventricular chambers. miR-133, a muscle-specific miRNA that is abundantly expressed in the heart, regulates myogenesis. Decreased cardiac expression of miR-133 in both mouse and human models of cardiac hypertrophy suggests its implication in cardiac diseases Citation[83]. In vitro overexpression of miR-133 was shown to inhibit cardiac hypertrophy, while suppression of the miRNA expression induced hypertrophy. The same study identified miR-1 to behave similarly to miR-133. Glucose uptake into cardiomyocytes is primarily mediated by GLUT4. Horie et al. observed that overexpression of miR-133 reduced GLUT4 expression and thus insulin-induced glucose uptake in cardiomyocytes is compromised Citation[63]. Furthermore, miR-133 also targets the Kruppel-like transcription factor, which promotes GLUT4 expression. With that, introducing miR-133 decoy into cardiac myocytes increased the expression of both Kruppel-like transcription factor and GLUT4, which in turn facilitated glucose uptake. An independent study by Shan et al. also described the upregulation of miR-1 and miR-206 in the myocardium of hyperglycemic rat as well as in rat neonatal cardiomyocytes that were subjected to high glucose levels Citation[84]. The increase in miR-1 and miR-206 suppressed heat-shock protein 60 expression, an inhibitor of cardiomyocyte apoptosis, suggesting a role of miR-1 and miR-206 in diabetes-induced cardiomyocyte death.
Ectopic expression of miR-21, miR-29 and miR-320 has also been reported in diabetes-related cardiac diseases. Abnormal elevation of miR-21 in failing hearts is particularly observed in cardiac fibroblasts Citation[85]. Increased expression of miR-21 is also associated with increased ERK signaling via blocking Spry1 expression, an ERK inhibitor. Activated ERK resulted in increased collagen expression, while knocking down miR-21 to decrease ERK activity was found to reduce interstitial fibrosis. In vivo delivery of miR-29 inhibitors via tail-vein injection was found to induce collagen expression not only in the heart but also in the kidney and liver Citation[86]. Enhanced expression of miR-320 expression is associated with several angiogenic factors in diabetic myocardial microvascular ECs Citation[87]. Some of these angiogenic factors, including VEGF, IGF-1 and IGF-1R, are related to diabetic cardiomyopathy.
miRNAs in obesity
It has long been established that obesity is a hallmark of T2D. Obesity is characterized with impaired adipocyte function and abnormalities in adipokine secretion. Obesity and T2D are associated through insulin resistance. Obese individuals released increased amounts of non esterified fatty acids, which contribute to insulin resistance. Furthermore, obese subjects who have their fat distributed predominantly in the central abdomen are less insulin sensitive than lean individuals with a more peripheral distribution of fat. Therefore, obesity-related miRNAs are also potential contributors to the development of diabetes. miRNA profiling of human fat cells and subcutaneous adipose tissue identified the increased expression of miR-100, miR-125b, miR-221 and miR-34a as well as the downregulation of miR-130b, miR-185 and miR-210 in obese individuals Citation[88]. Interestingly, the miRNA profiles in human pre-adipocytes and mature adipocytes revealed a different expression pattern. miR-130b and miR-210 showed reduced expression during adipocyte differentiation as well as in the subcutaneous fat depots of obese individuals. miR-100, miR-125b and miR-221, which were upregulated in the obese subjects, showed downregulation during adipocyte differentiation and maturation Citation[88]. miR-143, which regulates adipocyte differentiation, showed positive correlation with bodyweight as well as mesenteric fat weight Citation[89]. Investigation by Kloting et al. reported significant correlations between the expression levels of miR-17-5p, miR-132, miR-134, miR-145, miR-181a, miR-197 and miR-99a and adipose tissue morphology or key metabolic parameters such as visceral fat area, HbA1c, fasting plasma glucose and circulating leptin, adiponectin and IL-6 Citation[90]. In addition to these findings, expression levels of miR-17-5p and miR-132 were reported to show significant differences between obese and non obese omental fat Citation[91]. Dysregulation of these two miRNAs were also observed in the blood of obese subjects. Furthermore, the altered expression of miR-17-5p and miR-132 were found to correlate significantly with BMI, fasting blood glucose and glycosylated hemoglobin. Overall, these studies provided evidence for the implication of miRNAs in obesity.
Besides investigating the miRNAs that showed altered expression during obese conditions, it is also important to study the miRNAs that are implicated in lipid metabolism . Dysregulation of miRNAs controlling the cholesterol metabolism may contribute to triglyceride abnormalities and lipolysis. These two processes are early-stage manifestations that amplify many metabolic derangements as characterized in insulin resistance.
The adipose tissue emerges as the mediator in the pathophysiology of obesity in diabetes. Hence, miRNAs that have been discussed previously under ‘miRNAs in adipose tissue’ are also important key players in obesity and hyperlipidemia. In addition to adipose tissue, there have also been reports of miRNA dysregulation in other metabolic tissues that are affected by obesity. The expression of miR-122 in the liver regulates cholesterol synthesis. Beneficial effects of anti-miR-122 treatment in lowering plasma cholesterol levels have been proven in obese rodents and normal non-human primates Citation[92,93]. Furthermore, miR-122 has become one of the most extensively studied miRNAs and its therapeutic effect against hepatitis C virus (HCV) has progressed to a Phase IIa clinical trial Citation[201].
To date, miR-33, miR-122, miR-370 and miR-378/378* are the few miRNAs that have been reported as potent post-transcriptional regulators of cholesterol homeostasis, fatty acid metabolism and lipogenesis. miR-370 functions in a similar manner to miR-122 in regulating lipid metabolism. In vitro transfection of sense or antisense miR-122 or miR-370 was found to up- or downregulate, respectively, the expression levels of sterol-regulatory element-binding protein 1c, diacylglycerol acyltransferase 2, fatty acid synthase and acyl-CoA carboxylase 1, which are regulators of fatty acid and triglyceride synthesis Citation[94]. Furthermore, miR-370 reduces fatty acid oxidation by targeting carnitine palmitoyl transferase, a mediator for the transport of long-chain fatty acids across membrane. It was also found that the effects of miR-370 on lipogenic genes were blocked with antisense treatment against miR-122. These findings suggested miR-370 as an additional regulator of cholesterol metabolism, which possibly exerts its effects via miR-122 and subsequently leads to the accumulation of hepatic triglycerides. miR-378/miR-378* regulates lipid metabolism whereby its overexpression during adipogenesis enhanced triacylglycerol accumulation Citation[95]. Similarly, transfection of miR-378/378* in adipocytes increased the expression levels of fatty acid metabolism genes, including fatty acid-binding protein 4, fatty acid synthase and stearoyl-coenzyme A desaturase Citation[95].
Another key regulator of lipid metabolism is miR-33, which is located within intron 16 of human SREBF-2, a master switch in controlling genes involved in cholesterol uptake and synthesis Citation[96,97]. miR-33a is cotranscribed with SREBF-2 upon cholesterol depletion and their coexpression is highly conserved across mammals Citation[97–99]. In addition, miR-33 is known to repress several genes that are involved in cholesterol export and fatty acid oxidation. One of the validated gene targets of miR-33 is ATP-binding cassette transporter, subfamily A, member 1 (ABCA1), a transporter for cholesterol efflux Citation[96,97]. Functionally, introduction of sense miR-33 in macrophages resulted in reduced cholesterol efflux to ApoA1, a protein that promotes cholesterol efflux from tissues to the liver for excretion. By contrast, inhibition of endogenous miR-33 increased ABCA1 protein expression and a simultaneous increase in cholesterol efflux to ApoA1. Another target of miR-33 is Niemann–Pick disease, type C1 (NPC1) Citation[96,98]. There are two miR-33-binding sites in the 3′-UTR of NPC1 in humans. Interestingly, NPC1 acts along with ABCA1 for cholesterol efflux to ApoA1, thus, suppressive effects of miR-33 were coordinated in the regulation of both mRNA targets Citation[100]. A recent study by Rayner et al. investigated the effects of anti-miRNA oligonucleotides targeting both miR-33a/b on African green monkeys Citation[101]. Notably, increased hepatic ABCA1 expression and plasma high-density lipoprotein levels were observed over 12 weeks following treatment. Multiple miR-33 targets involved in fatty acid oxidation, including CROT, CPT1A, HADHB and PRKAA1, were increased while those that regulate fatty acid synthesis, such as SREBF1, FASN, ACLY and ACACA, showed reduced expression. Overall, this resulted in decreased plasma very-low-density lipoprotein-associated triglycerides, which may potentially be useful in treating dyslipidemias.
The clinical perspective
The discovery of miRNAs has brought the understanding of gene regulation to a whole new platform. The revelation of their roles in several pathologies, including diabetes, has revolutionized the otherwise traditional methods that scientists have been working on. Diabetes is a complex metabolic disease with several factors involved; thus, the identification of the disease biomarkers has remained a challenge. Recent studies employing clinical samples of diabetic patients have provided useful data as well as identifying potential early biomarkers of diabetes. More recently, several studies have taken the advantage of the stable extracellular existence of miRNAs in body fluids, including serum, plasma, urine and blood, to perform noninvasive miRNA profiling. Growing evidence suggests that blood-based miRNA profiling could be a fundamental way of identifying circulating miRNAs as fingerprints of any diseases. This finding is valuable in diabetes research since obtaining tissue samples of diabetic patients, particularly those in early stages, are rare.
Laterza et al. introduced the concept that disease biomarkers are secreted into the circulation upon tissue injury Citation[102]. At about the same time, Jeyaseelan et al. also showed that miRNA expressed in the injured brain could be found in the peripheral blood of stroke models Citation[103]. These circulating miRNAs could possibly serve as important indicators of what is happening at the tissue level. An independent study by Kosaka et al. reported how these miRNAs are packaged into a secretory vesicle to be transported to the recipient cells for them to resume their functions Citation[104]. These secretory vesicles could be in the form of exosomes, apoptotic bodies or shedding microvesicles. Within these secretory vesicles are cellular gene products, including miRNAs, mRNAs and proteins Citation[105]. As such, they serve as mediators for cell-to-cell communication by facilitating the exchange of molecular components. To date, the contents enclosed within the exosomes of multiple organisms have been collated in a database named ExoCarta Citation[202].
One of the earlier studies of miRNA characterization in serum with respect to diabetes was performed by Chen et al. Citation[9]. The team described an altered serum miRNA expression profile in diabetic patients compared with healthy controls. These findings showed that miRNAs are stably expressed in serum and their expression varies according to the disease state. Owing to insufficient details on the miRNA identities and clinical parameters of the patients under study, it is not possible to make correlations between miRNAs and disease progression. In the context of diabetes, the identification of early biomarkers would be beneficial in the management of the disease. A study by Zampetaki et al. profiled the plasma miRNA expression from a large cohort of T2D patients over a period of 10 years Citation[10]. Using age- and sex-matched controls, the study reported 13 differentially expressed miRNAs in the diabetic patients. Among these 13 miRNAs, the dysregulation of five miRNAs – miR-15a, miR-28-3p, miR-126, miR-223 and miR-320 – was observed well before the manifestation of the disease. This cluster of five miRNAs was able to identify up to 70% of the T2D patients. Interestingly, 52% of the healthy controls that developed T2D within the 10-year study were already categorized under the T2D group based on the expression of the five miRNAs. An independent study by our own group has also compared the global miRNA expression in healthy controls, impaired fasting glucose (prediabetes) and T2D patients Citation[8]. We identified eight miRNAs (miR-144, miR-146a, miR-150, miR-182, miR-192, miR-29a, miR-30d and miR-320) as potential ‘signature miRNAs’ that could distinguish prediabetic patients from those with overt T2D. The same pool of miRNAs was also found to be dysregulated in a rat model of T2D. By performing miRNA profiling in both blood and insulin-related tissues (pancreas, liver, adipose and skeletal muscle) of the rat model, we were able to compare the expression of the potential ‘signature miRNAs’ in both areas. This forms the basis of the connection between the events occurring at the tissue level and the observations made in the circulation. Notably, our data provided further evidence that circulating miRNAs in the blood could reflect those in the affected tissues. To further investigate the possible role of exosomes in miRNA transfer, we also examined the expression of the potential ‘signature miRNAs’ in the exosomes of the rat T2D model. As expected, the expression pattern of exosomal miRNAs was shown to be consistent with that in whole blood.
In contrast to T2D, there is less miRNA-based research focused on T1D pathogenesis, particularly in a clinical setting. Hezova et al. investigated the role of miRNAs in Tregs isolated from the peripheral blood samples of T1D patients Citation[106]. Tregs are important regulators of autoimmune diseases, such as in the case of T1D. Upregulation of miR-510 and underexpression of miR-191 and miR-342 were observed in the Tregs of T1D patients. For a more precise characterization of Tregs, the miRNA profile in Tregs was compared with that in conventional T cells. The study revealed that Tregs showed an increased miR-146a expression while eight other miRNAs (miR-20b, miR-31, miR-99a, miR-100, miR-125b, miR-151, miR-335 and miR-365) were found to be downregulated as compared with their expression in control T cells. Sebastiani et al. investigated the role of miRNAs in lymphocytes of patients with autoimmune diseases such as T1D Citation[107]. Overexpression of miR-326 was previously reported in multiple sclerosis and recently Sebastiani et al. reported similar upregulation of miR-326 in the peripheral blood lymphocytes of T1D patients in relationship with ongoing islet autoimmunity Citation[107]. In silico analysis revealed vitamin D receptor and erythroblastosis virus E26 oncogene homolog 1 as predicted targets of miR-326 that are highly involved in immune regulation.
These studies have provided evidence that circulating miRNAs could potentially serve not only as biomarkers of diseases but also as early prognostic markers. Nevertheless, there are some limitations to such approaches, particularly in measurement methods Citation[108]. Although circulating miRNAs can be easily measured with the many experimental techniques available, the consistency in miRNA expression usually differs across different platforms or manufacturers. This could be attributable to the short length of miRNAs and high sequence similarity among them. There is also no standard protocol available for miRNA isolation, which makes quantification and quality assessment challenging. Most importantly, normalization of miRNAs expression across samples remains a major issue for extracellular miRNAs. Global normalization against housekeeping genes can be used for cellular miRNAs but such approaches are not applicable for extracellular miRNAs. With no standard normalization method, a possible approach is to normalize the miRNA expression with other biomolecules in the body fluids, such as creatinine levels Citation[109,110].
Modulation of miRNAs could be the next RNA-based therapeutic tools against diabetes. This could be approached by first identifying the dysregulated miRNAs in the disease of interest. Following that, miRNAs that were upregulated can be inhibited by anti-miRs while the downregulated miRNAs can be restored via the reintroduction of the mature miRNAs. The biggest challenge of such approach would be the efficient delivery of miRNAs to the specific targeted sites while minimizing off-target effects. Although this is relatively at the stage of infancy, there are clinical reports on the successful delivery of miRNAs in the in vivo setting. miR-122 has shown an advancement in this area where its involvement in regulating cholesterol biosynthesis has been demonstrated in non human primates. miR-122 antagomirs were shown to bring down plasma cholesterol levels in African green monkeys to a maximum of 40% at 23 days after the treatment Citation[93]. In fact, miR-122 has now reached Phase II clinical trials for its implication in HCV infection. Miravirsen is the first miRNA-based drug to enter clinical trials, which works by inhibiting miR-122 expression, a miRNA that is important for HCV accumulation. So far, the results have been promising, as Miravirsen has been shown to be well tolerated in patients with chronic HCV infection and is able to suppress HCV RNA expression for a long period of time.
On the other hand, another research company, Mirna Therapeutics (TX, USA), is focusing more on miRNA replacement therapy in relation to cancer. For example, let-7 and miR-34, which are both tumor-suppressor miRNAs, showed loss of function in cancer and hence a possible therapeutic approach can be mediated by reintroducing an miRNA mimic into diseased tissue Citation[111]. When this miRNA expression is restored, it can reactivate pathways that interfere with cancer development, bringing about therapeutic benefits. The miRNA mimic simply replaces the depleted endogenous miRNA level and, since they share the same sequence as the naturally occurring miRNAs, any off-target effects are minimized. Both let-7 and miR-34 have shown promising laboratory-based results in the ‘miRNA replacement therapy’ project and researchers are currently investigating possible ways to enhance the delivery mechanism before proceeding to preclinical development. Although miRNA-based research in diabetes has not reached a similar level, current findings indicate a high potential for miRNAs to move towards a powerful clinical breakthrough in combating diseases, including diabetes.
Conclusion
miRNAs are rapidly emerging as key regulators of several biological and physiological functions. Alterations in miRNA expression have also been implicated in the pathogenesis of several diseases and conditions, including diabetes. Although several studies have identified miRNAs that are involved in glucose and lipid metabolism, only a handful of specific targets or pathways regulated by these miRNAs have been identified in diabetic conditions. Diabetes is a complex metabolic disorder, which chronically affects multiple organs as the disease progresses and is also known as the ‘silent killer’. The elucidation of the cross-talk between miRNAs, their multiple target genes and the disease development highlights the complexity of diabetes. The identification of circulating miRNAs could prove to be useful for diagnostic, prognostic and therapeutic applications in this debilitating condition. The current state-of-the-art technologies that we have at our disposal can be applied to modulate miRNA expression both in vitro and in vivo to reveal the therapeutic potential of individual miRNAs. Nevertheless, much work still remains to be done for the accurate identification of both miRNAs and their targets, as well as more refined approaches to target these molecules with high specificity.
Expert commentary
Diabetes is a chronic metabolic disorder depicted by altered glucose homeostasis. The diabetic population has been rapidly increasing globally, with 70% of cases found in low- and middle-income countries, as well as in countries where more people have transitioned from poverty to affluence. To date, diabetes represents the pharmaceutical companies' fastest growing market, with a huge sum of funds invested to prevent or treat this condition worldwide. Besides the heavy global burden of managing diabetes, it also imposes economic burdens such as lost productivity and slow economic growth to the nation. The antidiabetes drugs that are currently available only aid in managing diabetes but do not rectify the disorder permanently, thus, these treatments are lifelong. The huge economic burden imposed by the current management of diabetes and the lack of medications to resolve diabetes permanently call for novel and better therapeutics. miRNAs may represent a new hope that is worth investing in for future clinical development and advancement of diabetes.
miRNAs are a group of small, endogenous, noncoding RNA molecules discovered approximately a decade ago, and have now become key molecules in life sciences biomedical research. Functionally serving as regulators of gene expression, these small molecules have provided new insights into the RNA-based therapeutic approach. Throughout the years, a large number of reports have shown evidence of their involvement in various physiological and pathophysiological processes. It is now widely accepted that miRNA dysregulation affects the body's homeostasis and represents a common characteristic of several disease manifestations and health disorders. Despite the fact that miRNA research in the diabetes field is still in its early stages (ȣ10 years), laboratory results have generated very promising leads. Several miRNAs have been carefully identified to be key regulators of glucose and lipid metabolism (obesity) in relation to diabetes development and progression. Modulating the expression of these miRNAs has also shown both positive and negative effects in diabetes development. These findings suggest that manipulation of miRNA expression could also form part of the therapy for diabetic conditions. Recent reports on the identification of circulating miRNAs in the body fluids have also attracted the attention of scientists to utilize it as revenues for noninvasive methods of biomarker detection. This could prove to be extremely beneficial in diabetes research where biological samples are uncommon, especially in the early stages of the disorder. Taking advantage of the stable miRNA expression in the circulation, scientists are now working closely with clinicians to compare the findings from the bench with those observed at the bedside (patients) or vice versa.
Most importantly, research reports also suggest that miRNA signatures may not only serve as early biomarkers but also as both diagnostic and prognostic markers in diabetes mellitus. The discovery of the implication of miR-126 in diabetes is one such example. The inverse correlation between the circulating level of miR-126 and severity of diabetes suggested the strong potential of the miRNA as an early biomarker of diseases Citation[10]. In addition, miR-126 has also proven to be a significant indicator of diabetes manifestation in which its downregulation antedated the manifestation of the disease. Besides miR-126, reduced expression of miR-15a, miR-29b and miR-223 and elevation of miR-28-3p were also observed before the manifestation of diabetes Citation[10]. This strongly adds to the prognostic value of miRNAs in disease manifestation, particularly diabetes. Given the valuable and propitious findings collected thus far, miRNAs definitely offer a new promising direction for future diabetes management or even cure. Nevertheless, these results require validation in independent cohorts and more intensive research has to be carried out to develop miRNA-based drugs for diabetes.
Five-year view
The current treatment of diabetes, a chronic metabolic disorder that is rapidly growing across the world, relies on lifelong medications. It was not long ago that bariatric surgery was found to have anti-diabetic effects, whereby diabetes becomes resolved almost immediately within days of surgery. However, this weight loss procedure is only available to obese patients with a BMI above 35 kg/m2. The discovery of miRNAs has revolutionized the traditional manner of scientific research. miRNAs are small, non coding, endogenous RNA molecules that function as regulators of gene expression. This crucial role of miRNAs puts them at the crossroads of almost every biological process. With such important responsibilities on their shoulders, it is not surprising to see how dysregulated miRNA expression could result in disease manifestations. Moreover, miRNAs are now emerging as early biomarkers of diabetes whereby the expression of a group of miRNAs can now be used as significant predictors of diabetes manifestation. This is valuable to diabetes research because preventive measures would be more beneficial than corrective measures in a chronic disease such as diabetes.
Furthermore, the detection of altered expression of miRNAs in a temporal manner from early to advanced stages of diabetes will lay a platform for the identification of miRNAs that are useful in developing diabetes therapies. Less than a decade after their discovery, miRNAs are already making breakthroughs in clinical research. This is evident in miR-122, the first miRNA to be developed as a drug target. Miravirsen is an miRNA-based drug that targets miR-122, a miRNA required for HCV replication, and is currently showing favorable results in a Phase IIa clinical trial for HCV infection treatment. This rapid progression in miRNA-based clinical research raises the hope of discovering novel therapeutics to combat diabetes. The growing number of miRNA-based pharmaceutical companies, which is going hand-in-hand with the scientific community, is leading the development of innovative and novel therapeutics based on miRNAs. Results of such productive combinations are the development of miRNA-based cardiovascular drugs by miRagen Therapeutics, cancer therapy by Mirna Therapeutics as well as hepatitis C treatment by Santaris Pharma. miRNAs represent the base of a new generation of 21st century drugs in the medical field and, with such exponential progress, it will not be long until the invention of miRNA-targeted drug for diabetes that are available in the market.
Table 1. Implications of miRNAs in diabetes-associated hepatic pathophysiology and glucose metabolism.
Table 2. miRNAs associated with adipogenesis, adipocyte differentiation and obesity.
Table 3. Diabetes-related miRNAs in the skeletal muscle.
Key issues
• The incidence of diabetes cases has increased dramatically throughout the years, with approximately 6.4% of the world's population diagnosed with the metabolic disorder.
• The available treatment for diabetes involves lifelong drugs and its management imposes a huge economic burden globally.
• miRNAs are small, endogenously expressed, non-coding RNA molecules that are known to regulate gene expression. Since their discovery less than a decade ago, miRNAs have made their clinical breakthrough via the emergence of miravirsen, the first miRNA-based drug to reach clinical trials.
• Several miRNAs have been identified as being implicated in diabetes and a significant number of findings have been validated in both animal and human studies.
• The recent identification of circulating miRNAs provided valuable data in diabetes research. Altered miRNA expression can now be detected at early stages of diabetes or even before the manifestation of the disease by simply collecting body fluids of patients. This also constitutes a noninvasive method for the identification of early disease biomarkers.
• Furthermore, there has been increasing interest from industrial partners to develop miRNA-based therapeutics. Hence, miRNAs represent the base of a new generation of 21st century drugs in the medical field.
References
- Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc. Res. 93(4), 583–593 (2012).
- Poy MN, Eliasson L, Krutzfeldt J et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432(7014), 226–230 (2004).
- Ferland-McCollough D, Ozanne SE, Siddle K, Willis AE, Bushell M. The involvement of microRNAs in Type 2 diabetes. Biochem. Soc. Trans. 38(6), 1565–1570 (2010).
- Herrera BM, Lockstone HE, Taylor JM et al. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of Type 2 diabetes. Diabetologia 53(6), 1099–1109 (2010).
- Roggli E, Britan A, Gattesco S et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 59(4), 978–986 (2010).
- Li S, Chen X, Zhang H et al. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J. Lipid Res. 50(9), 1756–1765 (2009).
- Lovis P, Roggli E, Laybutt DR et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes 57(10), 2728–2736 (2008).
- Karolina DS, Armugam A, Tavintharan S et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in Type 2 diabetes mellitus. PLoS ONE 6(8), e22839 (2011).
- Chen X, Ba Y, Ma L et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18(10), 997–1006 (2008).
- Zampetaki A, Kiechl S, Drozdov I et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in Type 2 diabetes. Circ. Res. 107(6), 810–817 (2010).
- Kong L, Zhu J, Han W et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed Type 2 diabetes: a clinical study. Acta Diabetol. 48(1), 61–69 (2011).
- Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes 60(7), 1832–1837 (2011).
- Pandey AK, Agarwal P, Kaur K, Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell Physiol. Biochem. 23(4–6), 221–232 (2009).
- Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in Type 1 diabetes. Nature 464(7293), 1293–1300 (2010).
- Daneman D. Type 1 diabetes. Lancet 367(9513), 847–858 (2006).
- Jun HS, Yoon JW. A new look at viruses in Type 1 diabetes. Diabetes Metab. Res. Rev. 19(1), 8–31 (2003).
- Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann. NY Acad. Sci. 1084, 1–29 (2006).
- McCarthy MI. Genomics, Type 2 diabetes, and obesity. N. Engl. J. Med. 363(24), 2339–2350 (2010).
- Gastaldelli A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 93(Suppl. 1), S60–S65 (2011).
- Scheen AJ. Pathophysiology of Type 2 diabetes. Acta Clin. Belg. 58(6), 335–341 (2003).
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 29(9), 2114–2116 (2006).
- Scalea JR, Cooper M. Surgical strategies for Type II diabetes. Transplant. Rev. doi:10.1016/j.trre.2011.07.002 (2011) (Epub ahead of print).
- Schernthaner G, Morton JM. Bariatric surgery in patients with morbid obesity and Type 2 diabetes. Diabetes Care 31(Suppl. 2), S297–S302 (2008).
- Prevalence of overweight, obesity among adults with diagnosed diabetes – United States, 1988–1994, 1999–2002. MMWR Morbid. Mortal. Wkly Rep. 53(45), 1066–1068 (2004).
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of Type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54(10), 2506–2514 (2011).
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 6(5), 376–385 (2005).
- Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat. Genet. 38(Suppl.), S2–S7 (2006).
- Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood 108(12), 3646–3653 (2006).
- Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell 115(2), 209–216 (2003).
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2), 281–297 (2004).
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19(1), 92–105 (2009).
- Lim LP, Lau NC, Garrett-Engele P et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433(7027), 769–773 (2005).
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl Acad. Sci. USA 105(5), 1608–1613 (2008).
- Li LC, Okino ST, Zhao H et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl Acad. Sci. USA 103(46), 17337–17342 (2006).
- Mortensen RD, Serra M, Steitz JA, Vasudevan S. Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA – protein complexes (microRNPs). Proc. Natl Acad. Sci. USA 108(20), 8281–8286 (2011).
- Majid S, Dar AA, Saini S et al. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer 116(24), 5637–5649 (2010).
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20(5), 515–524 (2006).
- Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8(1), 9–22 (2007).
- Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56(12), 2938–2945 (2007).
- Melkman-Zehavi T, Oren R, Kredo-Russo S et al. miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. EMBO J. 30(5), 835–845 (2011).
- Gauthier BR, Wollheim CB. MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nat. Med. 12(1), 36–38 (2006).
- Tang X, Tang G, Ozcan S. Role of microRNAs in diabetes. Biochim. Biophys. Acta 1779, 697–701 (2008).
- Poy MN, Hausser J, Trajkovski M et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl Acad. Sci. USA 106(14), 5813–5818 (2009).
- Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J. Biol. Chem. 281(37), 26932–26942 (2006).
- Huang B, Qin W, Zhao B et al. MicroRNA expression profiling in diabetic GK rat model. Acta Biochim. Biophys. Sin. 41(6), 472–477 (2009).
- Baroukh N, Ravier MA, Loder MK et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J. Biol. Chem. 282(27), 19575–19588 (2007).
- Hennessy E, Clynes M, Jeppesen PB, O'Driscoll L. Identification of microRNAs with a role in glucose stimulated insulin secretion by expression profiling of MIN6 cells. Biochem. Biophys. Res. Commun. 396(2), 457–462 (2010).
- Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription. RNA 15(2), 287–293 (2009).
- Sekine S, Ogawa R, Mcmanus MT, Kanai Y, Hebrok M. Dicer is required for proper liver zonation. J. Pathol. 219(3), 365–372 (2009).
- Herrera BM, Lockstone HE, Taylor JM et al. MicroRNA-125a is over-expressed in insulin target tissues in a spontaneous rat model of Type 2 diabetes. BMC Med. Genomics 2, 54 (2009).
- Alexander R, Lodish H, Sun L. MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin. Ther. Targets 15(5), 623–636 (2011).
- Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58(5), 1050–1057 (2009).
- Jordan SD, Krüger M, Willmes DM et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 13(4), 434–446 (2011).
- Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol. Genet. Metab. 91(3), 209–217 (2007).
- Zaragosi LE, Wdziekonski B, Brigand KL et al. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 12(7), R64 (2011).
- Karbiener M, Fischer C, Nowitsch S et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem. Biophys. Res. Commun. 390(2), 247–251 (2009).
- Martinelli R, Nardelli C, Pilone V et al. miR-519d overexpression is associated with human obesity. Obesity 18(11), 2170–2176 (2010).
- He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 21(11), 2785–2794 (2007).
- Ling HY, Ou HS, Feng SD et al. Changes in microRNA profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin. Exp. Pharmacol. Physiol. doi:10.1111/j.1440-1681.2009.05207.x (2009) (Epub ahead of print).
- Granjon A, Gustin MP, Rieusset J et al. The microRNA signature in response to insulin reveals its implication in the transcriptional action of insulin in human skeletal muscle and the role of a sterol regulatory element-binding protein-1c/myocyte enhancer factor 2C pathway. Diabetes 58(11), 2555–2564 (2009).
- Gallagher IJ, Scheele C, Keller P et al. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in Type 2 diabetes. Genome Med. 2(2), 9 (2010).
- Nielsen S, Scheele C, Yfanti C et al. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J. Physiol. 588(Pt 20), 4029–4037 (2010).
- Horie T, Ono K, Nishi H et al. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 389(2), 315–320 (2009).
- Luo X, Xiao J, Lin H et al. Transcriptional activation by stimulating protein 1 and post-transcriptional repression by muscle-specific microRNAs of IKs-encoding genes and potential implications in regional heterogeneity of their expressions. J. Cell. Physiol. 212(2), 358–367 (2007).
- Zhang Y, Xiao J, Lin H et al. Ionic mechanisms underlying abnormal QT prolongation and the associated arrhythmias in diabetic rabbits: a role of rapid delayed rectifier K+ current. Cell Physiol. Biochem. 19(5–6), 225–238 (2007).
- Yu XY, Song YH, Geng YJ et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem. Biophys. Res. Commun. 376(3), 548–552 (2008).
- Elia L, Contu R, Quintavalle M et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120(23), 2377–2385 (2009).
- Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 252(1–2), 224–230 (2006).
- Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer 47(11), 939–946 (2008).
- Li JY, Yong TY, Michael MZ, Gleadle JM. Review: the role of microRNAs in kidney disease. Nephrology 15(6), 599–608 (2010).
- Kato M, Zhang J, Wang M et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl Acad. Sci. USA 104(9), 3432–3437 (2007).
- Kato M, Wang L, Putta S et al. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J. Biol. Chem. 285(44), 34004–34015 (2010).
- Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of microRNA-192 promotes fibrogenesis in diabetic nephropathy. J. Am. Soc. Nephrol. 21(3), 438–447 (2010).
- Wang B, Herman-Edelstein M, Koh P et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59(7), 1794–1802 (2010).
- Gregory PA, Bert AG, Paterson EL et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10(5), 593–601 (2008).
- Wang B, Koh P, Winbanks C et al. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 60(1), 280–287 (2011).
- Liu Y, Taylor NE, Lu L et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55(4), 974–982 (2010).
- Zhang Z, Peng H, Chen J et al. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS Lett. 583(12), 2009–2014 (2009).
- Wang Q, Wang Y, Minto AW et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 22(12), 4126–4135 (2008).
- Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 285(30), 23457–23465 (2010).
- McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 60(4), 1314–1323 (2011).
- Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest. Ophthalmol. Vis. Sci. 52(7), 4402–4409 (2011).
- Carè A, Catalucci D, Felicetti F et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 13(5), 613–618 (2007).
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J. Mol. Cell. Cardiol. 35(9), 1135–1143 (2003).
- Thum T, Gross C, Fiedler J et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456(7224), 980–984 (2008).
- van Rooij E, Sutherland LB, Thatcher JE et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105(35), 13027–13032 (2008).
- Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in Type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 36(2), 181–188 (2009).
- Ortega FJ, Moreno-Navarrete JM, Pardo G et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS ONE 5(2), e9022 (2010).
- Takanabe R, Ono K, Abe Y et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem. Biophys. Res. Commun. 376(4), 728–732 (2008).
- Klöting N, Berthold S, Kovacs P et al. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS ONE 4(3), e4699 (2009).
- Heneghan HM, Miller N, McAnena OJ, O'Brien T, Kerin MJ. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J. Clin. Endocrinol. Metab. 96(5), E846–E850 (2011).
- Esau C, Davis S, Murray SF et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 3(2), 87–98 (2006).
- Elmén J, Lindow M, Schütz S et al. LNA-mediated microRNA silencing in non-human primates. Nature 452(7189), 896–899 (2008).
- Iliopoulos D, Drosatos K, Hiyama Y, Goldberg IJ, Zannis VI. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J. Lipid Res. 51(6), 1513–1523 (2010).
- Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am. J. Physiol. Endocrinol. Metab. 299(2), E198–E206 (2010).
- Rayner KJ, Suárez Y, Dávalos A et al. miR-33 contributes to the regulation of cholesterol homeostasis. Science 328(5985), 1570–1573 (2010).
- Najafi-Shoushtari SH, Kristo F, Li Y et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328(5985), 1566–1569 (2010).
- Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl Acad. Sci. USA 107(27), 12228–12232 (2010).
- Horie T, Ono K, Horiguchi M et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl Acad. Sci. USA 107(40), 17321–17326 (2010).
- Wang MD, Franklin V, Sundaram M et al. Differential regulation of ATP binding cassette protein A1 expression and ApoA-I lipidation by Niemann – Pick type C1 in murine hepatocytes and macrophages. J. Biol. Chem. 282(31), 22525–22533 (2007).
- Rayner KJ, Esau CC, Hussain FN et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 478(7369), 404–407 (2011).
- Laterza OF, Lim L, Garrett-Engele PW et al. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 55(11), 1977–1983 (2009).
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39(3), 959–966 (2008).
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452 (2010).
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73(10), 1907–1920 (2010).
- Hezova R, Slaby O, Faltejskova P et al. microRNA-342, microRNA-191 and microRNA-510 are differentially expressed in T regulatory cells of Type 1 diabetic patients. Cell. Immunol. 260(2), 70–74 (2010).
- Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F. Increased expression of microRNA miR-326 in Type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab. Res. Rev. 27(8), 862–866 (2011).
- Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat. Res. 717(1–2), 85–90 (2011).
- Goldstein SL. Urinary kidney injury biomarkers and urine creatinine normalization: a false premise or not? Kidney Int. 78(5), 433–435 (2010).
- Wagner BD, Accurso FJ, Laguna TA. The applicability of urinary creatinine as a method of specimen normalization in the cystic fibrosis population. J. Cyst. Fibros. 9(3), 212–216 (2010).
- Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 70(18), 7027–7030 (2010).
- Zhao E, Keller MP, Rabaglia ME et al. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm. Genome 20(8), 476–485 (2009).
- Nakanishi N, Nakagawa Y, Tokushige N et al. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem. Biophys. Res. Commun. 385(4), 492–496 (2009).
- El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3´-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes 57(10), 2708–2717 (2008).
- Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol. Chem. 389(3), 305–312 (2008).
- Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol. Endocrinol. 23(6), 925–931 (2009).
- Qin L, Chen Y, Niu Y et al. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 11, 320 (2010).
- Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 12(9), 1626–1632 (2006).
- Andersen DC, Jensen CH, Schneider M et al. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes. Exp. Cell Res. 316(10), 1681–1691 (2010).
- Wang Q, Li YC, Wang J et al. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc. Natl Acad. Sci. USA 105(8), 2889–2894 (2008).
- Strum JC, Johnson JH, Ward J et al. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol. 23(11), 1876–1884 (2009).
- Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J. Cell. Physiol. 218(2), 444–449 (2009).
- Yang Z, Bian C, Zhou H et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 20(2), 259–267 (2011).
- Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc. Natl Acad. Sci. USA 105(40), 15417–15422 (2008).
- Kim YJ, Hwang SJ, Bae YC, Jung JS. miR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27(12), 3093–3102 (2009).
- Sun F, Wang J, Pan Q et al. Characterization of function and regulation of miR-24-1 and miR-31. Biochem. Biophys. Res. Commun. 380(3), 660–665 (2009).
- Tang YF, Zhang Y, Li XY, Li C, Tian W, Liu L. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells. OMICS 13(4), 331–336 (2009).
- Kinoshita M, Ono K, Horie T et al. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol. Endocrinol. 24(10), 1978–1987 (2010).
Websites
- Santaris. www.santaris.com
- ExoCarta. www.exocarta.org
miRNAs and diabetes mellitus
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertendo. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. Which of the following statements regarding microRNAs (miRNAs) is most accurate?
□ A They usually include several hundred nucleotides
□ B Genes coding for specific miRNAs are spread widely across chromosomes
□ C miRNAs regulate up to 60% of the human genome
□ D miRNAs act as translational inhibitors only
2. Which of the following statements regarding the roles of miRNAs within the pancreas is most accurate?
□ A miR-27a is the most studied
□ B Overexpression of miR-375 inhibits insulin secretion
□ C Only one or two miRNAs function to regulate insulin secretion
□ D Gene knockout studies of miRNAs have failed to demonstrate a difference in glucose-stimulated insulin secretion
3. miRNAs have which of the following roles in organs important in the pathogenesis of diabetes?
□ A miR-195 is the most abundant miRNA in the liver
□ B Inhibition of miR-143 increases adipogenesis
□ C miR-1 and miR-133a are overexpressed among individuals with diabetes compared with healthy controls
□ D Upregulation of miR-144 is evident in liver, adipose, and skeletal muscle tissues among individuals with Type 2 diabetes (T2D)
4. What should you consider regarding the clinical use of miRNAs among patients at risk for diabetes?
□ A The dysregulation of 5 miRNAs can identify up to 70% of T2D patients
□ B There is more research regarding miRNAs among Type 1 diabetes (T1D) vs T2D patients
□ C The devices to measure miRNAs have now been standardized across manufacturers
□ D Miravirsen works by inhibiting the expression of miR-375